Heat-Resistant Microporous Ag Die-Attach Structure for Wide Band-Gap Power Semiconductors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SiCp-Doped Ag Sinter Joining Paste
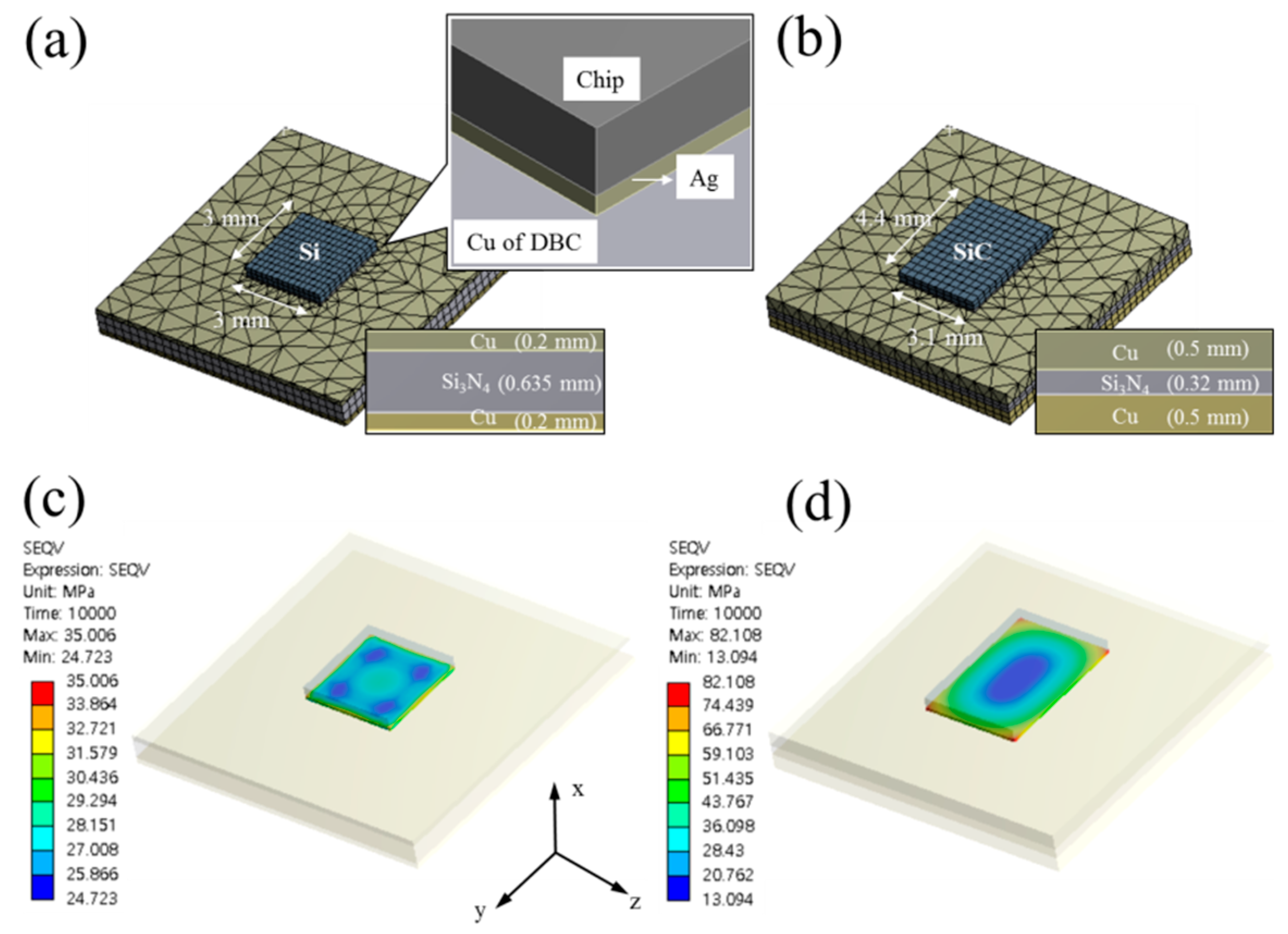
2.2. Bonding Components and Procedures
2.3. Characterization of Sintered Joints
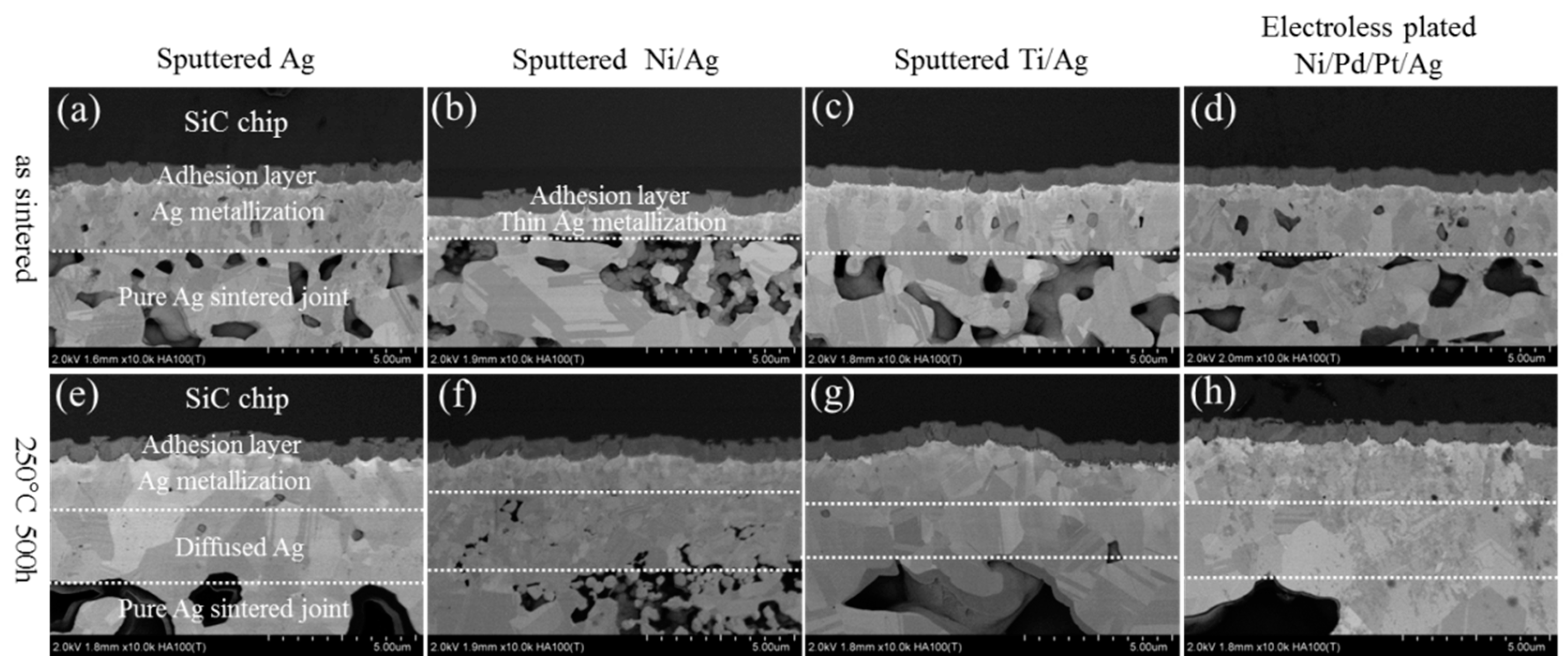
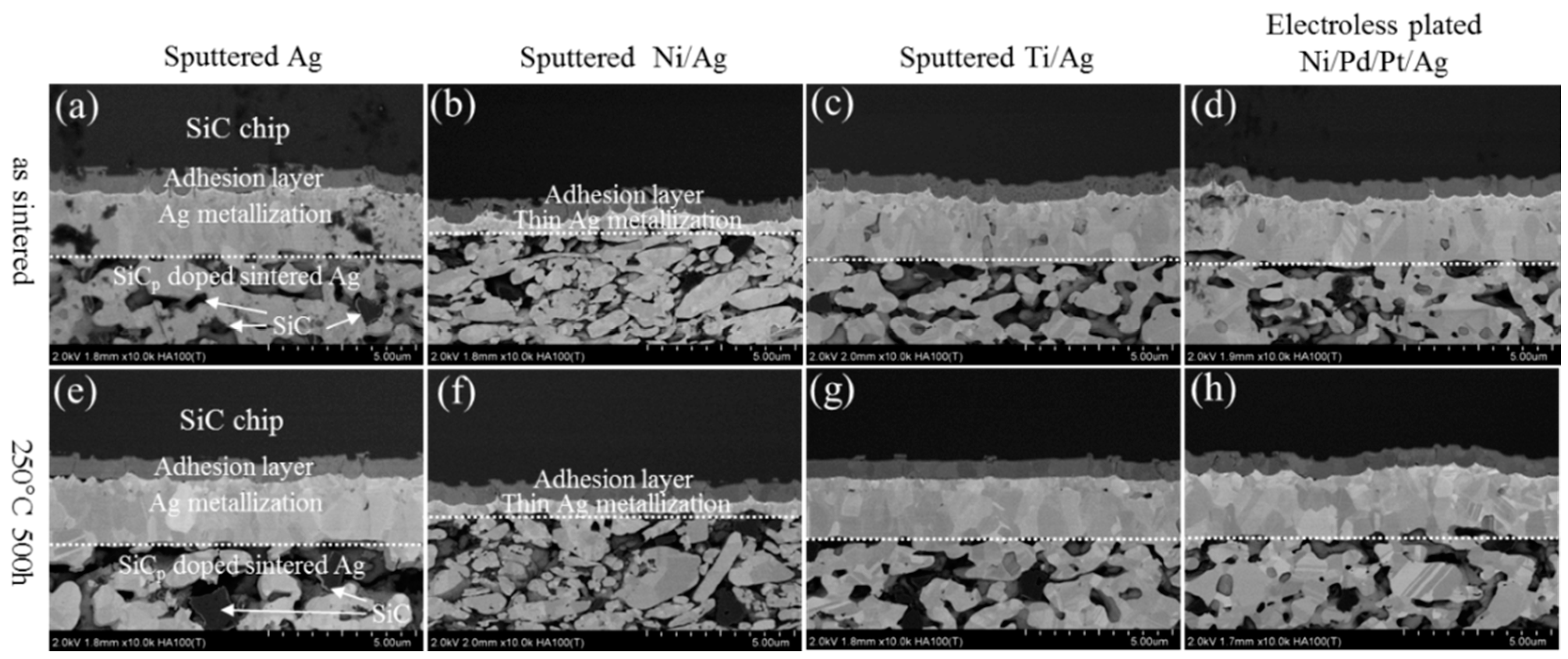
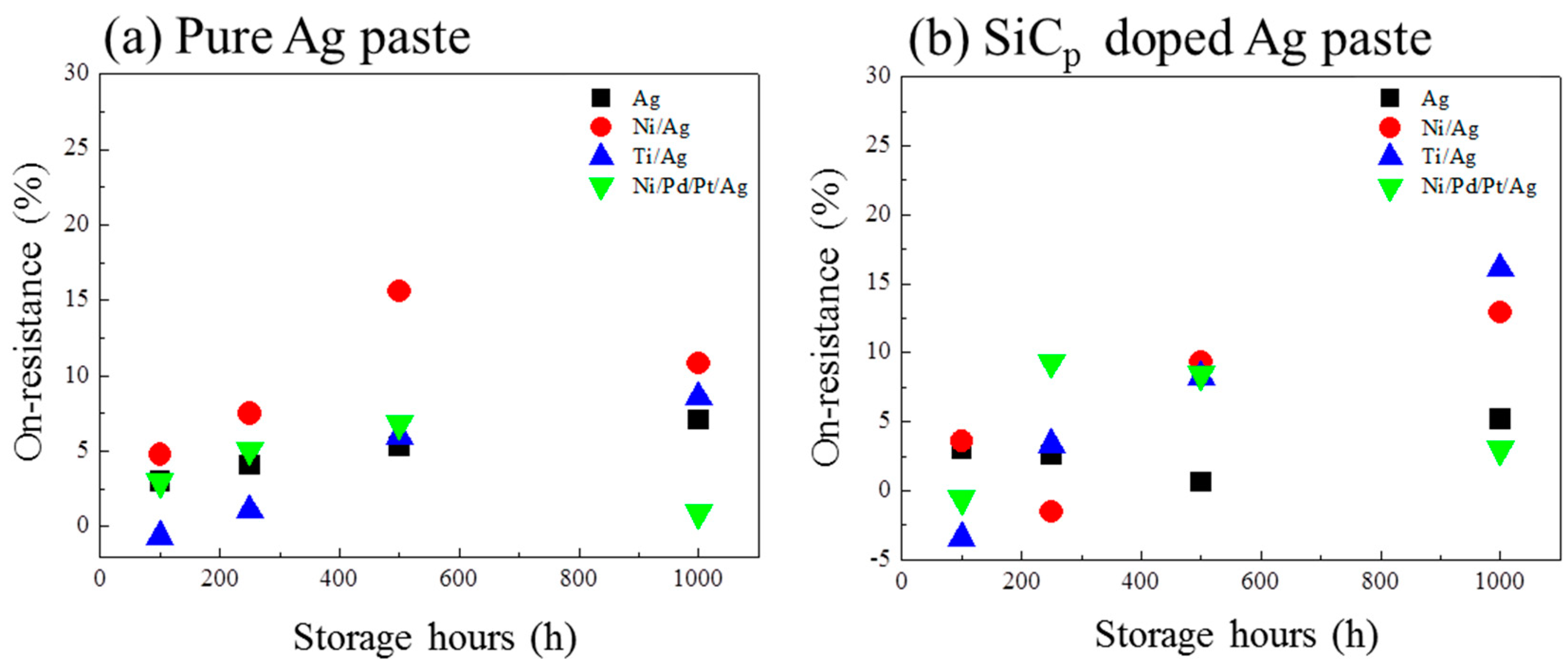
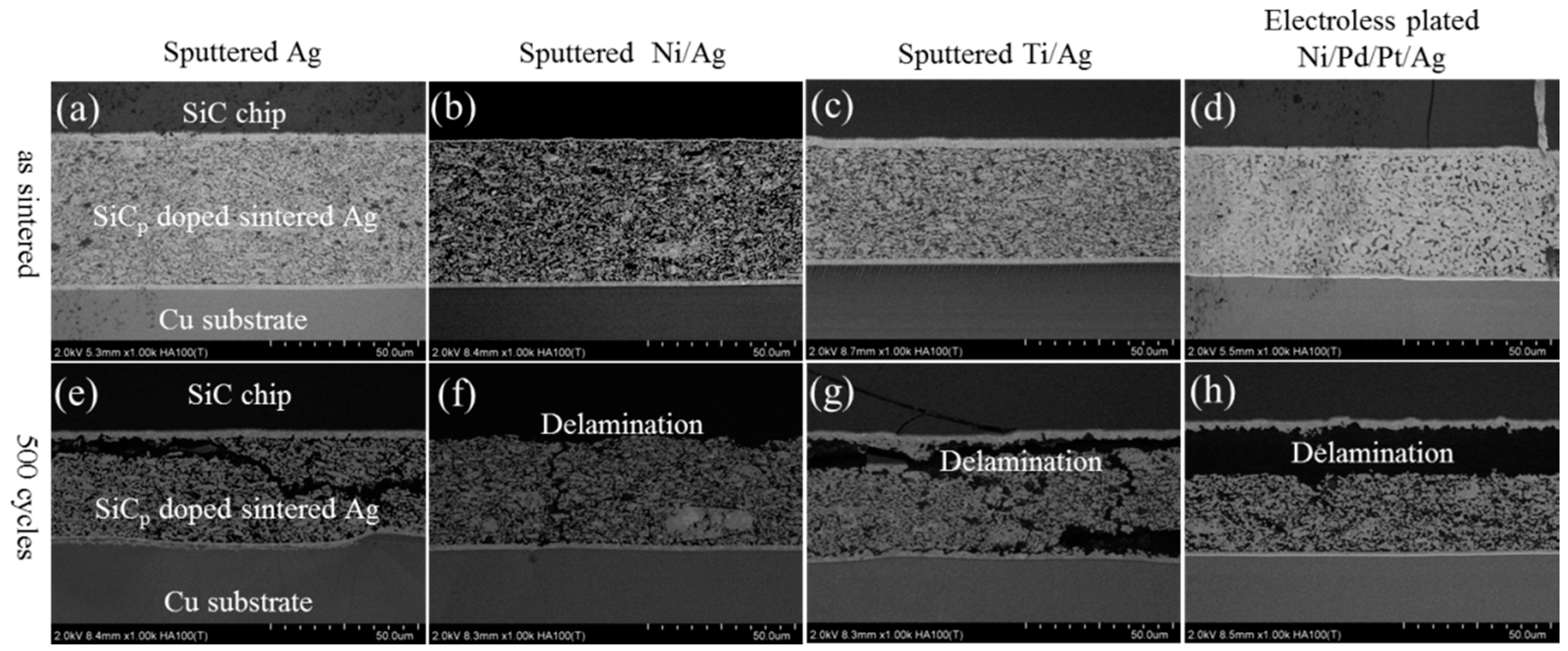
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Navarro, L.A.; Perpina, X.; Godignon, P.; Montserrat, J.; Banu, V.; Vellvehi, M.; Jorda, X. Thermomechanical assessment of die-attach materials for wide bandgap semiconductor devices and harsh environment applications. IEEE Trans. Power Electron. 2014, 29, 2261–2271. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Qin, Y.; Reiner, R.; Quay, R.; Wilde, J. Assembly and Packaging Technologies for Higherature and High-Power GaN Devices. IEEE Trans. Compon. Packag. Manuf. Technol. 2015, 5, 1402–1416. [Google Scholar] [CrossRef]
