Effect of Cation Ordering on the Performance and Chemical Stability of Layered Double Perovskite Cathodes
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the Materials
2.2. Electrochemical Characterization
2.3. Oxygen Deficiency and Chemical Stability
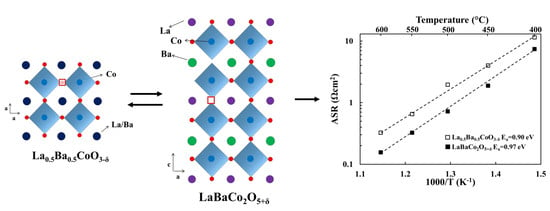
3. Results
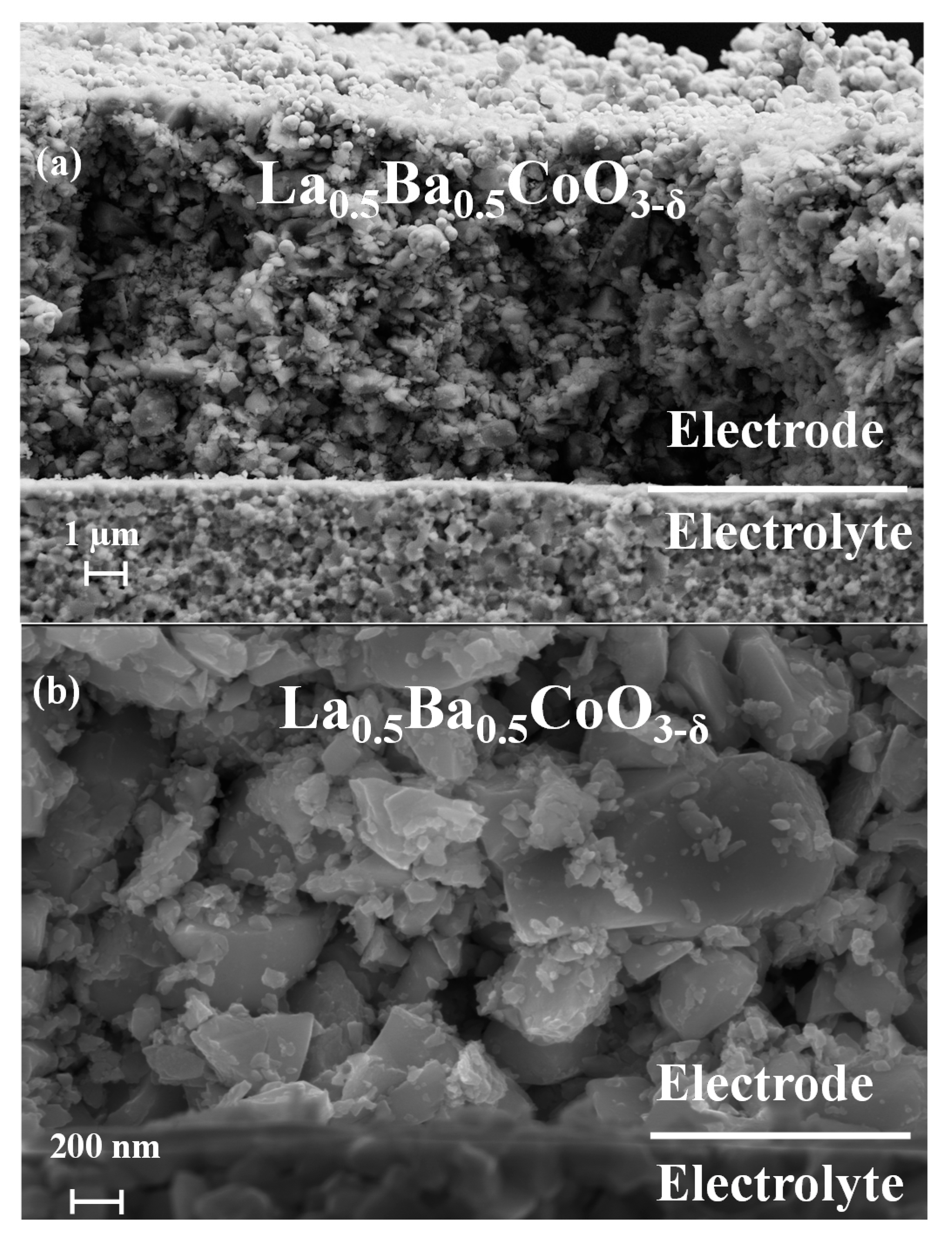
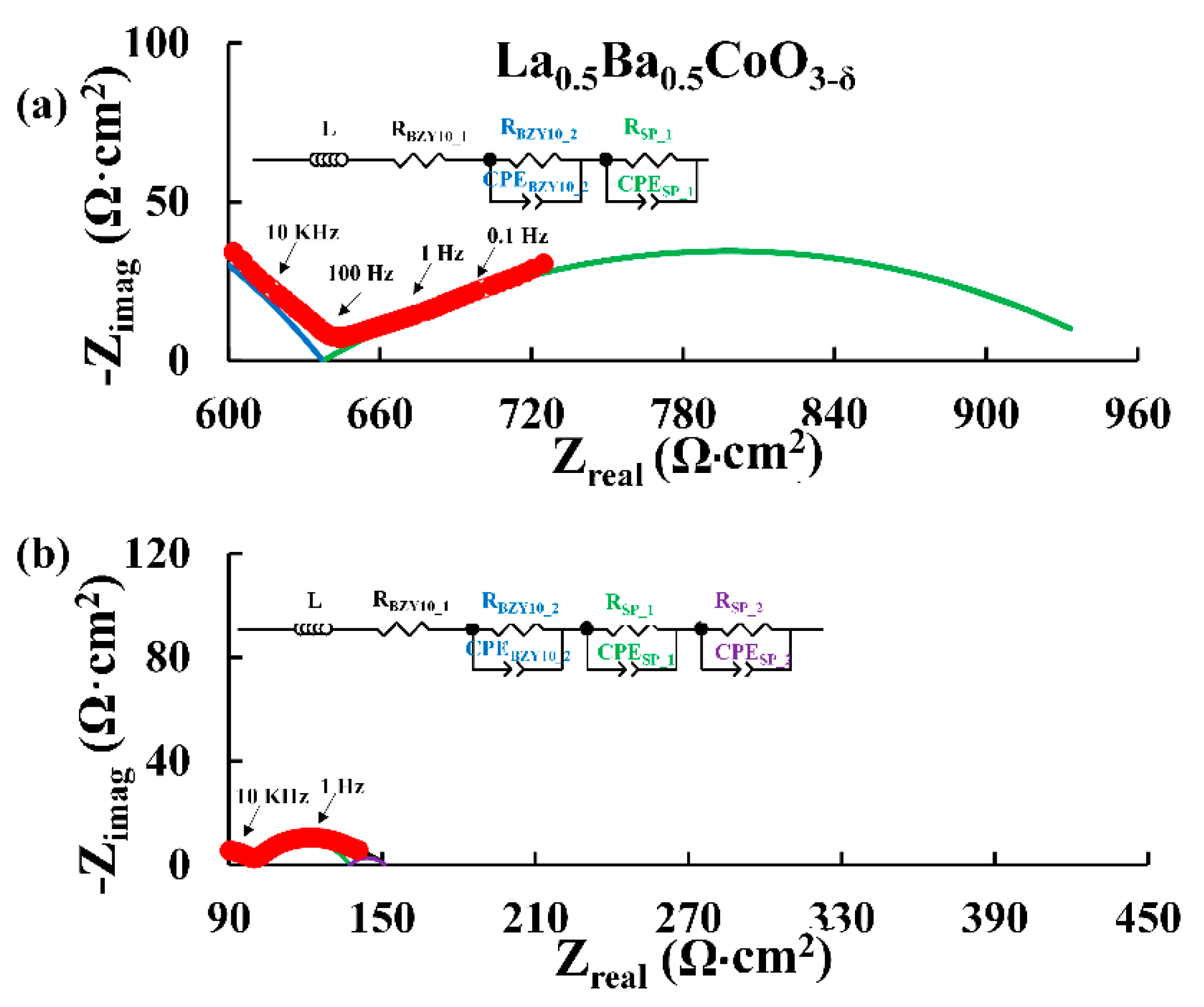
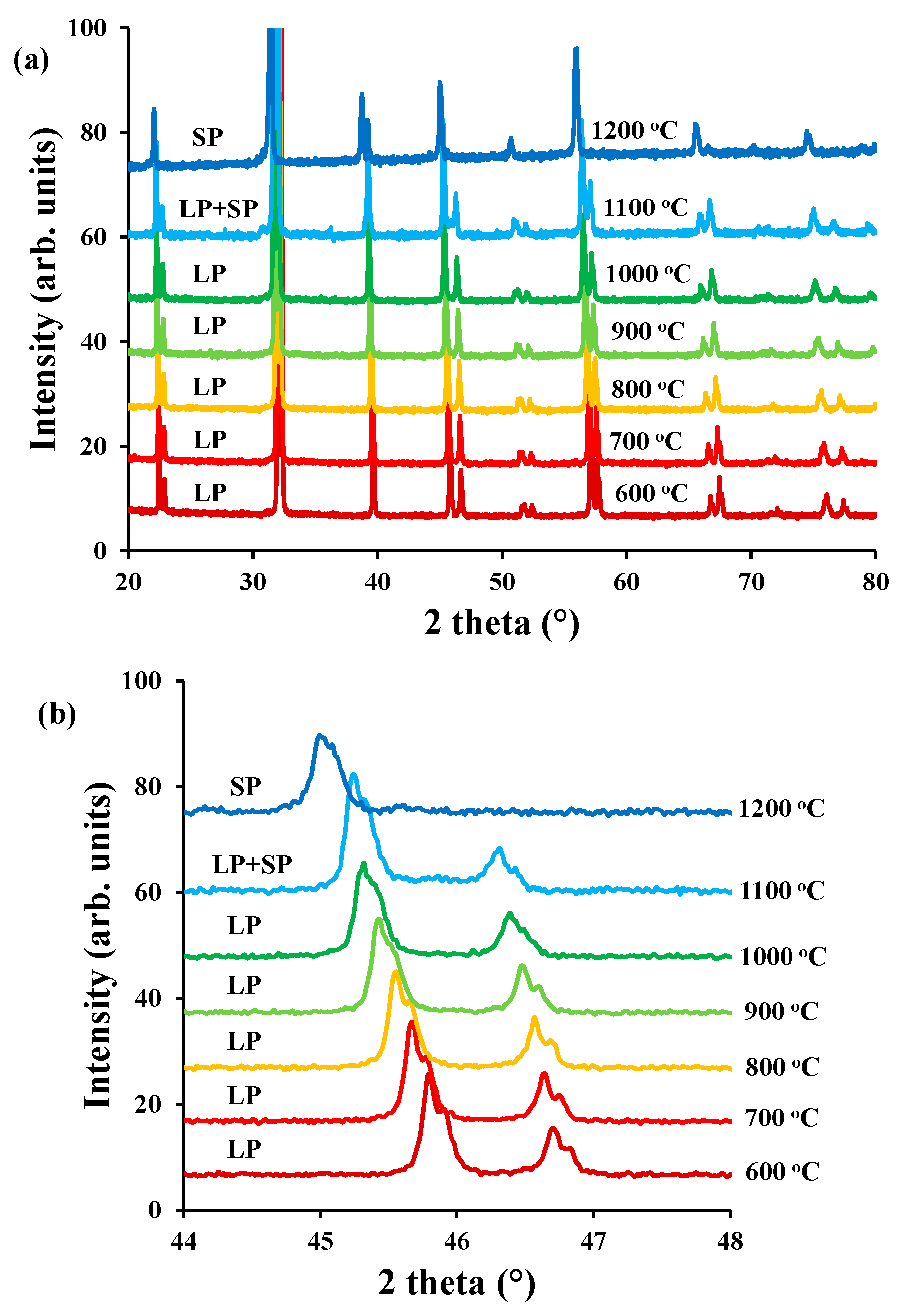
3.1. Microstructure of the Symmetric Cells
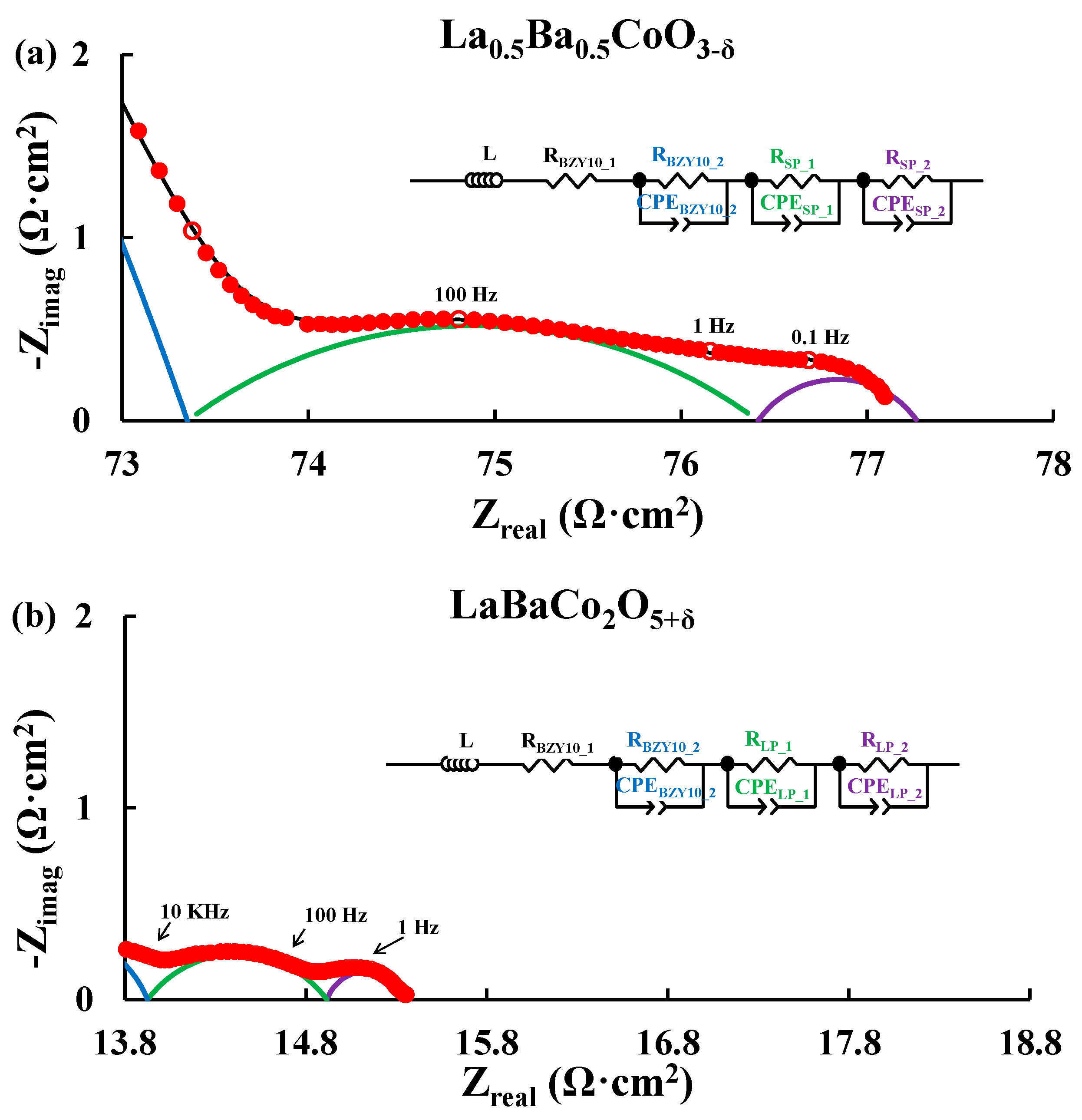
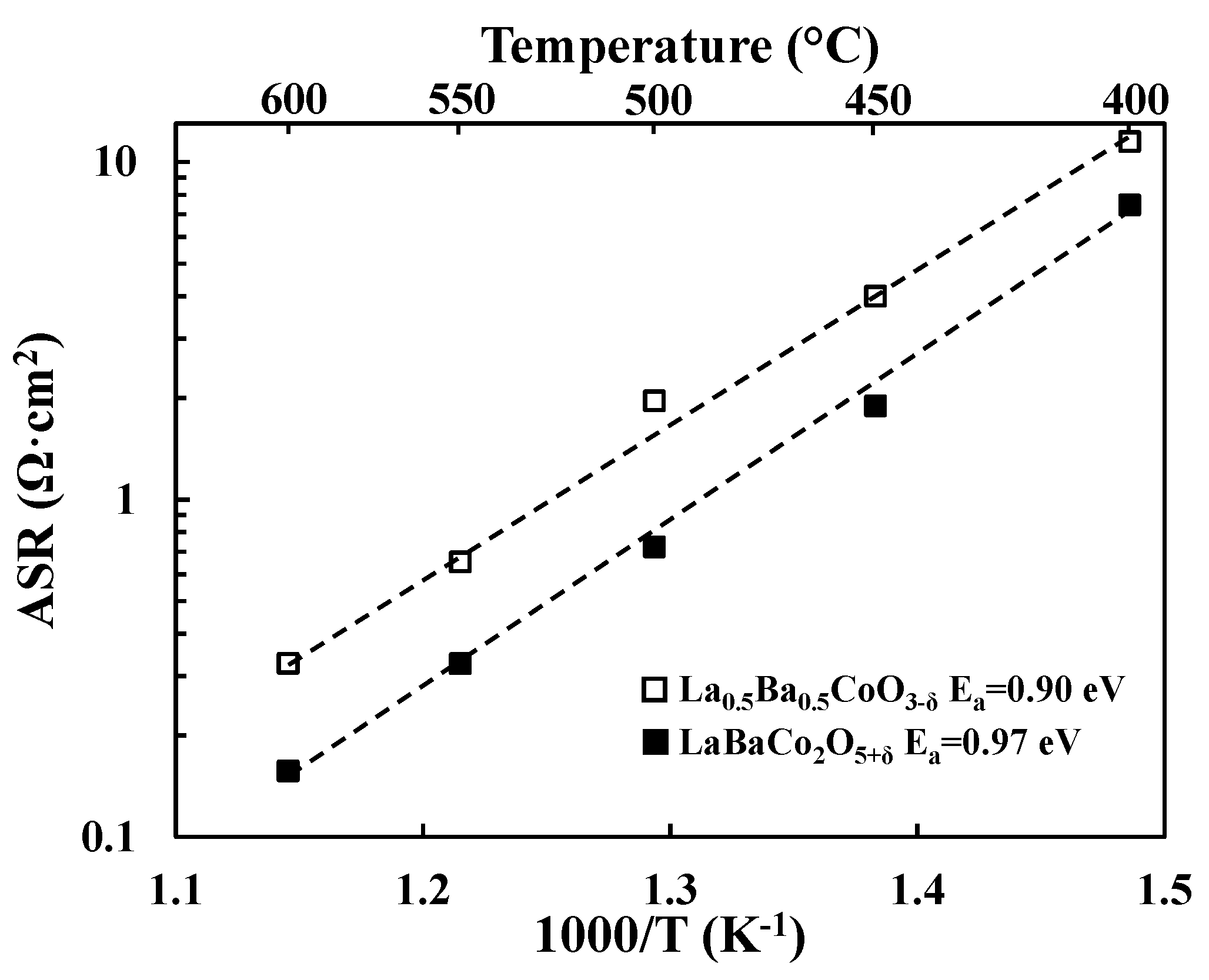
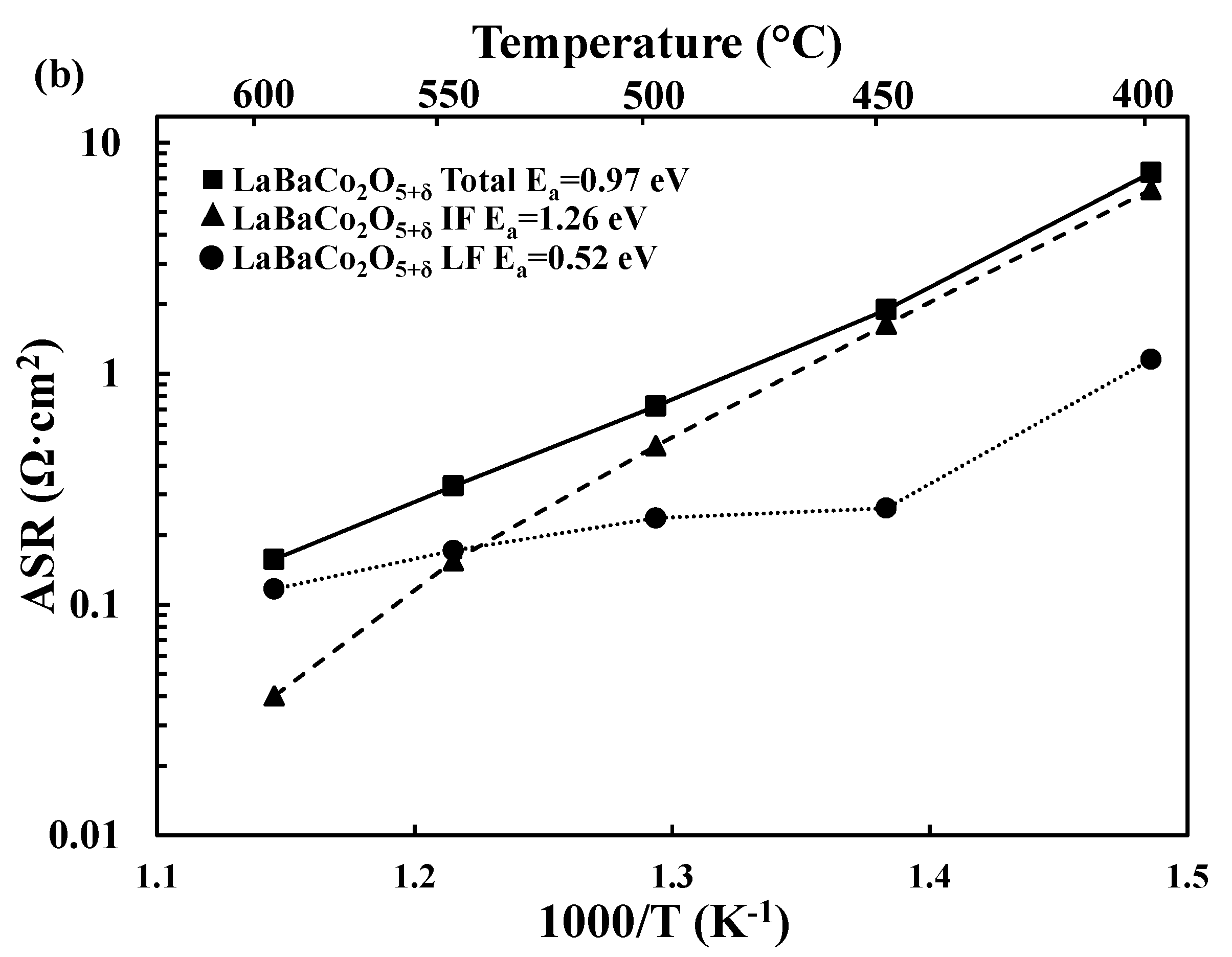
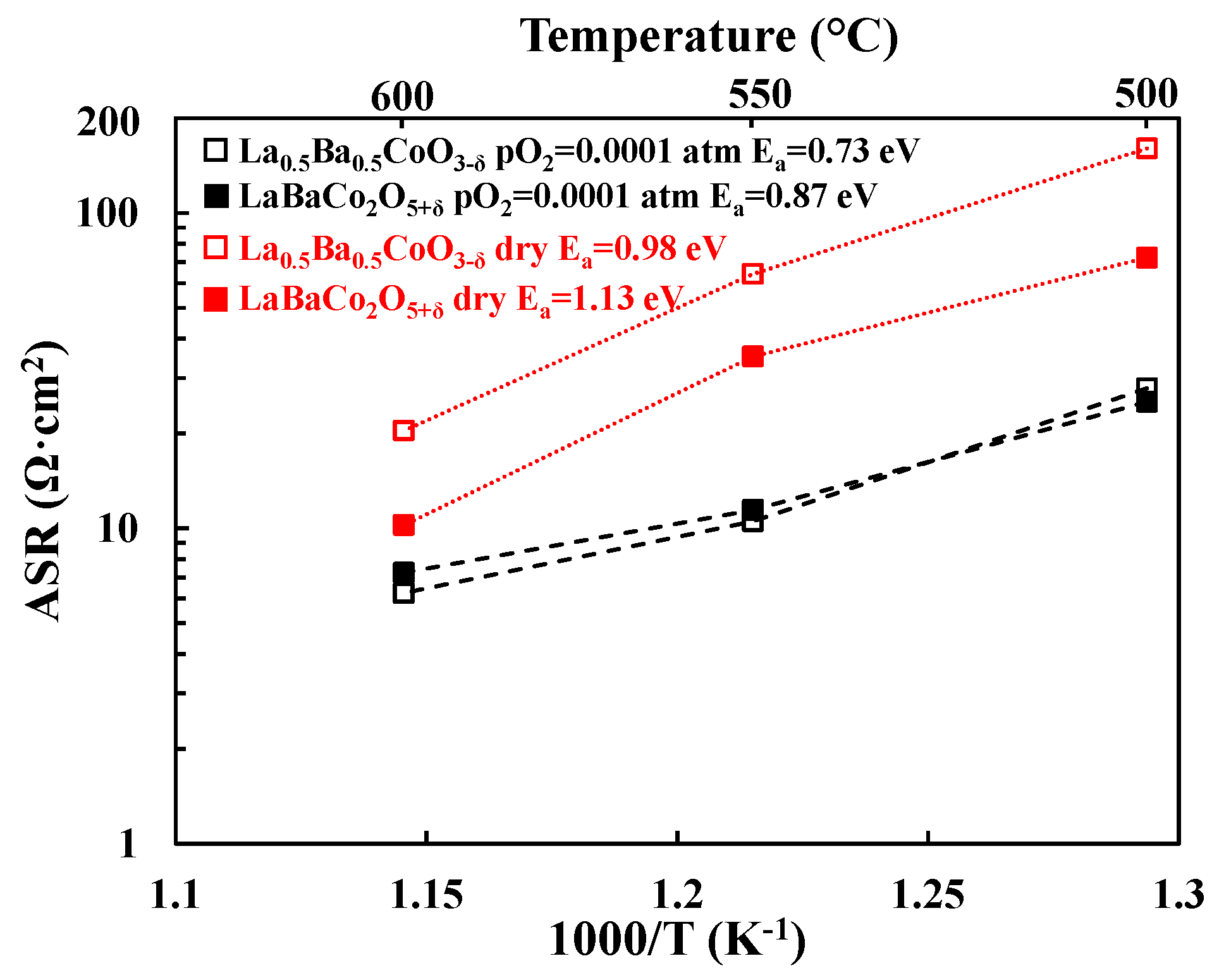
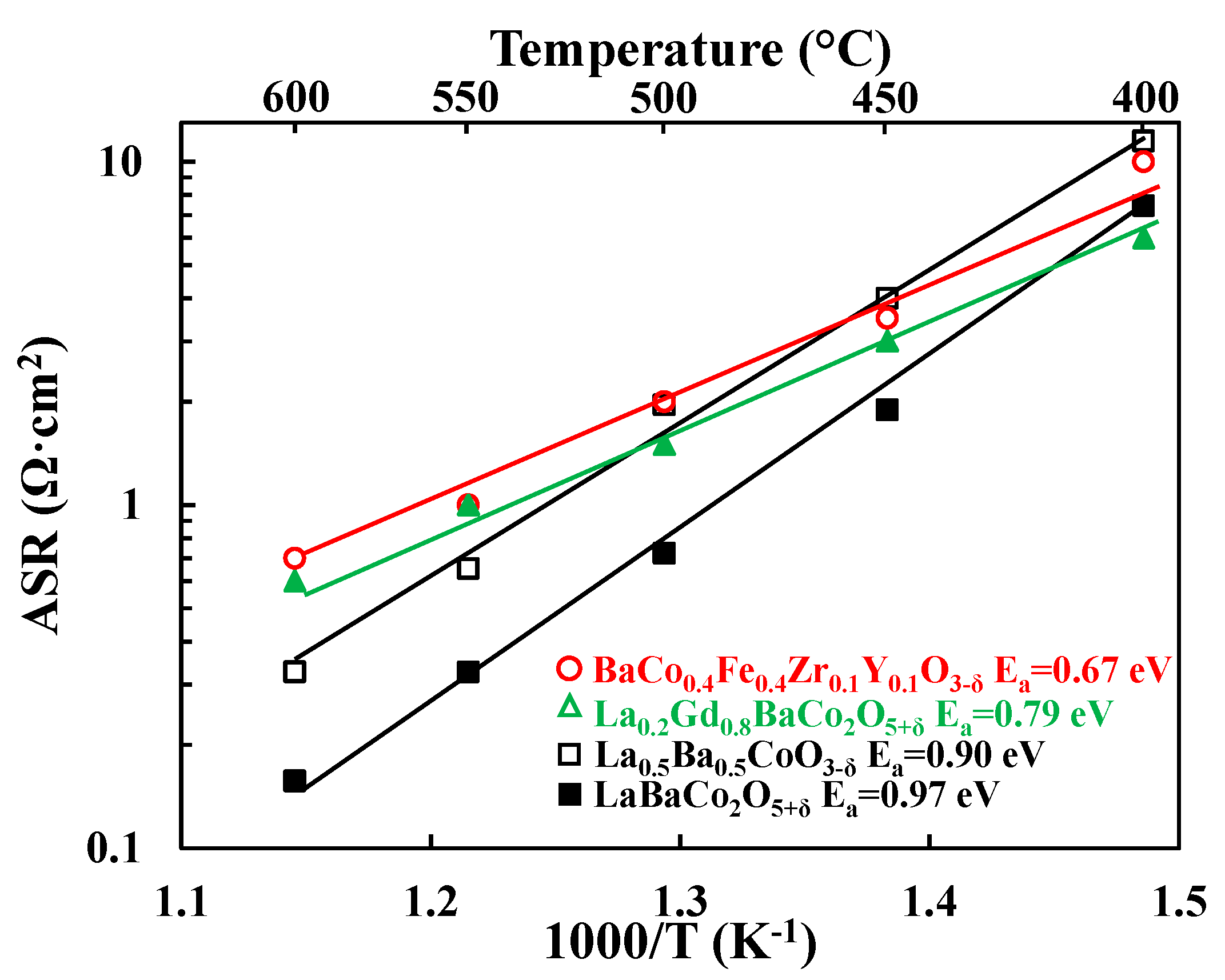
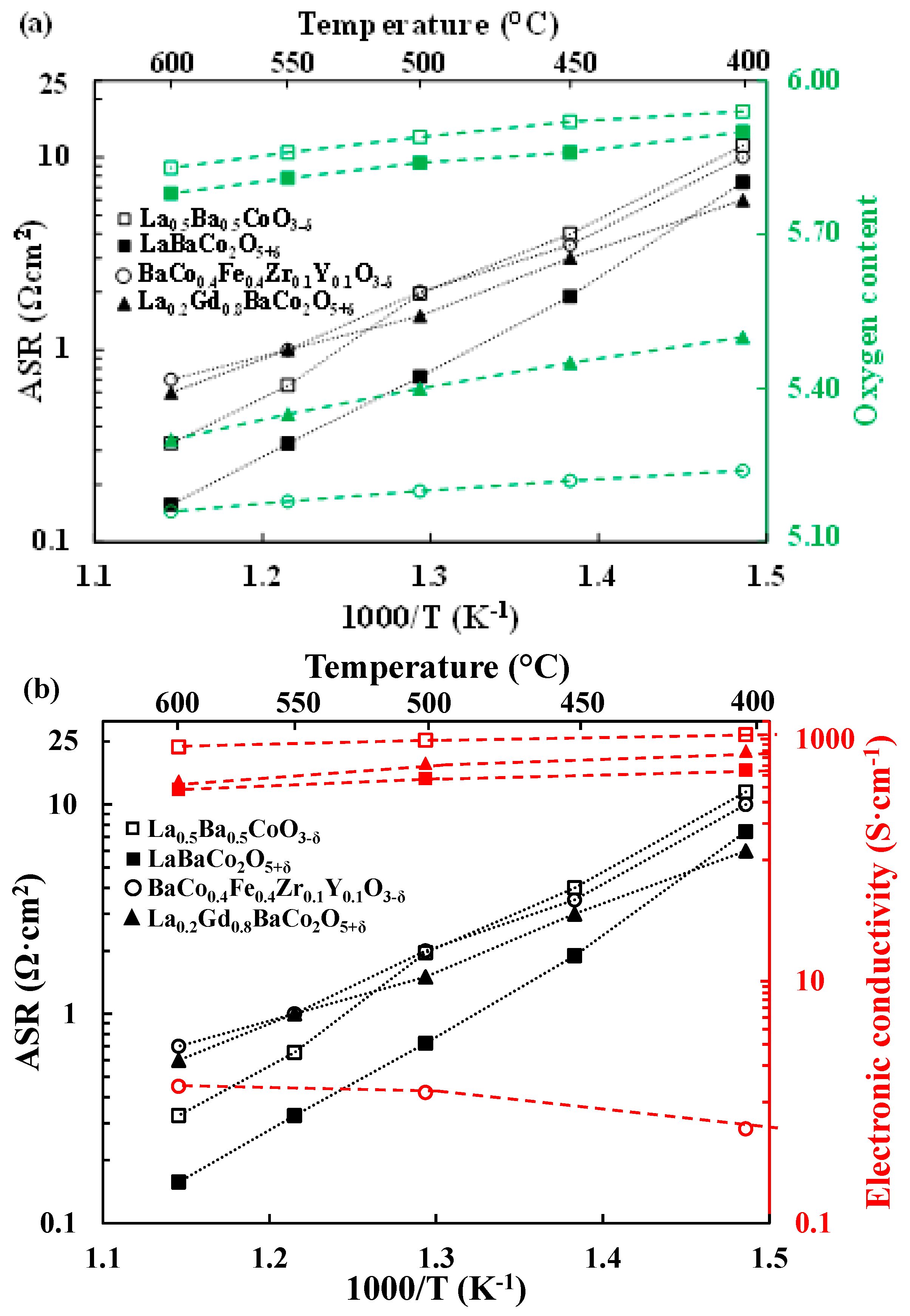
3.2. Electrochemical Performance
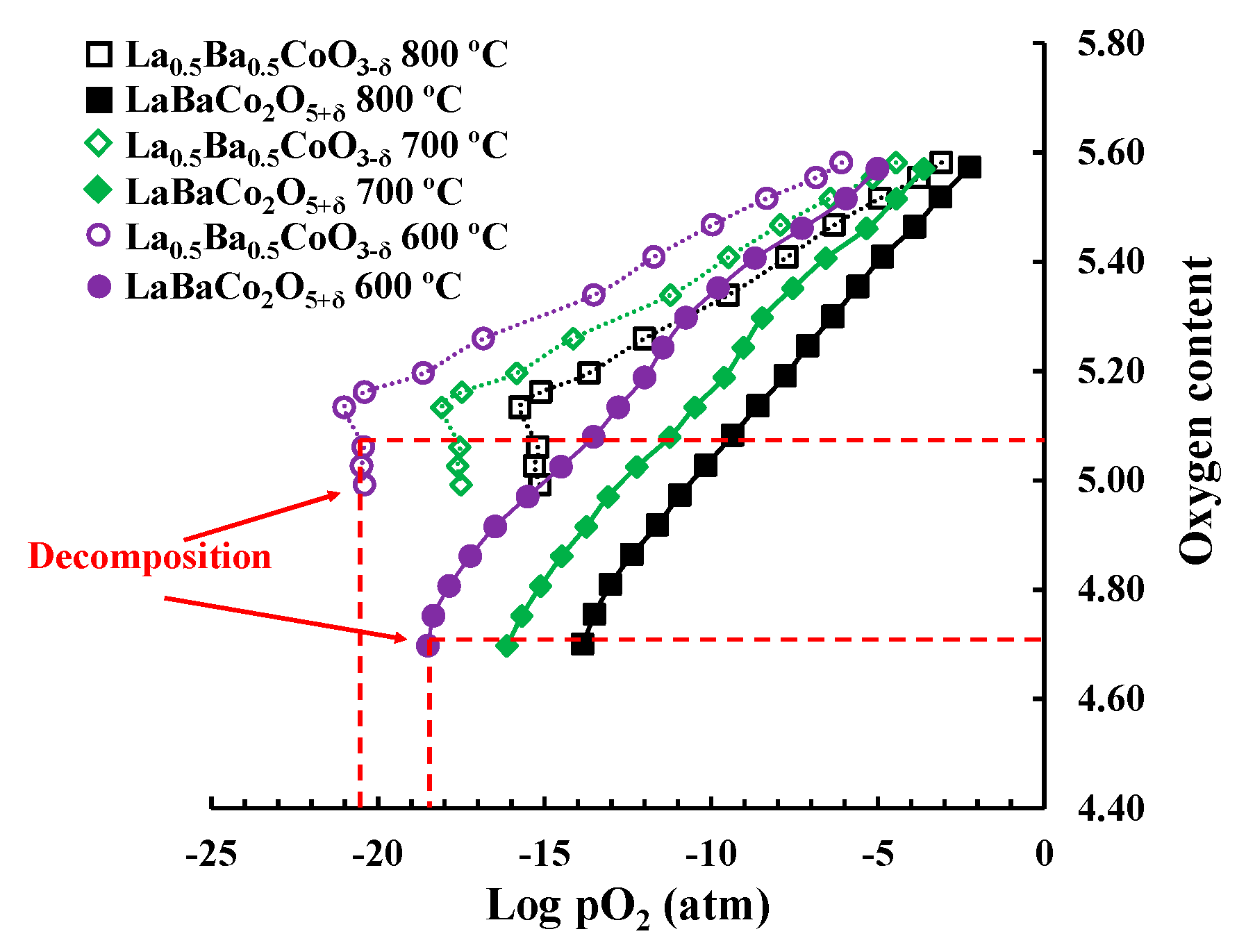
3.3. Chemical Stability
4. Discussion
4.1. Comparison with Literature
4.2. Correlation between ASR, Electrical Conductivity, Basicity and Oxygen Content
4.3. Chemical Stability of the Two Polymorphs
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ebbesen, S.D.; Jensen, S.H.; Hauch, A.; Mogensen, M.B. High Temperature Electrolysis in Alkaline Cells, Solid Proton Conducting Cells, and Solid Oxide Cells. Chem. Rev. 2014, 114, 10697–10734. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Boulfrad, S.; Traversa, E. Steam electrolysis by solid oxide electrolysis cells (SOECs) with proton-conducting oxides. Chem. Soc. Rev. 2014, 43, 8255–8270. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; O’Hayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Sengodan, S.; Kwon, G.; Ding, D.; Shin, J.; Liu, M.; Kim, G. Triple-Conducting Layered Perovskites as Cathode Materials for Proton-Conducting Solid Oxide Fuel Cells. ChemSusChem 2014, 7, 2811–2815. [Google Scholar] [CrossRef] [PubMed]
