Green Synthesis of Fluorescent Palladium Nanoclusters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instruments
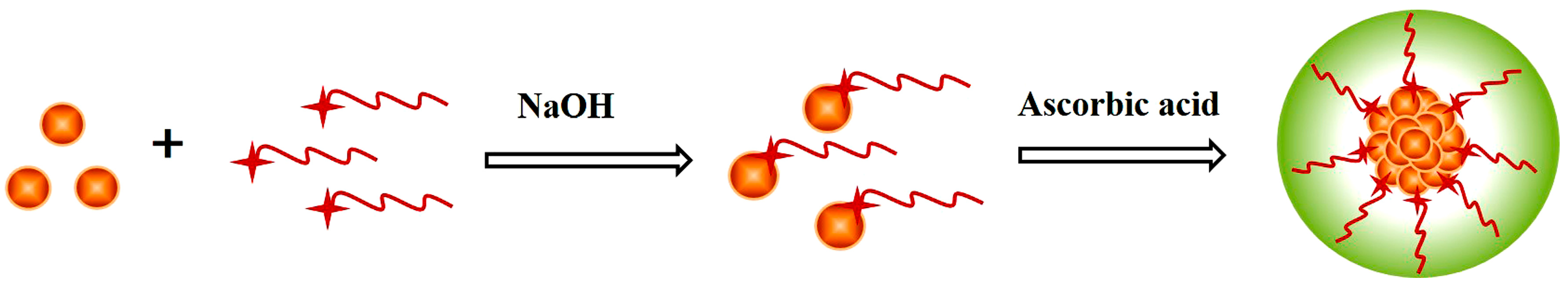
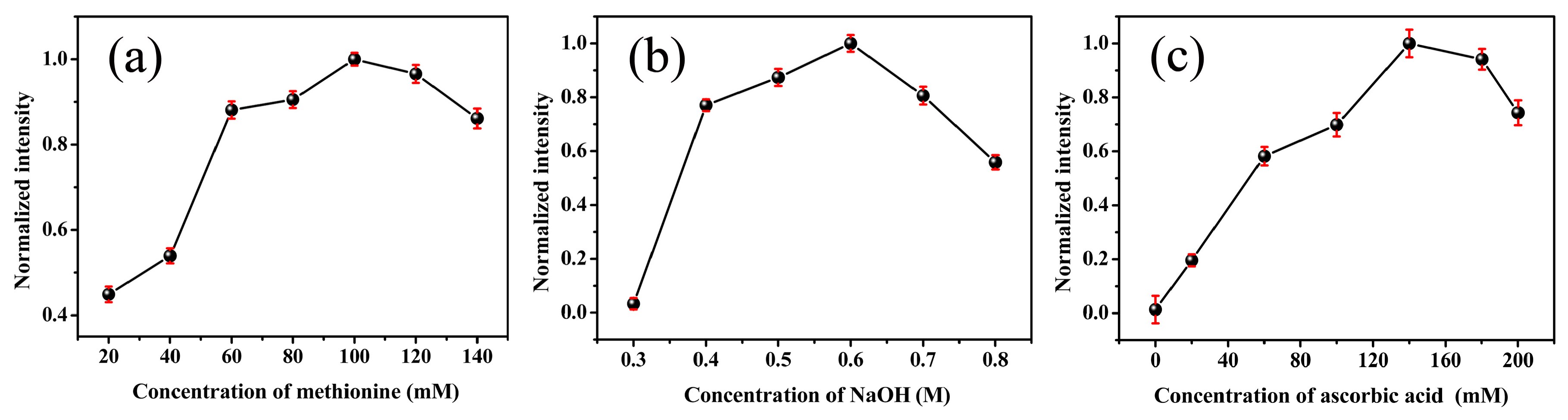
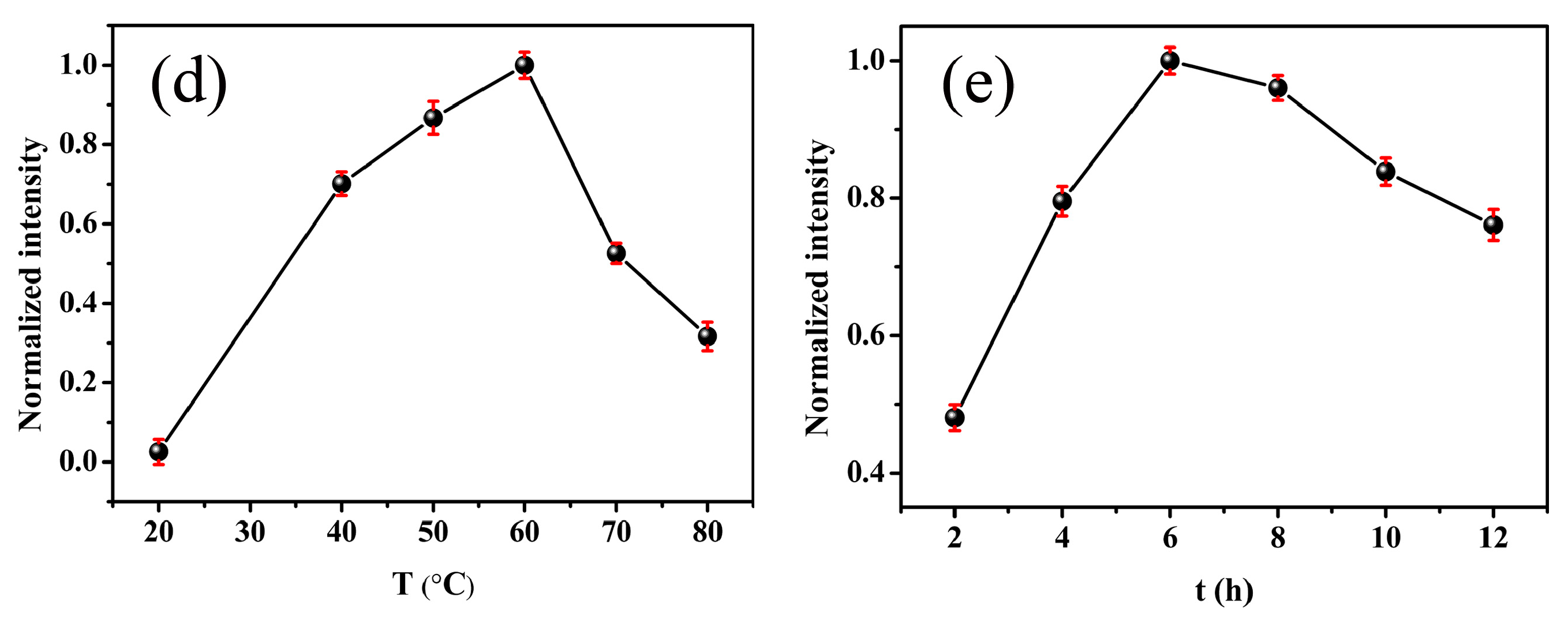
2.3. Synthesis of Pd NCs
2.4. Fluorescent Detection of Hemoglobin
3. Results and Discussion
3.1. Synthesis of Pd NCs
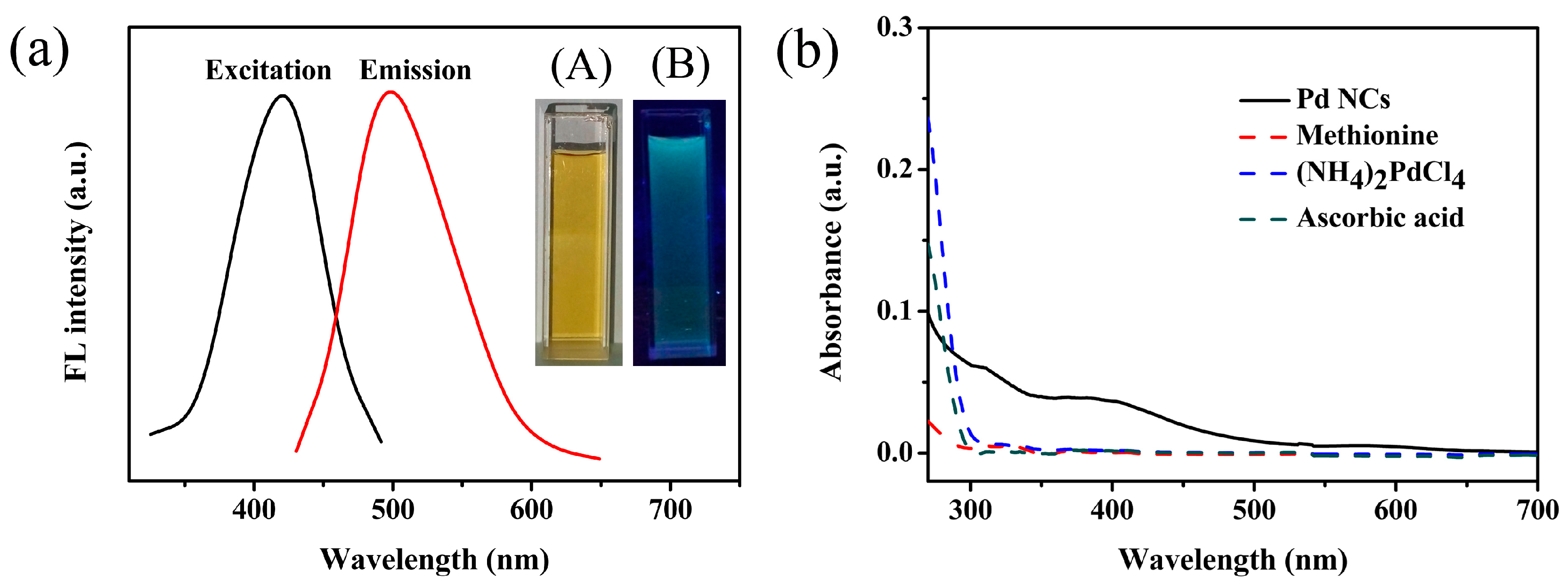
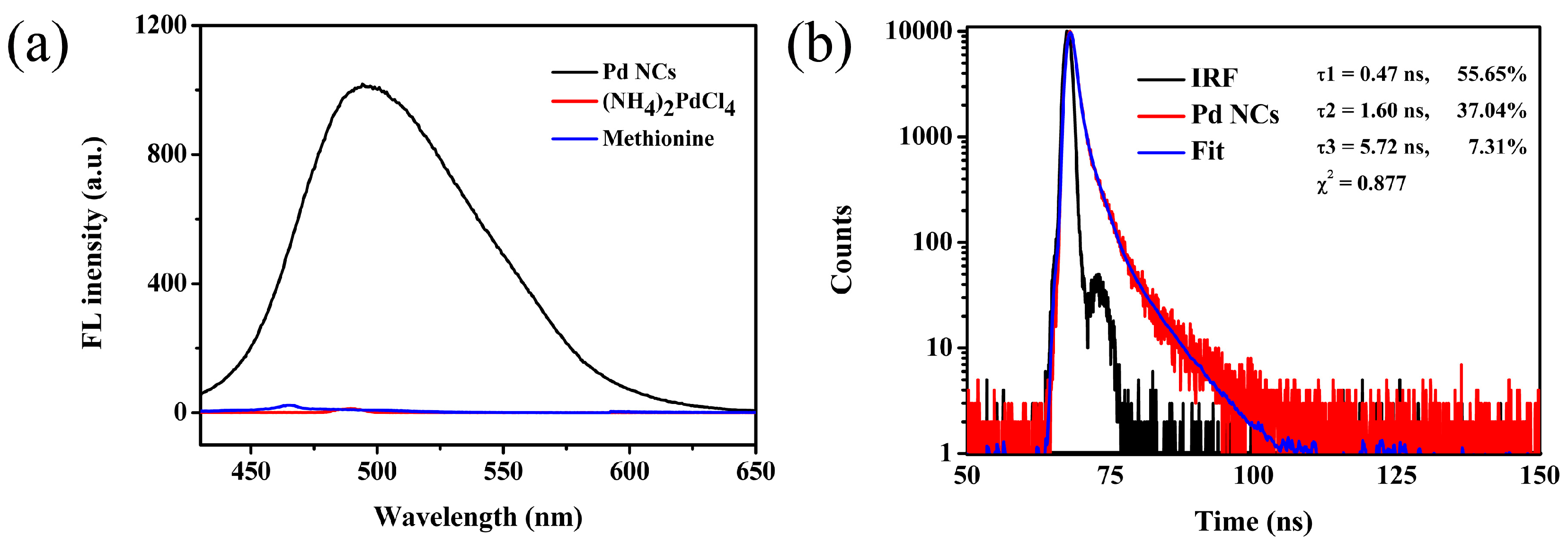
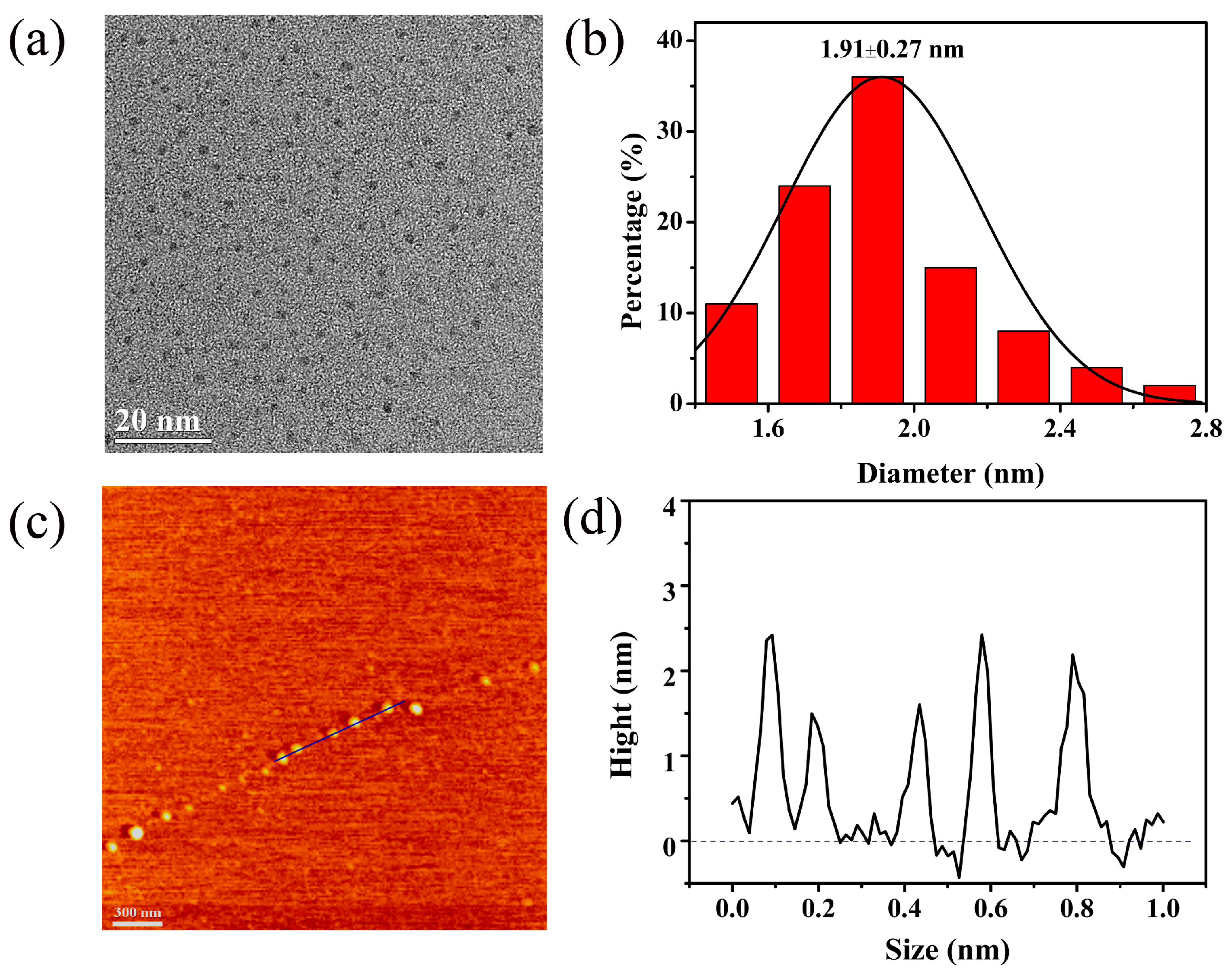
3.2. Characterization of Pd NCs
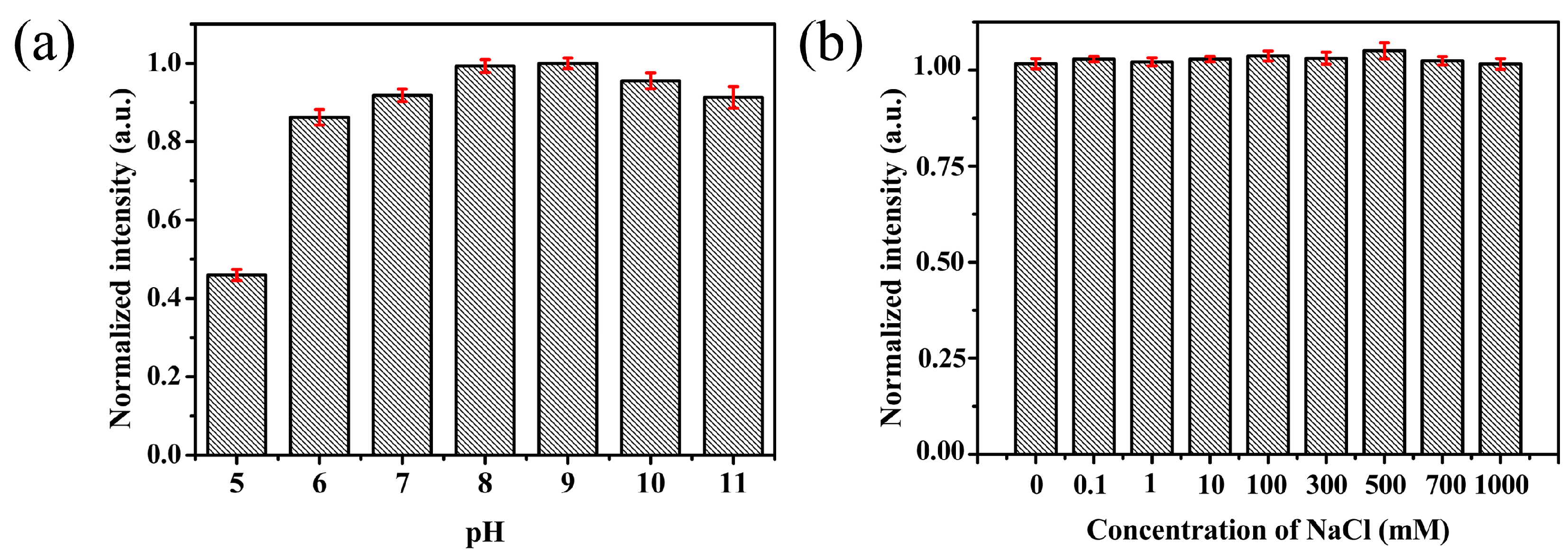
3.3. Stability of Fluorescent Pd NCs
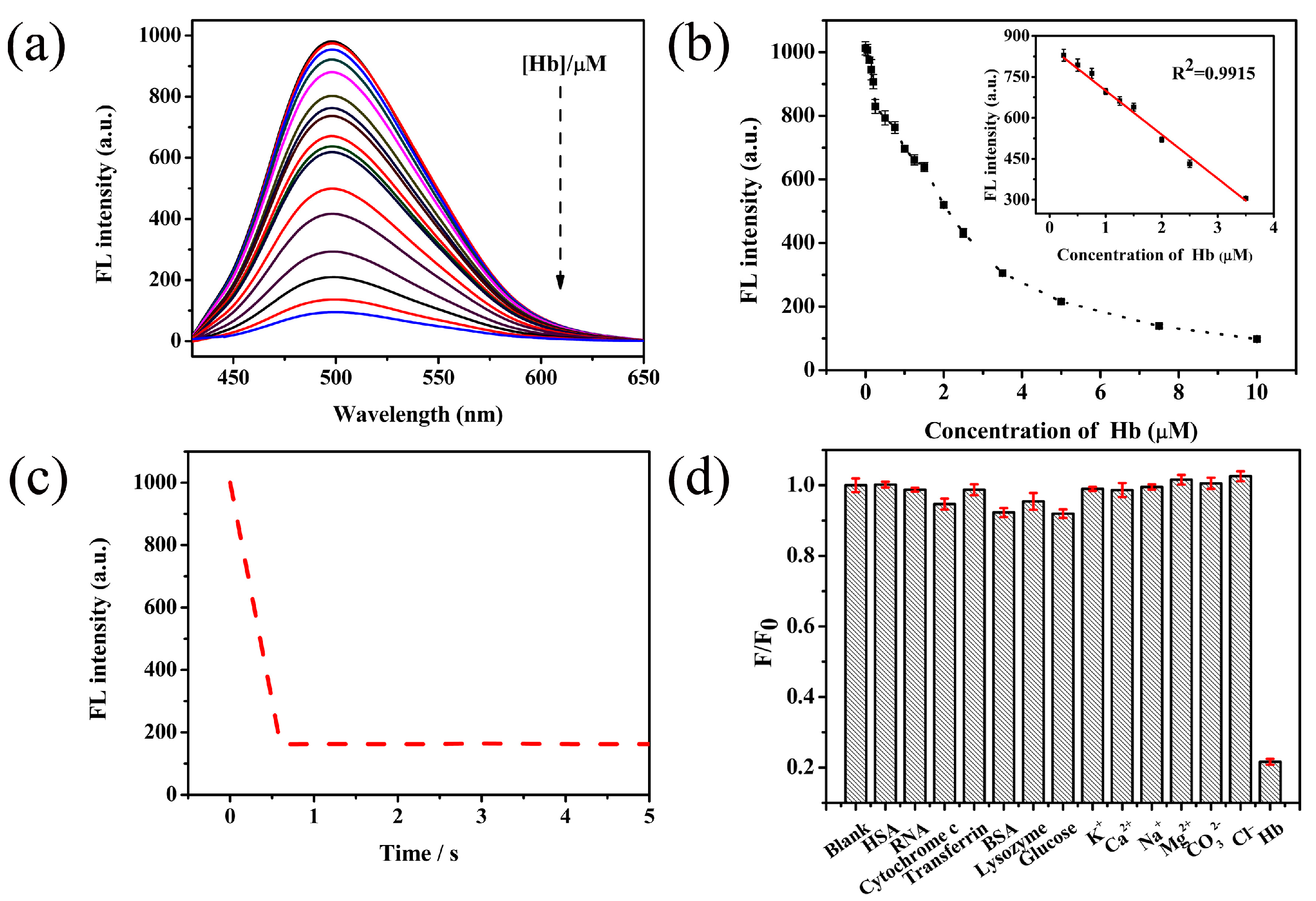
3.4. Fluorescent Sensing of Hemolobin Using Pd NCs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Jin, R. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2010, 2, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Sattler, K.; Mühlbach, J.; Recknagel, E. Generation of metal clusters containing from 2 to 500 atoms. Phys. Rev. Lett. 1980, 45, 821–824. [Google Scholar] [CrossRef]
- Tan, X.; Jin, R. Ultrasmall metal nanoclusters for bio-related applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zeng, C.; Chen, Y.; Zhao, S.; Sfeir, M.; Zhu, M.; Jin, R. Evolution from the plasmon to exciton state in atomically precise gold nanoparticles. Nat. Commun. 2016, 7, 13240. [Google Scholar] [CrossRef] [PubMed]