- Biela, J.; Schweizer, M.; Waffler, S.; Kolar, J.W. SiC versus Si—Evaluation of potentials for performance improvement of inverter and DCDC converter systems by SiC power semiconductors. IEEE Trans. Ind. Electron. 2011, 58, 2872–2882. [Google Scholar] [CrossRef]
- Chin, H.S.; Cheong, K.Y.; Ismail, A.B. A Review on Die Attach Materials for SiC-Based High-Temperature Power Devices. Metall. Mater. Trans. B 2010, 41, 824–832. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, A.Q.; Krishnaswami, S.; Richmond, J.; Agarwal, A.K. Comparison of static and switching characteristics of 1200 V 4H-SiC BJT and 1200 V Si-IGBT. IEEE Trans. Ind. Appl. 2008, 44, 887–893. [Google Scholar] [CrossRef]
- Kim, K.S.; Yu, C.H.; Kim, N.H.; Kim, N.K.; Chang, H.J.; Chang, E.G. Isothermal aging characteristics of Sn-Pb micro solder bumps. Microelectron. Reliab. 2003, 43, 757–763. [Google Scholar] [CrossRef]
- Laurila, T.; Vuorinen, V.; Kivilahti, J.K. Interfacial reactions between lead-free solders and common base materials. Mater. Sci. Eng. R Rep. 2005, 49, 1–60. [Google Scholar] [CrossRef]
