Analysis of Trauma Intensity during Surgical Bone Procedures Using NF-κB Expression Levels as a Stress Sensor: An Experimental Study in a Wistar Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Model and Care
2.3. Animals Surgery
2.4. Sample Preparation
2.5. Immunohistochemical Process
2.6. Data Collect
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- The installation of an implant at the bone perforation site did not result in an increase of inflammatory response, since no increase in NF-κB levels were evidenced at the perforation site compared to a control group.
- The irrigation process in bone perforation procedures resulted in a decrease of NF-κB levels, evidencing a reduction in trauma and inflammation processes.
- The vitaminic compound constitutes a promising alternative in the control of the surgical trauma, since a decrease in NF-κB levels were also observed.
Author Contributions
Funding
Conflicts of Interest
References
- Gehrke, S.A. Evaluation of the Cortical Bone Reaction around of Implants Using a Single-Use Final Drill: A Histologic Study. J. Craniofac. Surg. 2015, 26, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Loffredo Neto, H.; Mardegan, F.E. Investigation of the effect of movement and irrigation systems on temperature in the conventional drilling of cortical bone. Br. J. Oral. Maxillofac. Surg. 2013, 51, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Pazetto, M.K.; De Oliveira, S.; Corbella, S.; Taschieri, S.; Mardegan, F.E. Study of temperature variation in cortical bone during osteotomies with trephine drills. Clin. Oral. Investig. 2014, 18, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Marković, A.; Mišić, T.; Miličić, B.; Calvo-Guirado, J.L.; Aleksić, Z.; Ðinić, A. Heat generation during implant placement in low-density bone: Effect of surgical technique, insertion torque and implant macro design. Clin. Oral Implants Res. 2013, 24, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Piattelli, A.; Assenza, B.; Carinci, F.; Di Donato, L.; Romani, G.L.; Merla, A. Infrared thermographic evaluation of temperature modifications induced during implant site preparation with cylindrical versus conical drills. Clin. Implant Dent. Relat. Res. 2011, 13, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Bettach, R.; Taschieri, S.; Boukhris, G.; Corbella, S.; Del Fabbro, M. Temperature Changes in Cortical Bone after Implant Site Preparation Using a Single Bur versus Multiple Drilling Steps: An In Vitro Investigation. Clin. Implant Dent. Relat. Res. 2015, 17, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Kurokouchi, K.; Jacobs, C.R.; Donahue, H.J. Oscillating fluid flow inhibits TNF-alpha -induced NF-kappa B activation via an Ikappa B kinase pathway in osteoblast-like UMR106 cells. J. Biol. Chem. 2001, 276, 13499–13504. [Google Scholar] [CrossRef]
- Kumar, A.; Chaudhry, I.; Reid, M.B.; Boriek, A.M. Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers. J. Biol. Chem. 2002, 277, 46493–46503. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Buk, L.T.; Doll, C.J.; Land, S.C. Unifying theory of hypoxia tolerance: Molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc. Natl. Acad. Sci. USA 1996, 93, 9493–9498. [Google Scholar] [CrossRef]
- Arrigo, A.P. Gene expression and the thiol redox state. Free Radic. Biol. Med. 1999, 27, 936–944. [Google Scholar] [CrossRef]
- Adler, V.; Yin, Z.; Tew, K.D.; Ronai, Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene 1999, 18, 6104–6111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, Y.; Maruyama, M.; Fujita, T.; Arai, N.; Hayashi, R.; Araya, J.; Matsui, S.; Yamashita, N.; Sugiyama, E.; Kobayashi, M. Reactive oxygen intermediates stimulate interleukin-6 production in human bronchial epithelial cells. Am. J. Physiol. 1999, 276, L900–L908. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J.; Land, S.C. O(2)-evoked regulation of HIF-1alpha and NF-kappaB in perinatal lung epithelium requires glutathione biosynthesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L492–L503. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J.; Land, S.C.; Tarnow-Mordi, W.O.; Zembala, M.; Kowalczyk, D.; Lauterbach, R. Immunopharmacological potential of selective phosphodiesterase inhibition. II. Evidence for the involvement of an inhibitory-kappaB/nuclear factor-kappaB-sensitive pathway in alveolar epithelial cells. J. Pharmacol. Exp. Ther. 2002, 300, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S., Jr. Series introduction: The transcription factor NF-kappaB and human disease. J. Clin. Investig. 