- Yau, S.H.; Varnavski, O.; Goodson, T., III. An ultrafast look at Au nanoclusters. Acc. Chem. Res. 2013, 46, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Wang, C.-W.; Yuan, Z.; Chang, H.-T. Fluorescent gold nanoclusters: Recent advances in sensing and imaging. Anal. Chem. 2014, 87, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.; Xie, J. Luminescent metal nanoclusters with aggregation-induced emission. J. Phys. Chem. Lett. 2016, 7, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, X.; Zheng, M.; Yang, R.; Yang, H.; Jia, L.; Yang, M. A 4,5-quinolimide-based fluorescent sensor for the turn-on detection of Cd2+ with live-cell imaging. Org. Biomol. Chem. 2017, 15, 2211–2216. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.A.; Yang, T.Y.; Lee, C.H.; Huang, S.H.; Sperling, R.A.; Zanella, M.; Li, J.K.; Shen, J.L.; Wang, H.H.; Yeh, H.I. Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS Nano 2009, 3, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Pradeep, T. Noble metal clusters: Applications in energy, environment, and biology. Part. Part. Syst. Charact. 2014, 31, 1017–1053. [Google Scholar] [CrossRef]
- Lin, J.; Lee, C.H.; Hsieh, J.T.; Wang, H.H.; Li, J.K.; Shen, J.L.; Chan, W.H.; Yeh, H.I.; Chang, W.H. Review: Synthesis of fluorescent metallic nanoclusters toward biomedical application: Recent progress and present challenges. J. Med. Biol. Eng. 2009, 29, 276–283. [Google Scholar]
- Yuan, X.; Luo, Z.; Yu, Y.; Yao, Q.; Xie, J. Luminescent noble metal nanoclusters as an emerging optical probe for sensor development. Chem. Asian J. 2013, 8, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, T.; Pan, H.; Yuan, Y.; Chen, L.; Liu, M.; Zhang, K.; Zhang, S.; Wu, P.; Xu, J. Photoemission mechanism of water-soluble silver nanoclusters: Ligand-to-metal–metal charge transfer vs strong coupling between surface plasmon and emitters. J. Am. Chem. Soc. 2014, 136, 1686–1689. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.W.; Zhu, B.; Zeng, X.C.; Gao, Y. A grand unified model for liganded gold clusters. Nat. Commun. 2016, 7, 13574. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Sun, L.; Crooks, R.M. Preparation of Cu nanoclusters within dendrimer templates. J. Am. Chem. Soc. 2015, 120, 4877–4878. [Google Scholar] [CrossRef]
