Rheological Behavior and Microstructure Characteristics of SCC Incorporating Metakaolin and Silica Fume
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Design
2.3. Testing Methods
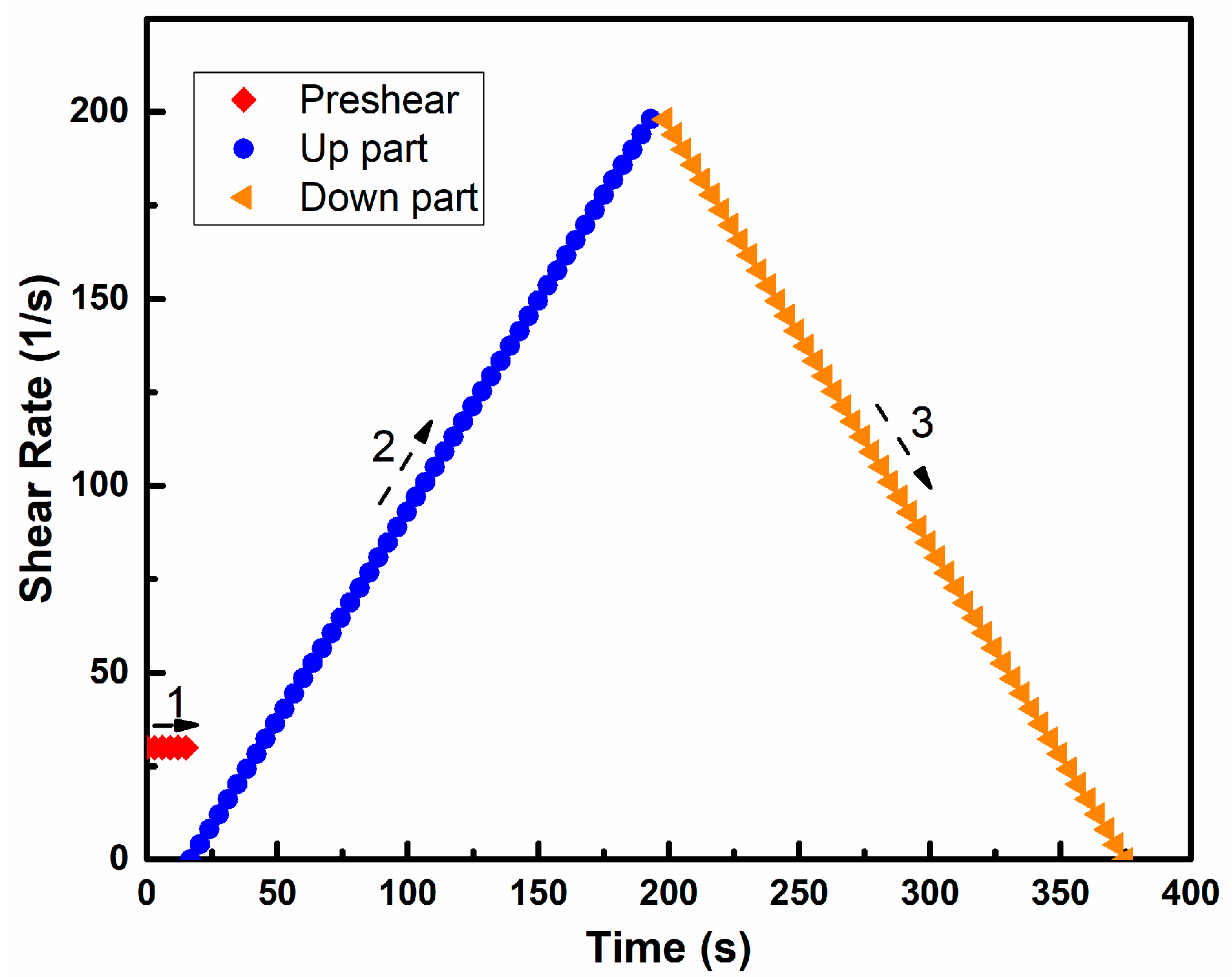
2.3.1. Rheology Behavior
2.3.2. Workability
2.3.3. Compressive Strength
2.3.4. Hydration Products
2.3.5. Pore Size Distribution
3. Results and Discussions
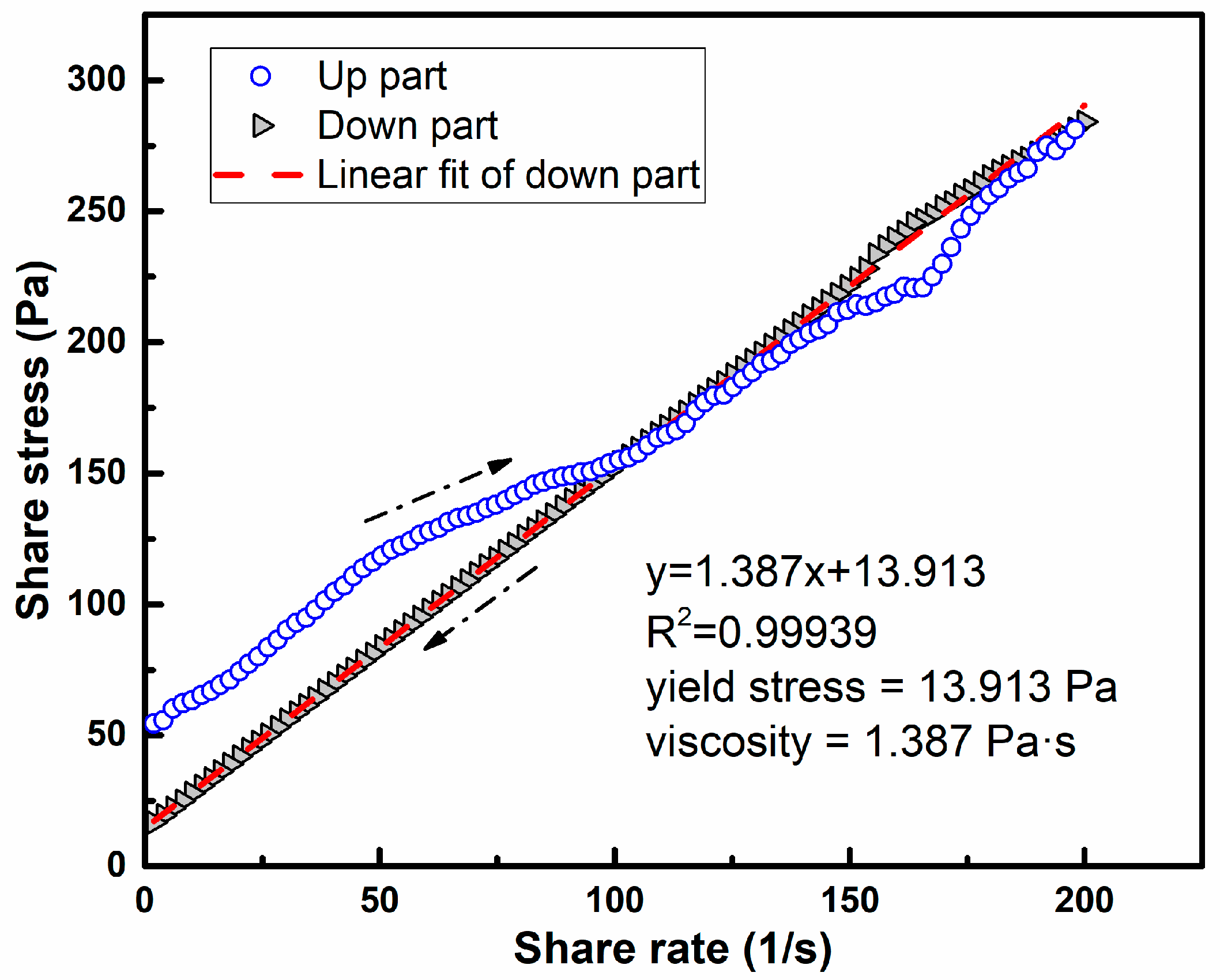
3.1. Rheology Behavior
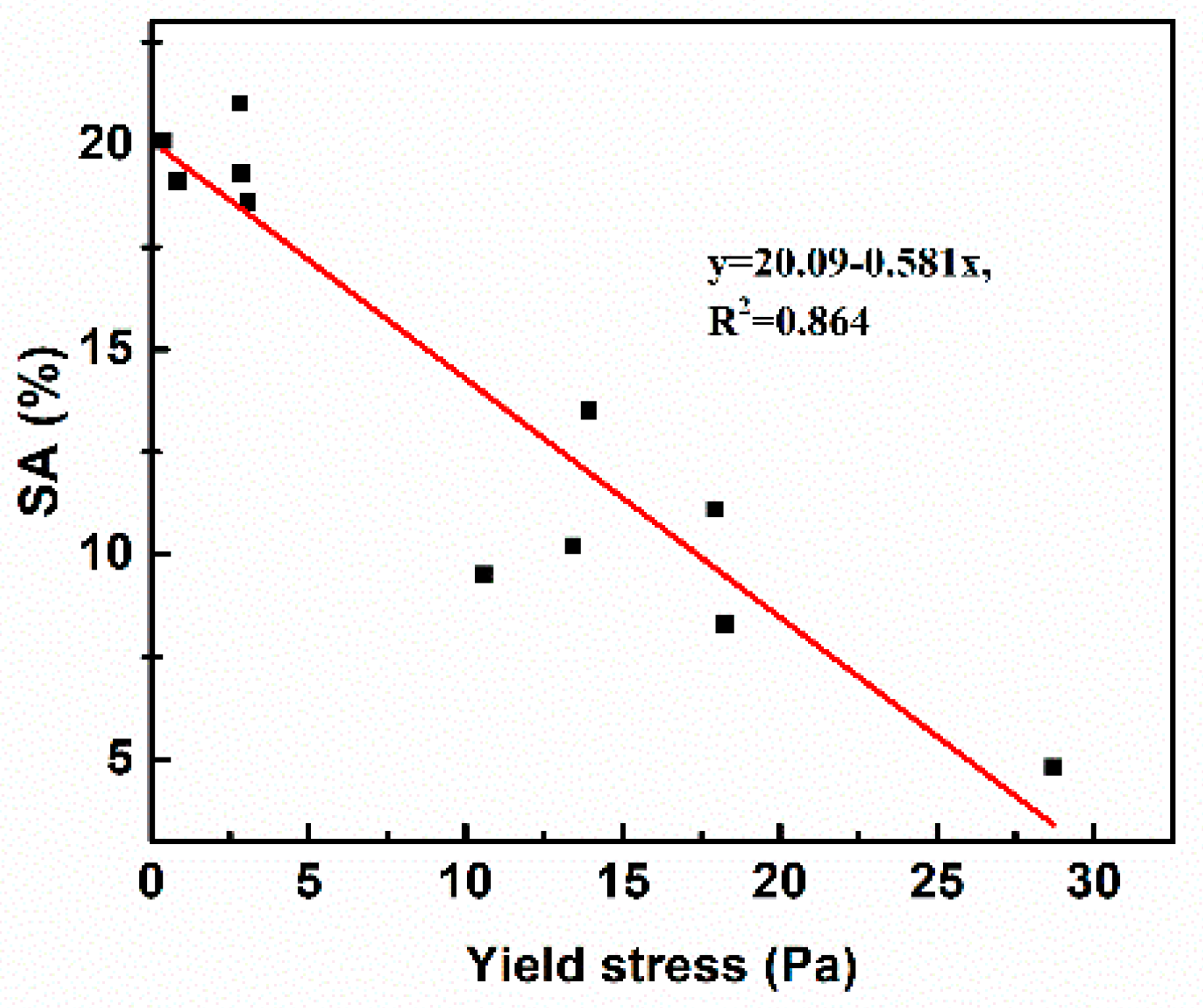
3.2. Workability
3.3. Compressive Strength
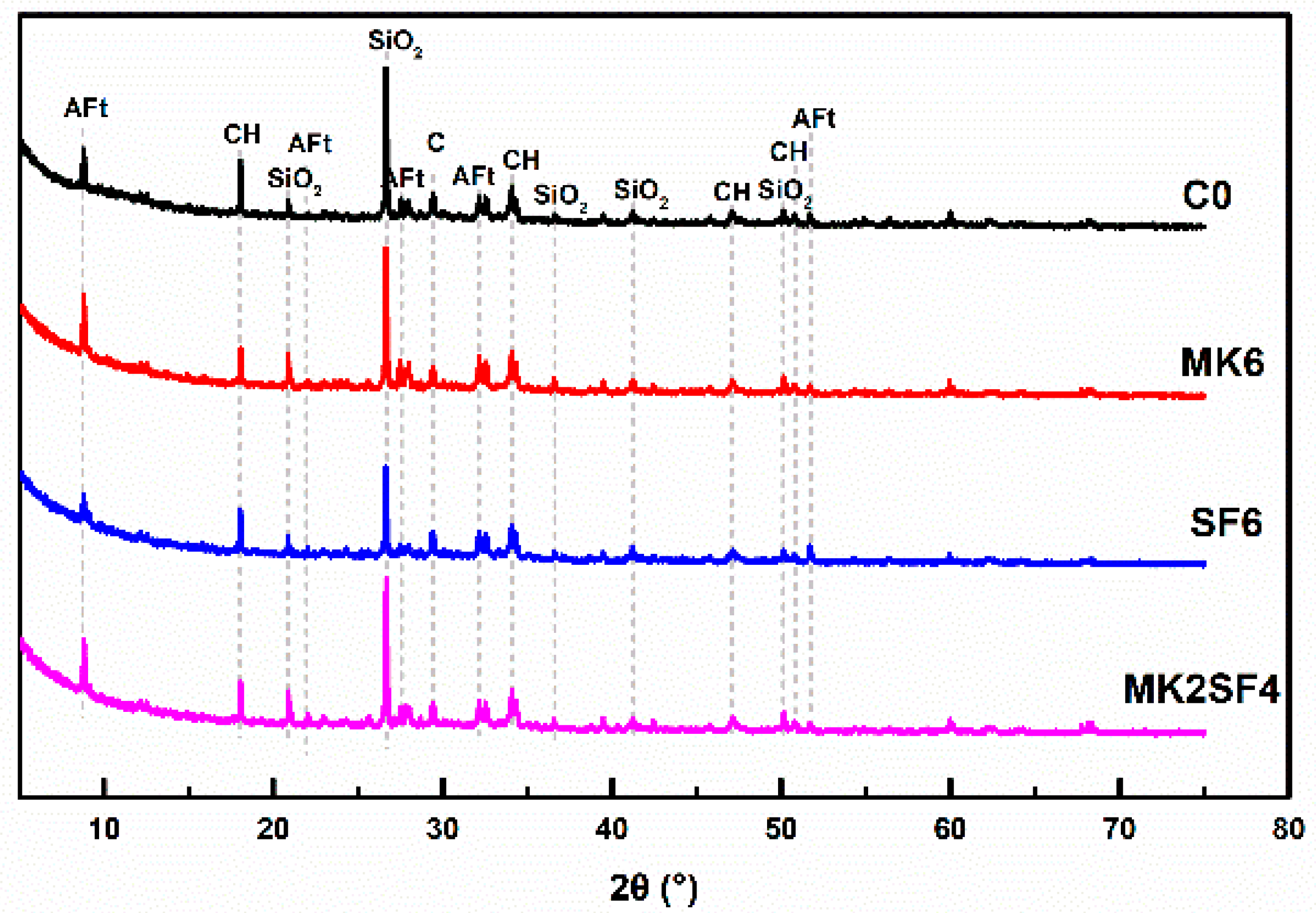
3.4. Hydration Products
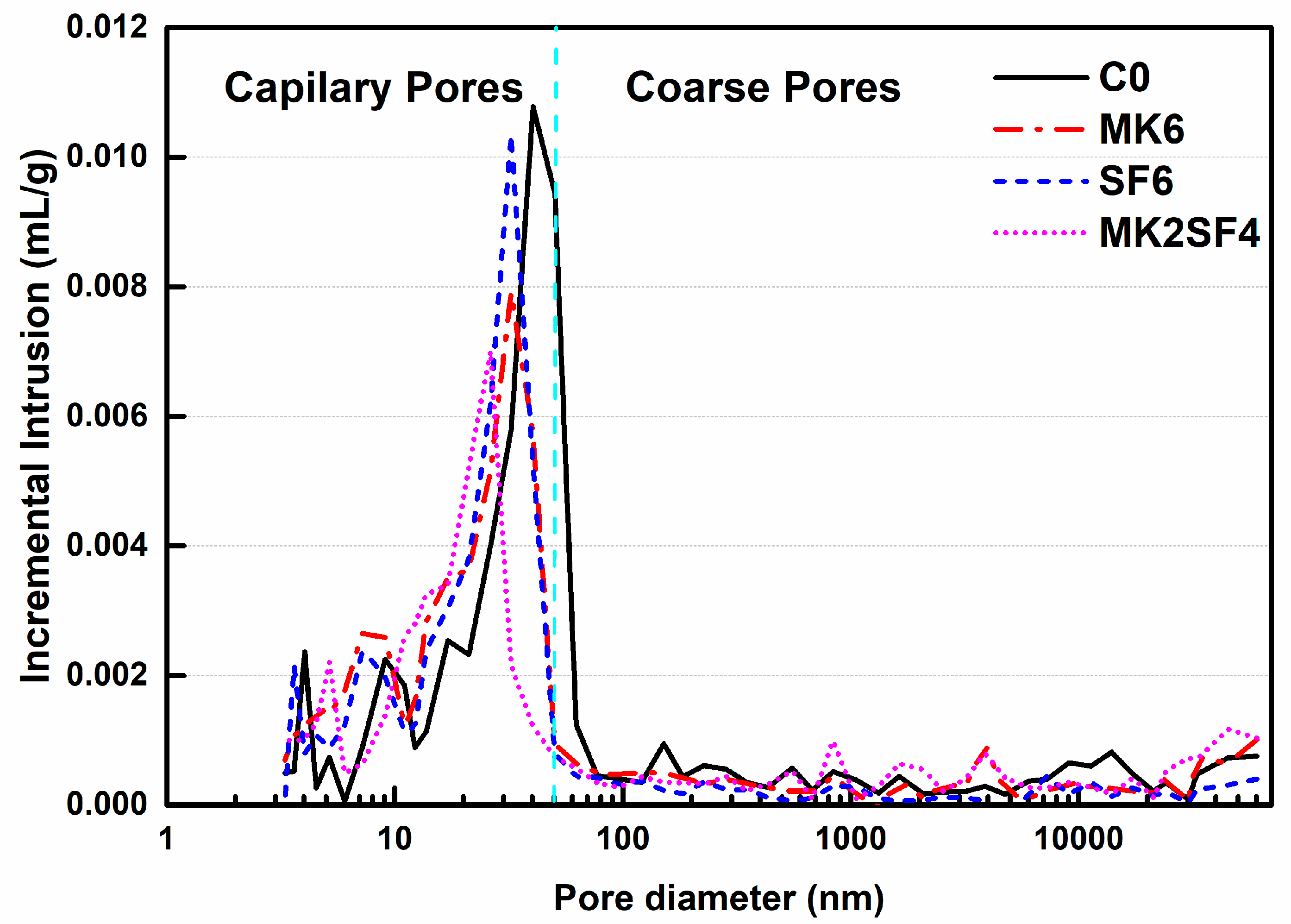
3.5. Pore Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hunger, M.; Entrop, A.G.; Mandilaras, I.; Brouwers, H.J.H.; Founti, M. The behavior of self-compacting concrete containing micro-encapsulated phase change materials. Cem. Concr. Comp. 2009, 31, 731–743. [Google Scholar] [CrossRef]
- Zhu, W.; Bartos, P.J.M. Permeation properties of self-compacting concrete. Cem. Concr. Res. 2003, 33, 921–926. [Google Scholar] [CrossRef]
- Burak, F.; Selçuk, T.; Bülent, B. Effect of water/cement ratio on the fresh and hardened properties of self-compacting concrete. Build. Environ. 2007, 42, 1795–1802. [Google Scholar]
- Persson, B. A comparison between mechanical properties of self-compacting concrete and the corresponding properties of normal concrete. Cem. Concr. Res. 2001, 31, 193–198. [Google Scholar] [CrossRef]
- Ouch, M.; SAKAI, E.; Sugiyama, T.; Mitsui, K.; Shindo, T.; Maekawa, K.; Noguchi, T. Self-Compacting Concrete in Japan. In Proceedings of the 8th International Symposium on Utilization of High-Strength and High-Performance Concrete, Tokyo, Japan, 27–29 October 2008; pp. 72–91. [Google Scholar]
- Saak, A.W.; Jennings, H.M.; Shah, S.P. New methodology for designing self-compacting concrete. ACI Mater. J. 2001, 98, 429–439. [Google Scholar]
- Loukili, A. Self-compacting concrete. Adv. Concr. Technol. 2003, 1, 1–23. [Google Scholar]