- Kreuer, K.D. Proton-Conducting Oxides. Ann. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Shang, M.; Tong, J.; O’Hayre, R. A promising cathode for intermediate temperature protonic ceramic fuel cells: BaCo0.4Fe0.4Zr0.2O3−δ. RSC Adv. 2013, 3, 15769–15775. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, H. A novel cobalt-free cathode material for proton-conducting solid oxide fuel cells. J. Mater. Chem. 2012, 22, 18387–18394. [Google Scholar] [CrossRef]
- Dailly, J.; Fourcade, S.; Largeteau, A.; Mauvy, F.; Grenier, J.C.; Marrony, M. Perovskite and A2MO4-type oxides as new cathode materials for protonic solid oxide fuel cells. Electrochim. Acta 2010, 55, 5847–5853. [Google Scholar] [CrossRef]
- Merkle, R.; Poetzsch, D.; Maier, J. Oxygen Reduction Reaction at Cathodes on Proton Conducting Oxide Electrolytes: Contribution from Three Phase Boundary Compared to Bulk Path. ECS Trans. 2015, 66, 95–102. [Google Scholar] [CrossRef]
- Poetzsch, D.; Merkle, R.; Maier, J. Oxygen Reduction at Dense Thin-Film Microelectrodes on a Proton-Conducting Electrolyte: I. Considerations on Reaction Mechanism and Electronic Leakage Effects. J. Electrochem. Soc. 2015, 162, F939–F950. [Google Scholar] [CrossRef]
- Bahout, M.; Pramana, S.S.; Hanlon, J.M.; Dorcet, V.; Smith, R.I.; Paofai, S.; Skinner, S.J. Stability of NdBaCo2−xMnxO5+δ (x = 0, 0.5) layered perovskites under humid conditions investigated by high-temperature in situ neutron powder diffraction. J. Mater. Chem. A 2015, 3, 15420–15431. [Google Scholar] [CrossRef]