2001, 107, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Kuo, Y.R.; Yang, K.D.; Wang, C.J.; Sheen Chen, S.M.; Huang, H.C.; Yang, Y.J.; Yi-Chih, S.; Wang, F.S. Activation of extracellular signal-regulated kinase (ERK) and p38 kinase in shock wave-promoted bone formation of segmental defect in rats. Bone 2004, 34, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Mouthuy, P.A.; Snelling, S.J.B.; Dakin, S.G.; Milkovic, L.; Gasparovic, A.C.; Carr, A.J.; Zarkovic, N. Biocompatibility of implantable materials: An oxidative stress viewpoint. Biomaterials 2016, 109, 55–68. [Google Scholar] [CrossRef]
- D’Angio, C.T.; Finkelstein, J.N. Oxygen regulation of gene expression: A study in opposites. Mol. Genet. Metab. 2000, 71, 371–380. [Google Scholar] [CrossRef]
- Baichwal, V.R.; Baeuerle, P.A. Activate NF-kappa B or die? Curr. Biol. 1997, 7, R94–R96. [Google Scholar] [CrossRef]
- Boyce, B.F.; Yao, Z.; Xing, L. Functions of nuclear factor kappaB in bone. Ann. N. Y. Acad. Sci. 2010, 1192, 367–375. [Google Scholar] [CrossRef]
- Lin, T.H.; Tamaki, Y.; Pajarinen, J.; Waters, H.A.; Woo, D.K.; Yao, Z.; Goodman, S.B. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-kappaB as a therapeutic target. Acta Biomater. 2014, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Novack, D.V. Role of NF-kappaB in the skeleton. Cell Res. 2011, 21, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Pajarinen, J.; Lu, L.; Nabeshima, A.; Cordova, L.A.; Yao, Z.; Goodman, S.B. NF-kappaB as a Therapeutic Target in Inflammatory-Associated Bone Diseases. Adv. Protein Chem. Struct. Biol. 2017, 107, 117–154. [Google Scholar] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-kappaB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Karin, M. Is NF-kappaB the sensor of oxidative stress? FASEB J. 1999, 13, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, F.; Leotoing, L.; Coxam, V.; Guicheux, J.; Wittrant, Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009, 15, 468–477. [Google Scholar] [CrossRef]
- Struck, R.; Wittmann, M.; Recht, T.; Baumgarten, G.; Meybohm, P.; Muller, A.; Bagci, S. Effect of remote ischemic preconditioning on the melatonin and antioxidative status: A pilot study in patients undergoing cardiac surgery. J. Cardiovasc. Surg. 2017, 58, 909–915. [Google Scholar]
- Mao, L.; Qian, Q.; Li, Q.; Wei, S.; Cao, Y.; Hao, Y.; Liu, N.; Wang, Q.; Bai, Y.; Zheng, G. Lead selenide nanoparticles-induced oxidative damage of kidney in rats. Environ. Toxicol. Pharmacol. 2016, 45, 63–67. [Google Scholar] [CrossRef]
- Ilyas, A.; Odatsu, T.; Shah, A.; Monte, F.; Kim, H.K.; Kramer, P.; Aswath, P.B.; Varanasi, V.G. Amorphous Silica: A New Antioxidant Role for Rapid Critical-Sized Bone Defect Healing. Adv. Healthc. Mater. 2016, 5, 2199–2213. [Google Scholar] [CrossRef]
- Banche, G.; Allizond, V.; Bracco, P.; Bistolfi, A.; Boffano, M.; Cimino, A.; Brach Del Prever, E.M.; Cuffini, A.M. Interplay between surface properties of standard, vitamin E blended and oxidised ultra high molecular weight polyethylene used in total joint replacement and adhesion of Staphylococcus aureus and Escherichia coli. Bone Joint J. 2014, 96-B, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Kamath, M.S.; Ahmed, S.S.; Dhanasekaran, M.; Santosh, S.W. Polycaprolactone scaffold engineered for sustained release of resveratrol: Therapeutic enhancement in bone tissue engineering. Int. J. Nanomedicine 2014, 9, 183–195. [Google Scholar] [PubMed]
- Salles, M.B.; Gehrke, S.A.; Koo, S.; Allegrini, S., Jr.; Rogero, S.O.; Ikeda, T.I.; Cruz, A.S.; Shinohara, E.H.; Yoshimoto, M. An alternative to nerve repair using an antioxidant compound: A histological study in rats. J. Mater. Sci. Mater. Med. 2015, 26, 5340. [Google Scholar] [CrossRef] [PubMed]
- Gerhke, S.A.; Shibli, J.A.; Salles, M.B. Potential of the use of an antioxidant compound to promote peripheral nerve regeneration after injury. Neural. Regen. Res. 2015, 10, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Brazilian Law No. 11,794, of October 8, 2008. (In English). Available online: http://www.planalto.gov.br/ccivil_03/_Ato2007-2010/2008/Lei/L11794.htm. (accessed on 8 October 2018).