- Brouwers, H.J.H.; Radix, H.J. Self-compacting concrete: Theoretical and experimental study. Cem. Concr. Res. 2005, 35, 2116–2136. [Google Scholar] [CrossRef]
- Zerbino, R.; Barragán, B.; Garcia, T.; Agulló, L.; Gettu, R. Workability tests and rheological parameters in self-compacting concrete. Mater. Struct. 2009, 42, 947–960. [Google Scholar] [CrossRef]
- Roussel, N. A theoretical frame to study stability of fresh concrete. Mater. Struct. 2006, 39, 81–91. [Google Scholar] [CrossRef]
- Bui, V.K.; Akkaya, Y.; Shah, S.P. Rheological model for self-consolidating concrete. ACI Mater. J. 2002, 99, 549–559. [Google Scholar]
- Chidiac, S.E.; Mahmoodzadeh, F. Plastic viscosity of fresh concrete—A critical review of predictions methods. Cem. Concr. Compos. 2009, 31, 535–544. [Google Scholar] [CrossRef]
- Hu, C.; Larrard, D.F. The rheology of fresh high-performance concrete. Cem. Concr. Res. 1996, 26, 283–294. [Google Scholar] [CrossRef]
- Gołaszewski, J. Influence of cement properties on new generation superplasticizers performance. Constr. Build. Mater. 2012, 35, 586–596. [Google Scholar] [CrossRef]
- Papo, A.; Piani, L. Effect of various superplasticizers on the rheological properties of Portland cement pastes. Cem. Concr. Res. 2004, 34, 2097–2101. [Google Scholar] [CrossRef]
- Xie, H.; Liu, F.; Fan, Y.R.; Yang, H.; Chen, J.; Zhang, J.; Zuo, C. Workability and proportion design of pumping concrete based on rheological parameters. Constr. Build. Mater. 2013, 44, 267–275. [Google Scholar] [CrossRef]
- Flatt, R.J.; Larosa, D.; Roussel, N. Linking yield stress measurements: Spread test versus Viskomat. Cem. Concr. Res. 2006, 36, 99–109. [Google Scholar] [CrossRef]
- Lootens, D.; Jousset, P.; Martinie, L.; Roussel, N.; Flatt, R.J. Yield stress during setting of cement pastes from penetration tests. Cem. Concr. Res. 2009, 39, 401–408. [Google Scholar] [CrossRef]
- Wan, B.; Gadalamaria, F.; Petrou, M.F. Influence of mortar rheology on aggregate settlement. ACI Mater. J. 2000, 97, 479–485. [Google Scholar]