- Poetzsch, D.; Merkle, R.; Maier, J. Proton conductivity in mixed-conducting BSFZ perovskite from thermogravimetric relaxation. Phys. Chem. Chem. Phys. 2014, 16, 16446–16453. [Google Scholar] [CrossRef] [PubMed]
- Zohourian, R.; Merkle, R.; Maier, J. Proton uptake into the protonic cathode material BaCo0.4Fe0.4Zr0.2O3−δ and comparison to protonic electrolyte materials. Solid State Ion. 2017, 299, 64–69. [Google Scholar] [CrossRef]
- Gryaznov, D.; Merkle, R.; Kotomin, E.A.; Maier, J. Ab initio modelling of oxygen vacancies and protonic defects in La1−xSrxFeO3−δ perovskite solid solutions. J. Mater. Chem. A 2016, 4, 13093–13104. [Google Scholar] [CrossRef]
- Grimaud, A.; Bassat, J.M.; Mauvy, F.; Pollet, M.; Wattiaux, A.; Marrony, M.; Grenier, J.C. Oxygen reduction reaction of PrBaCo2−xFexO5+δ compounds as H+-SOFC cathodes: Correlation with physical properties. J. Mater. Chem. A 2014, 2, 3594–3604. [Google Scholar] [CrossRef]
- Strandbakke, R.; Cherepanov, V.A.; Zuev, A.Y.; Tsvetkov, D.S.; Argirusis, C.; Sourkouni, G.; Prünte, S.; Norby, T. Gd- and Pr-based double perovskite cobaltites as oxygen electrodes for proton ceramic fuel cells and electrolyser cells. Solid State Ion. 2015, 278, 120–132. [Google Scholar] [CrossRef]
- Mao, X.; Yu, T.; Ma, G. Performance of cobalt-free double-perovskite NdBaFe2−xMnxO5+δ cathode materials for proton-conducting IT-SOFC. J. Alloys Compd. 2015, 637, 286–290. [Google Scholar] [CrossRef]
- Singh, K.; Baral, A.K.; Thangadurai, V. Electrochemical Studies of Gd0.5Pr0.5BaCo2O5+δ (GPBC) Cathode for Oxide Ion and Proton Conducting Solid Oxide Fuel Cells. Solid State Ion. 2016, 288, 351–356. [Google Scholar] [CrossRef]