- Udaya Bhaskara Rao, T.; Pradeep, T. Luminescent Ag7 and Ag8 clusters by interfacial synthesis. Angew. Chem. Int. Ed. 2010, 49, 3925–3929. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Fink, J.; Bethell, D.; Schiffrin, D.J.; Kiely, C. Synthesis and reactions of functionalised gold nanoparticles. J. Chem. Soc. Chem. Commun. 1995, 16, 1655–1656. [Google Scholar] [CrossRef]
- Brust, M. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Zheng, J.; Petty, J.T.; Dickson, R.M. High quantum yield blue emission from water-soluble Au8 nanodots. J. Am. Chem. Soc. 2003, 34, 7780–7781. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ren, J.; Yang, W.; Qu, X. Molecular crowding-facilitated synthesis of DNA-templated Ag nanoclusters with enhanced fluorescence emission and quantum yield. Chem. Commun. (Camb.) 2013, 49, 10856–10858. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, Z.; Zheng, H.; Huang, Y. Copper nanoclusters as a highly sensitive and selective fluorescence sensor for ferric ions in serum and living cells by imaging. Biosens. Bioelectron. 2014, 62, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.I.; Miyazaki, J.; Tiwari, D.K.; Jin, D.T.; Inouye, D.Y. Fluorescent platinum nanoclusters: Synthesis, purification, characterization, and application to bioimaging. Angew. Chem. Int. Ed. Engl. 2011, 50, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Duan, Y.; Wang, F.; Wang, C. Fluorescent Au nanoclusters stabilized by silane: Facile synthesis, color-tunability and photocatalytic properties. Nanoscale 2017, 9, 4981–4988. [Google Scholar] [CrossRef] [PubMed]
- Pérezlorenzo, M. Palladium nanoparticles as efficient catalysts for suzuki cross-coupling reactions. J. Phys. Chem. Lett. 2012, 43, 167–174. [Google Scholar] [CrossRef]
- Teschner, D.; Borsodi, J.; Wootsch, A.; Révay, Z.; Hävecker, M.; Knop-Gericke, A.; Jackson, S.D.; Schlögl, R. The roles of subsurface carbon and hydrogen in palladium-catalyzed alkyne hydrogenation. Science 2008, 320, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Kaden, W.E.; Wu, T.; Kunkel, W.A.; Anderson, S.L. Electronic structure controls reactivity of size-selected Pd clusters adsorbed on TiO2 surfaces. Science 2009, 326, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Wilson, O.M.; Knecht, M.R.; Garcia-Martinez, J.C.; Crooks, R.M. Effect of Pd nanoparticle size