- Tregger, N.A.; Pakula, M.E.; Shah, S.P. Influence of clays on the rheology of cement pastes. Cem. Concr. Res. 2010, 40, 384–391. [Google Scholar] [CrossRef]
- Toutou, Z.; Roussel, N. Multi scale experimental study of concrete rheology: From water scale to gravel scale. Mater. Struct. 2006, 39, 189–199. [Google Scholar] [CrossRef]
- Wu, Q.; An, X. Development of a mix design method for SCC based on the rheological characteristics of paste. Constr. Build. Mater. 2014, 53, 642–651. [Google Scholar] [CrossRef]
- Dehwah, H.A.F. Mechanical properties of self-compacting concrete incorporating quarry dust powder, silica fume or fly ash. Constr. Build. Mater. 2012, 26, 547–551. [Google Scholar] [CrossRef]
- Yazici, H. The effect of silica fume and high-volume Class C fly ash on mechanical properties, chloride penetration and freeze-thaw resistance of self-compacting concrete. Constr. Build. Mater. 2008, 22, 456–462. [Google Scholar] [CrossRef]
- Faella, C.; Lima, C.; Martinelli, E.; Pepe, M.; Realfonzo, R. Mechanical and durability performance of sustainable structural concretes: An experimental study. Cem. Concr. Comp. 2016, 71, 85–96. [Google Scholar] [CrossRef]
- Lima, C.; Caggiano, A.; Faella, C.; Martinelli, E.; Pepe, M.; Realfonzo, R. Physical properties and mechanical behaviour of concrete made with recycled aggregates and fly ash. Constr. Build. Mater. 2013, 47, 547–559. [Google Scholar] [CrossRef]
- Sonebi, M. Medium strength self-compacting concrete containing fly ash: Modelling using factorial experimental plans. Cem. Concr. Res. 2004, 34, 1199–1208. [Google Scholar] [CrossRef]
- Behforooz, B.; Eftekhar, M.R.; Amin, E.; Ghias, A. Effects of using silica fume (SF) in improving cermeability properties of Self-compacting concrete (SCC). In Proceedings of the 9th International Congress on Civil Engineering, Isfahan, Iran, 8 May 2012. [Google Scholar]