- Ramanathan, L.N.; Jang, J.W.; Lin, J.K.; Frear, D.R. Solid-state annealing behavior of two high-Pb solders, 95Pb5Sn and 90Pb10Sn, on Cu under bump metallurgy. J. Electron. Mater. 2005, 34, 43–46. [Google Scholar] [CrossRef]
- Kisiel, R.; Szczepański, Z. Die-attachment solutions for SiC power devices. Microelectron. Reliab. 2009, 49, 627–629. [Google Scholar] [CrossRef]
- Bai, J.G.; Calata, J.N.; Lei, G.; Lu, G.Q. Thermomechanical reliability of low-temperature sintered silver die-attachment. In Proceedings of the Intersociety Conference on Thermomechanical Phenomena in Electronic Systems, San Diego, CA, USA, 30 May–2 June 2006; pp. 1126–1130. [Google Scholar] [CrossRef]
- Peng, P.; Hu, A.; Gerlich, A.P.; Zou, G.; Liu, L.; Zhou, Y.N. Joining of Silver Nanomaterials at Low Temperatures: Processes, Properties, and Applications. ACS Appl. Mater. Interfaces 2015, 7, 12597–12618. [Google Scholar] [CrossRef]
- Sakamoto, S.; Nagao, S.; Suganuma, K. Thermal fatigue of Ag flake sintering die-attachment for Si/SiC power devices. J. Mater. Sci. Mater. Electron. 2013, 24, 2593–2601. [Google Scholar] [CrossRef]