- Pelosato, R.; Cordaro, G.; Stucchi, D.; Cristiani, C.; Dotelli, G. Cobalt based layered perovskites as cathode material for intermediate temperature Solid Oxide Fuel Cells: A brief review. J. Power Sources 2015, 298, 46–67. [Google Scholar] [CrossRef]
- Sengodan, S.; Choi, S.; Jun, A.; Shin, T.H.; Ju, Y.W.; Jeong, H.Y.; Shin, J.; Irvine, J.T.S.; Kim, G. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 2015, 14, 205–209. [Google Scholar] [CrossRef] [PubMed]
- King, G.; Woodward, P.M. Cation ordering in perovskites. J. Mater. Chem. 2010, 20, 5785–5796. [Google Scholar] [CrossRef]
- Maignan, A.; Martin, C.; Pelloquin, D.; Nguyen, N.; Raveau, B. Structural and Magnetic Studies of Ordered Oxygen-Deficient Perovskites LnBaCo2O5+δ, Closely Related to the “112” Structure. J. Solid State Chem. 1999, 142, 247–260. [Google Scholar] [CrossRef]
- Muñoz-Gil, D.; Ávila-Brande, D.; Urones-Garrote, E.; García-Martín, S. Ordering effects in the crystal structure and electrochemical properties of the Gd0.5Ba0.5Mn0.5Fe0.5O3−δ perovskite. Dalton Trans. 2015, 44, 10867–10874. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Allix, M.; Ibberson, R.M.; Claridge, J.B.; Niu, H.; Rosseinsky, M.J. Oxygen Vacancy Ordering Phenomena in the Mixed-Conducting Hexagonal Perovskite Ba7Y2Mn3Ti2O20. Chem. Mater. 2007, 19, 2884–2893. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Høydalsvik, K.; Einarsrud, M.-A.; Grande, T. Effect of A-Site Cation Ordering on Chemical Stability, Oxygen Stoichiometry and Electrical Conductivity in Layered LaBaCo2O5+δ Double Perovskite. Materials 2016, 9, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setevich, C.; Mogni, L.; Caneiro, A.; Prado, F. Characterization of the La1−xBaxCoO3−δ (0 ≤ x≤ 1) System as Cathode Material for IT-SOFC. J. Electrochem. Soc. 2011, 159, B72–B79. [Google Scholar] [CrossRef]