on the catalytic hydrogenation of allyl alcohol. J. Am. Chem. Soc. 2006, 128, 4510–4511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, B.; Jiang, K.; Cai, W.B. Surfactant-free synthesis of carbon supported palladium nanoparticles and size dependent hydrogen production from formic acid-formate solution. ACS Appl. Mater. Interfaces 2017, 9, 24678–24687. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wei, Y.; Zhao, X.; Li, X.; Zhang, Y.; Li, B.; Liu, J. Palladium nanoparticles supported by amyloid fibrils: From size controllable synthesis to extremely high catalytic performance. Colloids Surf. A 2015, 482, 416–421. [Google Scholar] [CrossRef]
- Yano, H.; Nakajima, Y.; Obora, Y. N,N-dimethylformamide-stabilized palladium nanoclusters as catalyst for migita–kosugi–stille cross-coupling reactions. J. Organomet. Chem. 2013, 745, 258–261. [Google Scholar] [CrossRef]
- Asada, S.; Nito, A.; Miyagi, Y.; Ishida, J.; Obora, Y.; Sanda, F. Sonogashira–hagihara and mizoroki–heck coupling polymerizations catalyzed by Pd nanoclusters. Macromolecules 2017, 50, 4083–4087. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, E. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today 2014, 9, 132–157. [Google Scholar] [CrossRef]