- Suganuma, K.; Sakamoto, S.; Kagami, N.; Wakuda, D.; Kim, K.S.; Nogi, M. Low-temperature low-pressure die attach with hybrid silver particle paste. Microelectron. Reliab. 2012, 52, 375–380. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Jiu, J.; Suganuma, K. In situ bridging effect of Ag2O on pressureless and low-temperature sintering of micron-scale silver paste. J. Alloys Compd. 2017, 696, 123–129. [Google Scholar] [CrossRef]
- Quintero, P.O.; McCluskey, F.P. Temperature cycling reliability of high-temperature lead-free die-attach technologies. IEEE Trans. Device Mater. Reliab. 2011, 11, 531–539. [Google Scholar] [CrossRef]
- Tan, Y.; Li, X.; Chen, G.; Mei, Y.; Chen, X. Three-Dimensional Visualization of the Crack-Growth Behavior of Nano-Silver Joints During Shear Creep. J. Electron. Mater. 2014, 44, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, C.; Nagao, S.; Suganuma, K. Thermal Fatigue Behavior of Silicon-Carbide-Doped Silver Microflake Sinter Joints for Die Attachment in Silicon/Silicon Carbide Power Devices. J. Electron. Mater. 2016, 46, 1–6. [Google Scholar] [CrossRef]