- Setevich, C.F.; Mogni, L.V.; Caneiro, A.; Prado, F.D. Optimum cathode configuration for IT-SOFC using La0.4Ba0.6CoO3−δ and Ce0.9Gd0.1O1.95. Int. J. Hydrogen Energy 2012, 37, 14895–14901. [Google Scholar] [CrossRef]
- Pang, S.; Jiang, X.; Li, X.; Su, Z.; Xu, H.; Xu, Q.; Chen, C. Characterization of cation-ordered perovskite oxide LaBaCo2O5+δ as cathode of intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 6836–6843. [Google Scholar] [CrossRef]
- Garces, D.; Setevich, C.F.; Caneiro, A.; Cuello, G.J.; Mogni, L. Effect of cationic order-disorder on the transport properties of LaBaCo2O6−δ and La0.5Ba0.5CoO3−δ perovskites. J. Appl. Crystallogr. 2014, 47, 325–334. [Google Scholar] [CrossRef]
- Garcés, D.; Soldati, A.L.; Troiani, H.; Montenegro-Hernández, A.; Caneiro, A.; Mogni, L.V. La/Ba-based cobaltites as IT-SOFC cathodes: A discussion about the effect of crystal structure and microstructure on the O2-reduction reaction. Electrochim. Acta 2016, 215, 637–646. [Google Scholar] [CrossRef]
- Dahl, P.I.; Lein, H.L.; Yu, Y.; Tolchard, J.; Grande, T.; Einarsrud, M.-A.; Kjølseth, C.; Norby, T.; Haugsrud, R. Microstructural characterization and electrical properties of spray pyrolyzed conventionally sintered or hot-pressed BaZrO3 and BaZr0.9Y0.1O3−δ. Solid State Ion. 2011, 182, 32–40. [Google Scholar] [CrossRef]