- Dousti, A.; Beaudoin, J.J.; Shekarchi, M. Chloride binding in hydrated MK, SF and natural zeolite-lime mixtures. Constr. Build. Mater. 2017, 154, 1035–1047. [Google Scholar] [CrossRef]
- Majid, G.; Ali, K. An investigation on the fresh and hardened properties of self-compacting concrete incorporating magnetic water with various pozzolanic materials. Constr. Build. Mater. 2018, 15, 173–180. [Google Scholar]
- Poon, C.S.; Kou, S.C.; Lam, L. Compressive strength, chloride diffusivity and pore structure of high performance metakaolin and silica fume concrete. Constr. Build. Mater. 2006, 20, 858–865. [Google Scholar] [CrossRef]
- Zibara, H.; Hooton, R.D.; Thomas, M.D.A.; Stanish, K. Influence of the C/S and C/A ratios of hydration products on the chloride ion binding capacity of lime-SF and lime-MK mixtures. Cem. Concr. Res. 2008, 38, 422–426. [Google Scholar] [CrossRef]
- Dadsetan, S.; Bai, J.P. Mechanical and microstructural properties of self-compacting concrete blended with metakaolin, ground granulated blast furnace slag and fly ash. Constr. Build. Mater. 2017, 146, 658–667. [Google Scholar] [CrossRef]
- Madandoust, R.; Mousavi, S.Y. Fresh and hardened properties of self-compacting concrete containing metakaolin. Constr. Build. Mater. 2012, 35, 752–760. [Google Scholar] [CrossRef]
- Duan, P.; Shui, Z.H.; Chen, W.; Shen, C. Effects of metakaolin, silica fume and slag on pore structure, interfacial transition zone and compressive strength of concrete. Constr. Build. Mater. 2013, 44, 1–6. [Google Scholar] [CrossRef]
- Hassan, A.A.A.; Mayo, J.R. Influence of mixture composition on the properties of SCC incorporating metakaolin. Mag. Concr. Res. 2014, 66, 1–15. [Google Scholar] [CrossRef]
- Sfikas, I.P.; Badogiannis, E.G.; Trezos, K.G. Rheology and mechanical characteristics of self-compacting concrete mixtures containing metakaolin. Constr. Build. Mater. 2014, 64, 121–129. [Google Scholar] [CrossRef]