- Mei, Y.H.; Lian, J.Y.; Chen, X.; Chen, G.; Li, X.; Lu, G.Q. Thermo-mechanical reliability of double-sided IGBT assembly bonded by sintered nanosilver. IEEE Trans. Device Mater. Reliab. 2014, 14, 194–202. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, H.; Shang, P.; Li, C.; Chen, C.; Wang, J.; Liu, Z.; Zhang, H.; Suganuma, K. Effect of electroplated Au layer on bonding performance of Ag pastes. J. Alloys Compd. 2018, 731, 1280–1287. [Google Scholar] [CrossRef]
- Krishnan, K.H.; John, S.; Srinivasan, K.N.; Praveen, J.; Ganesan, M.; Kavimani, P.M. An Overall Aspect of Electroless Ni-P Depositions—A Review Article. Metall. Mater. Trans. 2006, 37A, 1917–1926. [Google Scholar] [CrossRef]
- Noh, S.; Choe, C.; Chen, C.; Zhang, H.; Suganuma, K. Printed wire interconnection using Ag sinter paste for wide band gap power semiconductors. J. Mater. Sci. Mater. Electron. 2018, 29, 15223–15232. [Google Scholar] [CrossRef]
- Zhang, H.; Nagao, S.; Suganuma, K.; Albrecht, H.J.; Wilke, K. Thermostable Ag die-attach structure for high-temperature power devices. J. Mater. Sci. Mater. Electron. 2016, 27, 1337–1344. [Google Scholar] [CrossRef]