- Sažinas, R.; Bernuy-López, C.; Einarsrud, M.A.; Grande, T. Effect of CO2 exposure on the chemical stability and mechanical properties of BaZrO3-ceramics. J. Am. Ceram. Soc. 2016, 99, 3685–3695. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, F.; Budiman, R.A.; Nakamura, T.; Amezawa, K. Oxygen nonstoichiometry, the defect equilibrium model and thermodynamic quantities of the Ruddlesden–Popper oxide Sr3Fe2O7−δ. Phys. Chem. Chem. Phys. 2015, 17, 7489–7497. [Google Scholar] [CrossRef] [PubMed]
- Mizusaki, J.; Mori, N.; Takai, H.; Yonemura, Y.; Minamiue, H.; Tagawa, H.; Dokiya, M.; Inaba, H.; Naraya, K.; Sasamoto, T.; et al. Oxygen nonstoichiometry and defect equilibrium in the perovskite-type oxides La1−xSrxMnO3+δ. Solid State Ion. 2000, 129, 163–177. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Sinclair, D.C.; West, A.R. Electroceramics: Characterization by Impedance Spectroscopy. Adv. Mater. 1990, 2, 132–138. [Google Scholar] [CrossRef]
- Bausá, N.; Solís, C.; Strandbakke, R.; Serra, J.M. Development of composite steam electrodes for electrolyzers based on barium zirconate. Solid State Ion. 2017, 306, 62–68. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Lenrick, F.; Wallenberg, R. LaCoO3: Promising cathode material for protonic ceramic fuel cells based on a BaCe0.2Zr0.7Y0.1O3−δ electrolyte. J. Power Sources 2012, 218, 313–319. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Rørvik, P.M.; Haavik, C. Microstructure and performance of La0.58Sr0.4Co0.2Fe0.8O3−δ cathodes deposited on BaCe0.2Zr0.7Y0.1O3−δ by infiltration and spray pyrolysis. J. Power Sources 2012, 209, 172–179. [Google Scholar] [CrossRef]
- Peng, R.; Wu, T.; Liu, W.; Liu, X.; Meng, G. Cathode processes and materials for solid oxide fuel cells with proton conductors as electrolytes. J. Mater. Chem. 2010, 20, 6218–6225. [Google Scholar] [CrossRef]