- Quercia, G.G.; Spiesz, P.P.; Hüsken, G.G.; Brouwers, H.J.H. Effects of amorphous nano-silica additions on mechanical and durability performance of SCC mixtures. In Durability of Reinforced Concrete from Composition to Protectio; Andrade, C., Gulikers, J., Polder, R., Eds.; Springer Publishing: New York, NY, USA, 2013; pp. 125–143. [Google Scholar]
- Łaźniewska-Piekarczyk, B. The influence of admixtures type on the air-voids parameters of non-air-entrained and air-entrained high-performance SCC. Constr. Build. Mater. 2013, 41, 109–124. [Google Scholar] [CrossRef]
- Song, Q.; Yu, R.; Wang, X.; Rao, S.; Shui, Z. A novel self-compacting ultra-high performance fibre reinforced concrete (SCUHPFRC) derived from compounded high-active powders. Constr. Build. Mater. 2018, 158, 883–893. [Google Scholar] [CrossRef]
- ASTM C192. Standard Practice for Making and Curing Concrete Test Specimens in the Laboratory; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- BS EN 12350. Testing fresh concrete, self-compacting concrete; Technical committee, British Standards Institution: London, UK, 2010. [Google Scholar]
- ASTM C39. Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Ye, Q.; Shiho, K. Distinguishing dynamic and static yield stress of fresh cement mortars through thixotropy. Cem. Concr. Compos. 2018, 86, 288–296. [Google Scholar]
- Ferraris, C.F.; Obla, K.H.; Hill, R. The influence of mineral admixtures on the rheology of cement paste and concrete. Cem. Concr. Res. 2001, 31, 245–255. [Google Scholar] [CrossRef]
- Janotka, I.; Puertas, F.; Palacios, M.; Kuliffayová, M.; Varga, C. Metakaolin sand–blended-cement pastes: Rheology, hydration process and mechanical properties. Constr. Build. Mater. 2010, 24, 791–802. [Google Scholar] [CrossRef]
- Schowalter, W.R.; Christensen, G. Toward a rationalization of the slump test for fresh concrete: Comparisons of calculations and experiments. J. Rheol. 1998, 42, 865–870. [Google Scholar] [CrossRef]
- Ng, I.Y.T.; Ng, P.L.; Kwan, A.K.H. Rheology of mortar and its influences on performance of self-consolidating concrete. Key Eng. Mater. 2008, 400–402, 421–426. [Google Scholar] [CrossRef]