- Qu, X.; Li, Y.; Li, L.; Wang, Y.; Liang, J.; Liang, J. Fluorescent gold nanoclusters: Synthesis and recent biological application. J. Nanomater. 2015, 2015, 784097. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, C.; Yu, M.; Liu, J. Different sized luminescent gold nanoparticles. Nanoscale 2012, 4, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Jing, L.; Lei, H.; Ren, J.; Xuan, Y.; Wang, E. DNA-hosted copper nanoclusters for fluorescent identification of single nucleotide polymorphisms. ACS Nano 2012, 6, 3311–3317. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.-H.; Zhang, L.-N.; He, S.-B.; Liu, A.-L.; Li, G.-W.; Lin, X.-H.; Xia, X.-H.; Chen, W. Methionine-directed fabrication of gold nanoclusters with yellow fluorescent emission for Cu2+ sensing. Biosens. Bioelectron. 2015, 65, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Sagasti, A.; Bouropoulos, N.; Kouzoudis, D.; Panagiotopoulos, A.; Topoglidis, E.; Gutierrez, J. Nanostructured ZnO in a metglas/ZnO/hemoglobin modified electrode to detect the oxidation of the hemoglobin simultaneously by cyclic voltammetry and magnetoelastic resonance. Materials 2017, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Barati, A.; Shamsipur, M.; Abdollahi, H. Hemoglobin detection using carbon dots as a fluorescence probe. Biosens. Bioelectron. 2015, 71, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, X.; Zhang, Y.; Xu, Y.; Lu, K.; Li, C.; Yan, Y. Rapid and sensitive detection of hemoglobin with gold nanoparticles based fluorescence sensor in aqueous solution. J. Alloys Compd. 2016, 685, 820–827. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, S. Sensitive detection of trace hemoglobin using fluorescence method based on functionalized quantum dots. Anal. Bioanal. Chem. 2013, 405, 4989–4991. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Wang, P.; Luo, L.; Liu, L.; Wang, F. Green Synthesis of Fluorescent Palladium Nanoclusters. Materials 2018, 11, 191. https://doi.org/10.3390/ma11020191
Peng Y, Wang P, Luo L, Liu L, Wang F. Green Synthesis of Fluorescent Palladium Nanoclusters. Materials. 2018; 11(2):191. https://doi.org/10.3390/ma11020191
Chicago/Turabian StylePeng, Yan, Pei Wang, Liang Luo, Lang Liu, and Fu Wang. 2018. "Green Synthesis of Fluorescent Palladium Nanoclusters" Materials 11, no. 2: 191. https://doi.org/10.3390/ma11020191