- Salles, M.B.; Gehrke, S.A.; Shibli, J.A.; Allegrini, S., Jr.; Yoshimoto, M.; Konig, B., Jr. Evaluating Nuclear Factor NF-kappaB Activation following Bone Trauma: A Pilot Study in a Wistar Rats Model. PLoS ONE 2015, 10, e0140630. [Google Scholar] [CrossRef] [PubMed]
- Salles, M.B.; Konig, B., Jr.; Allegrini, S., Jr.; Yoshimoto, M.; Martins, M.T.; Coelho, P.G. Identification of the nuclear factor kappa-beta (NF-κB) in cortical of mice Wistar using Technovit 7200 VCR(R). Med. Oral Patol. Oral Cir. Bucal 2011, 16, e124–e131. [Google Scholar] [CrossRef]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J. Oral. Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Aramburú Júnior, J.S.; Pérez-Albacete Martínez, C.; Ramirez Fernandez, M.P.; Maté Sánchez de Val, J.E.; Calvo-Guirado, J.L. The influence of drill length and irrigation system on heat production during osteotomy preparation for dental implants: An ex vivo study. Clin. Oral Implants Res. 2018, 29, 772–778. [Google Scholar] [CrossRef]
- Hellweg, C.E.; Arenz, A.; Bogner, S.; Schmitz, C.; Baumstark-Khan, C. Activation of nuclear factor kappa B by different agents: influence of culture conditions in a cell-based assay. Ann. N. Y. Acad. Sci. 2006, 1091, 191–204. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Perez, K.C.; Hyman, S.; Brunski, J.B.; Tulu, U.; Bao, C.; Salmon, B.; Helms, J.A. Effects of Condensation on Peri-implant Bone Density and Remodeling. J. Dent. Res. 2017, 96, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Insua, A.; Monje, A.; Wang, H.L.; Miron, R.J. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J. Biomed. Mater. Res. A 2017, 105, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Todisco, M.; Consolo, U.; Travaglini, D. High versus low implant insertion torque: A histologic, histomorphometric, and biomechanical study in the sheep mandible. Int. J. Oral Maxillofac. Implants 2011, 26, 837–849. [Google Scholar] [PubMed]
- Yellowley, C.E.; Li, Z.; Zhou, Z.; Jacobs, C.R.; Donahue, H.J. Functional gap junctions between osteocytic and osteoblastic cells. J. Bone Miner. Res. 2000, 15, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Park, P.S.; Baek, S.H.; Yang, I.H. Histomorphometric analysis of microcrack healing after the installation of mini-implants. J. Periodontal Implant Sci. 2015, 45, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Takano-Yamamoto, T. Molecular events caused by mechanical stress in bone. Matrix Biol. 2000, 19, 91–96. [Google Scholar] [CrossRef]
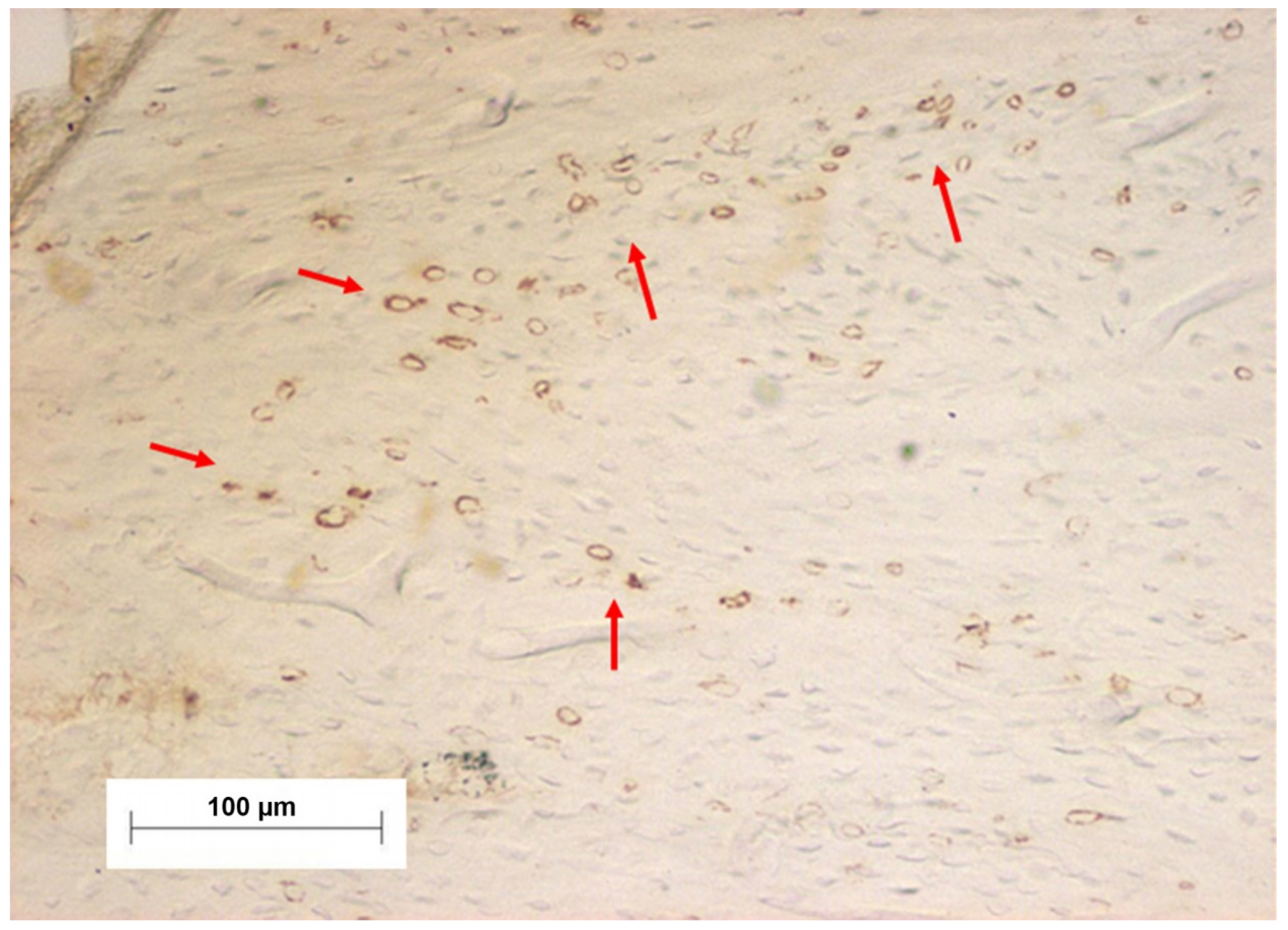

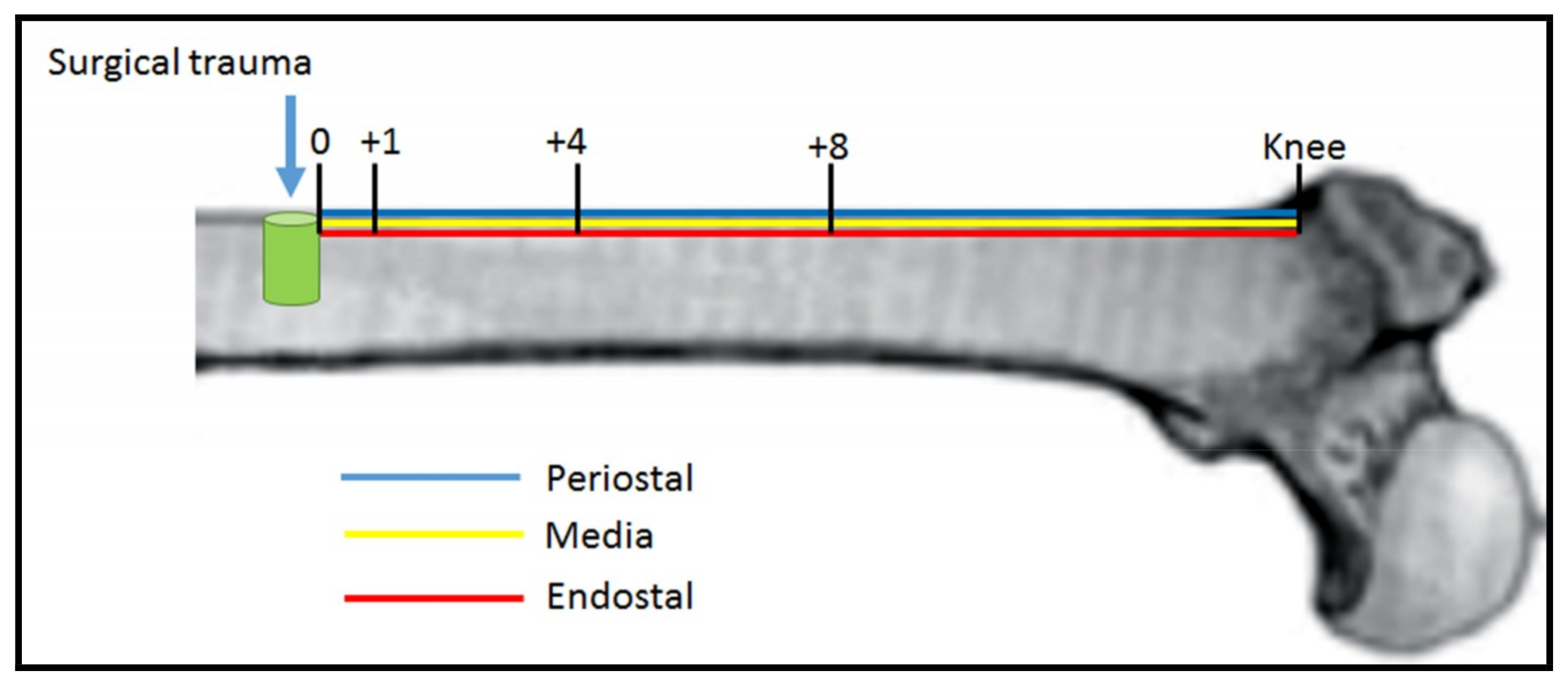


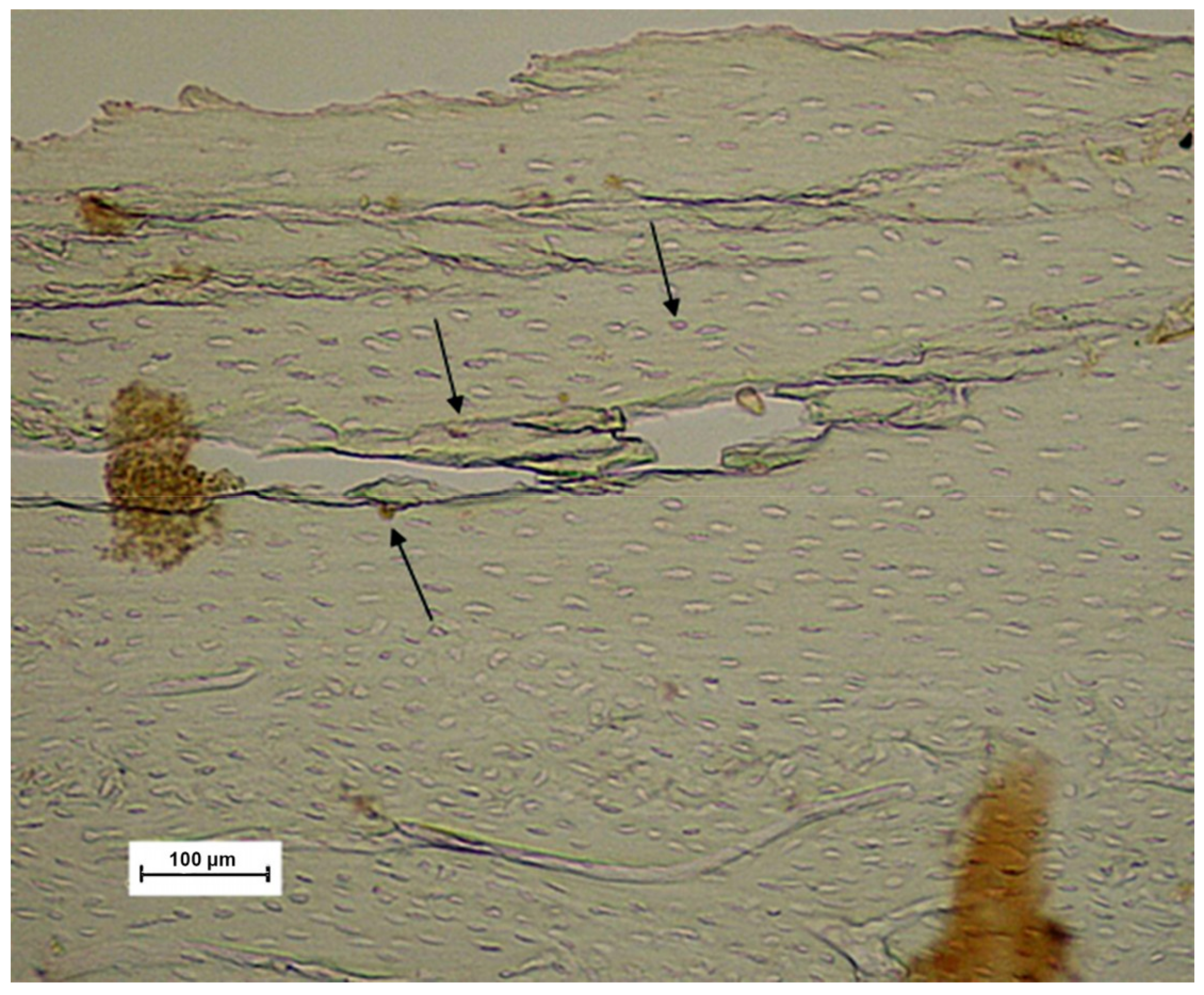
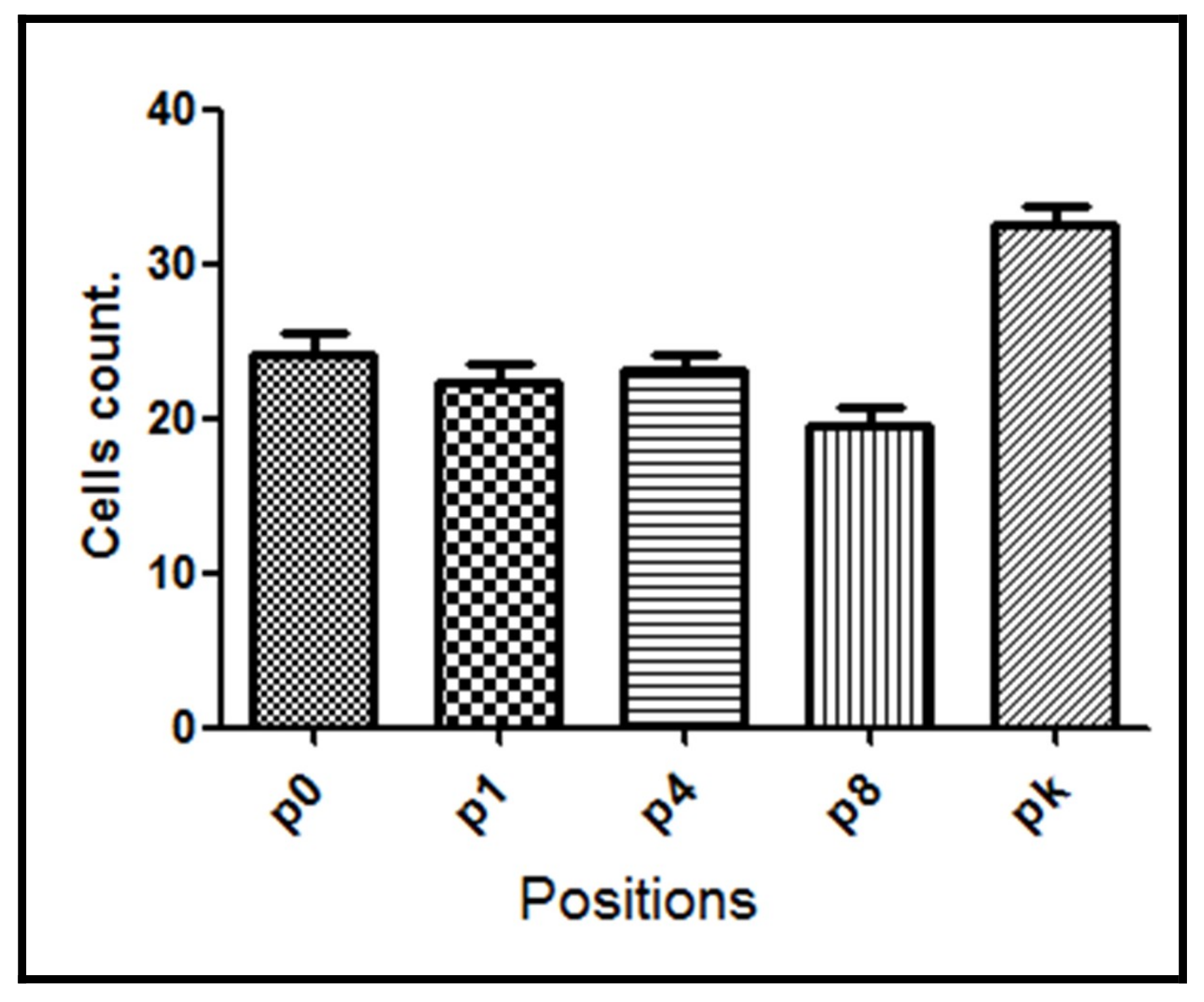

| Group | p0 | p1 | p4 | p8 | pk | Average |
|---|---|---|---|---|---|---|
| G1 | 30.4 ± 2.24 | 35.4 ± 2.38 | 34.5 ± 2.56 | 35.8 ± 4.07 | 30.7 ± 0.98 | 33.4 ± 2.45 |
| G2 | 30.3 ± 2.03 | 27.4 ± 2.97 | 26.6 ± 3.00 | 24.0 ± 2.68 | 35.9 ± 2.85 | 28.9 ± 2.70 |
| G3 | 24.0 ± 3.47 | 22.4 ± 2.67 | 23.1 ± 2.29 | 19.5 ± 2.47 | 32.6 ± 2.70 | 24.3 ± 2.72 |
| G4 | 18.2 ± 2.36 | 21.2 ± 2.75 | 26.2 ± 2.48 | 33.2 ± 2.45 | 33.5 ± 2.94 | 26.5 ± 2.60 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salles, M.B.; Allegrini Jr., S.; Yoshimoto, M.; Pérez-Díaz, L.; Calvo-Guirado, J.L.; Gehrke, S.A. Analysis of Trauma Intensity during Surgical Bone Procedures Using NF-κB Expression Levels as a Stress Sensor: An Experimental Study in a Wistar Rat Model. Materials 2018, 11, 2532. https://doi.org/10.3390/ma11122532
Salles MB, Allegrini Jr. S, Yoshimoto M, Pérez-Díaz L, Calvo-Guirado JL, Gehrke SA. Analysis of Trauma Intensity during Surgical Bone Procedures Using NF-κB Expression Levels as a Stress Sensor: An Experimental Study in a Wistar Rat Model. Materials. 2018; 11(12):2532. https://doi.org/10.3390/ma11122532
Chicago/Turabian StyleSalles, Marcos Barbosa, Sergio Allegrini Jr., Marcelo Yoshimoto, Leticia Pérez-Díaz, José Luis Calvo-Guirado, and Sergio Alexandre Gehrke. 2018. "Analysis of Trauma Intensity during Surgical Bone Procedures Using NF-κB Expression Levels as a Stress Sensor: An Experimental Study in a Wistar Rat Model" Materials 11, no. 12: 2532. https://doi.org/10.3390/ma11122532