- Lothenbach, B.; Rentsch, D.; Wieland, E. Hydration of a silica fume blended low-alkali shotcrete cement. Phys. Chem. Earth 2014, 70–71, 3–16. [Google Scholar] [CrossRef]
- Szeląg, M. Development of cracking patterns in modified cement matrix with microsilica. Materials 2018, 11, 1928. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.M.; Arjunan, P.; Silsbee, M.R. Effect of silica fume, metakaolin, and low-calcium fly ash on chemical resistance of concrete. Cem. Concr. Res. 2001, 31, 1809–1813. [Google Scholar] [CrossRef]
- Nili, M.; Ehsani, A. Investigating the effect of the cement paste and transition zone on strength development of concrete containing nanosilica and silica fume. Mater. Des. 2015, 75, 174–183. [Google Scholar] [CrossRef]
- Karen, S.; Ruben, S.; Barbara, L. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Zhou, Q.; Glasser, F.P. Thermal stability and decomposition mechanisms of ettringite at <120 °C. Cem. Concr. Res. 2001, 31, 1333–1339. [Google Scholar]
- Nonnet, E.; Lequeux, N.; Boch, P. Elastic properties of high alumina cement castables from room temperature to 1600 °C. J. Eur. Ceram. Soc. 1999, 19, 1575–1583. [Google Scholar] [CrossRef]
- Siddique, R.; Klaus, J. Influence of metakaolin on the properties of mortar and concrete: A review. Appl. Clay Sci. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Lothenbach, B.; Bary, B.; Bescop, P.L.; Schmidt, T.; Leterrier, N. Sulfate ingress in Portland cement. Cem. Concr. Res. 2010, 40, 1211–1225. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Sun, T.; Huang, Y.; Wang, G. Effect of fly ash, sinking beads and metakaolin on the workability, strength, free shrinkage and chloride resistance of concretes: A comparative Study. Arab. J. Sci. Eng. 2018, 43, 5243–5254. [Google Scholar] [CrossRef]