| Average Diameter (μm) | Thickness (nm) | Specific Surface Area (m2/g) | |
|---|---|---|---|
| Ag flakes | 6.0 | 80 | 5.0 |
| SiCp | 0.6 | N/A | N/A |
| SiC Metallization Scheme | DBC Metallization Scheme |
|---|---|
| Ti/Ni/Au/Ag: Ag thickness 2000 nm | Ag: 2000 nm |
| Ti/Ni/Au/Ag: Ag thickness 300 nm | Ni/Ag: 2000 nm/2000 nm |
| Ti/Ni/Au/Ag: Ag thickness 2000 nm | Ti/Ag: 500nm/2000 nm |
| Ti/Ni/Au/Ag: Ag thickness 2000 nm | Ni/Pd/Pt/Ag: 5000 nm/30 nm/300 nm/2000 nm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, S.; Zhang, H.; Suganuma, K. Heat-Resistant Microporous Ag Die-Attach Structure for Wide Band-Gap Power Semiconductors. Materials 2018, 11, 2531. https://doi.org/10.3390/ma11122531
Noh S, Zhang H, Suganuma K. Heat-Resistant Microporous Ag Die-Attach Structure for Wide Band-Gap Power Semiconductors. Materials. 2018; 11(12):2531. https://doi.org/10.3390/ma11122531
Chicago/Turabian StyleNoh, Seungjun, Hao Zhang, and Katsuaki Suganuma. 2018. "Heat-Resistant Microporous Ag Die-Attach Structure for Wide Band-Gap Power Semiconductors" Materials 11, no. 12: 2531. https://doi.org/10.3390/ma11122531