- Fabbri, E.; Bi, L.; Pergolesi, D.; Traversa, E. High-performance composite cathodes with tailored mixed conductivity for intermediate temperature solid oxide fuel cells using proton conducting electrolytes. Energy Environ. Sci. 2011, 4, 4984–4993. [Google Scholar] [CrossRef]
- Solis, C.; Navarrete, L.; Roitsch, S.; Serra, J.M. Electrochemical properties of composite fuel cell cathodes for La5.5WO12−δ proton conducting electrolytes. J. Mater. Chem. 2012, 22, 16051–16059. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Wang, H.J.; Haugsrud, R. Conductivity, transport number measurements and hydration thermodynamics of BaCe0.2Zr0.7Y(0.1−ξ)NiξO(3−δ). Solid State Ion. 2011, 185, 11–17. [Google Scholar] [CrossRef]
- Adler, S.B.; Lane, J.A.; Steele, C.H. Electrode Kinetics of Porous Mixed-Conducting Oxygen Electrodes. J. Electrochem. Soc. 1996, 143, 3554–3564. [Google Scholar] [CrossRef]
- Strandbakke, R.; Dyrlie, O.; Hage, F.S.; Norby, T. Reaction kinetics of protons and oxide ions in LSM/lanthanum tungstate cathodes with Pt nanoparticle activation. J. Electrochem. Soc. 2016, 163, F507–B515. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. LnBaCo2O5+δ Oxides as Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells. J. Electrochem. Soc. 2008, 155, B385–B390. [Google Scholar] [CrossRef]
- Duan, C.; Hook, D.; Chen, Y.; Tong, J.; O’Hayre, R. Zr and Y co-doped perovskite as a stable, high performance cathode for solid oxide fuel cells operating below 500 °C. Energy Environ. Sci. 2017, 10, 176–182. [Google Scholar] [CrossRef]