- Wild, S.; Khatib, J.M. Portlandite consumption in metakaolin cement pastes and mortars. Cem. Concr. Res. 1997, 27, 137–146. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Sun, T.; Duan, P. Properties of coral waste-based mortar incorporating metakaolin: Part II. Chloride migration and binding behaviors. Constr. Build. Mater. 2018, 174, 433–442. [Google Scholar] [CrossRef]







| Compositions | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | LOI | Specific Surface Area (m2/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 21.86 | 4.45 | 2.35 | 63.51 | 1.67 | 0.55 | 0.26 | 2.91 | 1.89 | 353 |
| Fly Ash | 46.43 | 38.02 | 3.11 | 7.51 | 0.23 | 0.89 | 0.34 | 0.68 | 2.78 | 372 |
| Metakaolin | 52.27 | 44.58 | 0.70 | 0.02 | 0.13 | 0.34 | 0.53 | 0.22 | 1.02 | 14,600 |
| Silica Fume | 94.65 | 0.15 | 0.25 | 0.33 | 0.49 | 0.85 | 0.16 | 0.66 | 2.21 | 46,100 |
| NO. | Water | Cement | Fly Ash | Metakaolin | Silica Fume | Superplasticizer |
|---|---|---|---|---|---|---|
| P0 | 552 | 920 | 753 | 0 | 0 | 10 |
| PMK2 | 552 | 920 | 720 | 33 | 0 | 10 |
| PMK4 | 552 | 920 | 687 | 66 | 0 | 10 |
| PMK6 | 552 | 920 | 654 | 99 | 0 | 10 |
| PMK8 | 552 | 920 | 621 | 132 | 0 | 10 |
| PSF2 | 552 | 920 | 720 | 0 | 33 | 10 |
| PSF4 | 552 | 920 | 687 | 0 | 66 | 10 |
| PSF6 | 552 | 920 | 654 | 0 | 99 | 10 |
| PSF8 | 552 | 920 | 621 | 0 | 132 | 10 |
| PMK2SF4 | 552 | 920 | 654 | 66 | 33 | 10 |
| PMK4SF2 | 552 | 920 | 654 | 33 | 66 | 10 |
| NO. | Water | Cement | Fly Ash | Metakaolin | Silica Fume | Sand | Gravel | Superplasticizer |
|---|---|---|---|---|---|---|---|---|
| C0 | 165 | 275 | 255 | 0 | 0 | 859 | 762 | 5 |
| CMK2 | 165 | 275 | 215 | 10 | 0 | 859 | 762 | 5 |
| CMK4 | 165 | 275 | 205 | 20 | 0 | 859 | 762 | 5 |
| CMK6 | 165 | 275 | 195 | 30 | 0 | 859 | 762 | 5 |
| CMK8 | 165 | 275 | 185 | 40 | 0 | 859 | 762 | 5 |
| CSF2 | 165 | 275 | 215 | 0 | 10 | 859 | 762 | 5 |
| CSF4 | 165 | 275 | 205 | 0 | 20 | 859 | 762 | 5 |
| CSF6 | 165 | 275 | 195 | 0 | 30 | 859 | 762 | 5 |
| CSF8 | 165 | 275 | 185 | 0 | 40 | 859 | 762 | 5 |
| CMK2SF4 | 165 | 275 | 195 | 10 | 20 | 859 | 762 | 5 |
| CMK4SF2 | 165 | 275 | 195 | 20 | 10 | 859 | 762 | 5 |
| No. | Metakaolin (%) | Silica Fume (%) | Slump (mm) | Bingham Equation | Viscosity (Pa·s) | Yield Stress (Pa) |
|---|---|---|---|---|---|---|
| P0 | 0 | 0 | 275 | τ = 1.140γ + 2.804 | 1.140 | 2.804 |
| PMK2 | 2 | 0 | 280 | τ = 0.704γ + 0.385 | 0.704 | 0.385 |
| PMK4 | 4 | 0 | 250 | τ = 1.341γ + 2.858 | 1.341 | 2.858 |
| PMK6 | 6 | 0 | 200 | τ = 1.387γ + 13.913 | 1.387 | 13.913 |
| PMK8 | 8 | 0 | 190 | τ = 1.582γ + 18.266 | 1.582 | 18.266 |
| PSF2 | 0 | 2 | 275 | τ = 1.130γ + 0.823 | 1.130 | 0.823 |
| PSF4 | 0 | 4 | 260 | τ = 1.416γ + 3.061 | 1.416 | 3.061 |
| PSF6 | 0 | 6 | 210 | τ = 1.611γ + 17.939 | 1.611 | 17.939 |
| PSF8 | 0 | 8 | 165 | τ = 1.988γ + 28.711 | 1.988 | 28.711 |
| PMK4SF2 | 4 | 2 | 220 | τ = 1.379γ + 5.568 | 1.379 | 5.568 |
| PMK2SF4 | 2 | 4 | 225 | τ = 1.318γ + 13.411 | 1.318 | 13.411 |
| Sample Code | C0 | MK6 | SF6 | MK2SF4 |
|---|---|---|---|---|
| Coarse pores (mL/g) | 0.022 | 0.018 | 0.019 | 0.010 |
| Capillary pores (mL/g) | 0.044 | 0.038 | 0.038 | 0.044 |
| Total porosity (%) | 11.66 | 10.49 | 10.58 | 9.92 |
| Average diameter (nm) | 24.4 | 18.0 | 20.0 | 15.4 |
| Most probate pore diameter (nm) | 40.3 | 32.4 | 32.4 | 26.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, G.; Shui, Z.; Sun, T.; Gao, X.; Wang, Y.; Sun, Y.; Wang, G.; Li, Z. Rheological Behavior and Microstructure Characteristics of SCC Incorporating Metakaolin and Silica Fume. Materials 2018, 11, 2576. https://doi.org/10.3390/ma11122576
Ling G, Shui Z, Sun T, Gao X, Wang Y, Sun Y, Wang G, Li Z. Rheological Behavior and Microstructure Characteristics of SCC Incorporating Metakaolin and Silica Fume. Materials. 2018; 11(12):2576. https://doi.org/10.3390/ma11122576
Chicago/Turabian StyleLing, Gang, Zhonghe Shui, Tao Sun, Xu Gao, Yunyao Wang, Yu Sun, Guiming Wang, and Zhiwei Li. 2018. "Rheological Behavior and Microstructure Characteristics of SCC Incorporating Metakaolin and Silica Fume" Materials 11, no. 12: 2576. https://doi.org/10.3390/ma11122576