| Ba per mol | Structure | Material | Performance (Ω·cm2) at 400 °C and 3% H2O in Air | Performance (Ω·cm2) at 600 °C and 3% H2O in Air | Oxygen Content at 400 °C in Air | Oxygen Content at 600 °C in Air | Electrical Conductivity at 400 °C in Air (S·cm−1) | Electrical Conductivity at 600 °C in Air (S·cm−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | Single perovskite | BaCo0.4Fe0.4Zr0.1Y0.1O3−δ (BCFZY) | 23.03 | 0.703 | 2.6713 | 2.5813 | 0.603 | 1.353 |
| 0.5 | La0.5Ba0.5CoO3−δ | 11.5 | 0.33 | 2.97 | 2.92 | 1085.0 | 857.0 | |
| Layered double perovskite | LaBaCo2O5+δ | 7.4 | 0.16 | 2.95 | 2.89 | 554.0 | 384.0 | |
| La0.2Gd0.8BaCo2O6−δ (LGBC) | 6.016 | 0.616 | 2.7516 | 2.6516 | <795 (GdBaCo2O5+δ)5 | <447 (GdBaCo2O5+δ)5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernuy-Lopez, C.; Rioja-Monllor, L.; Nakamura, T.; Ricote, S.; O’Hayre, R.; Amezawa, K.; Einarsrud, M.-A.; Grande, T. Effect of Cation Ordering on the Performance and Chemical Stability of Layered Double Perovskite Cathodes. Materials 2018, 11, 196. https://doi.org/10.3390/ma11020196
Bernuy-Lopez C, Rioja-Monllor L, Nakamura T, Ricote S, O’Hayre R, Amezawa K, Einarsrud M-A, Grande T. Effect of Cation Ordering on the Performance and Chemical Stability of Layered Double Perovskite Cathodes. Materials. 2018; 11(2):196. https://doi.org/10.3390/ma11020196
Chicago/Turabian StyleBernuy-Lopez, Carlos, Laura Rioja-Monllor, Takashi Nakamura, Sandrine Ricote, Ryan O’Hayre, Koji Amezawa, Mari-Ann Einarsrud, and Tor Grande. 2018. "Effect of Cation Ordering on the Performance and Chemical Stability of Layered Double Perovskite Cathodes" Materials 11, no. 2: 196. https://doi.org/10.3390/ma11020196