Regulating the Emission Spectrum of CsPbBr3 from Green to Blue via Controlling the Temperature and Velocity of Microchannel Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
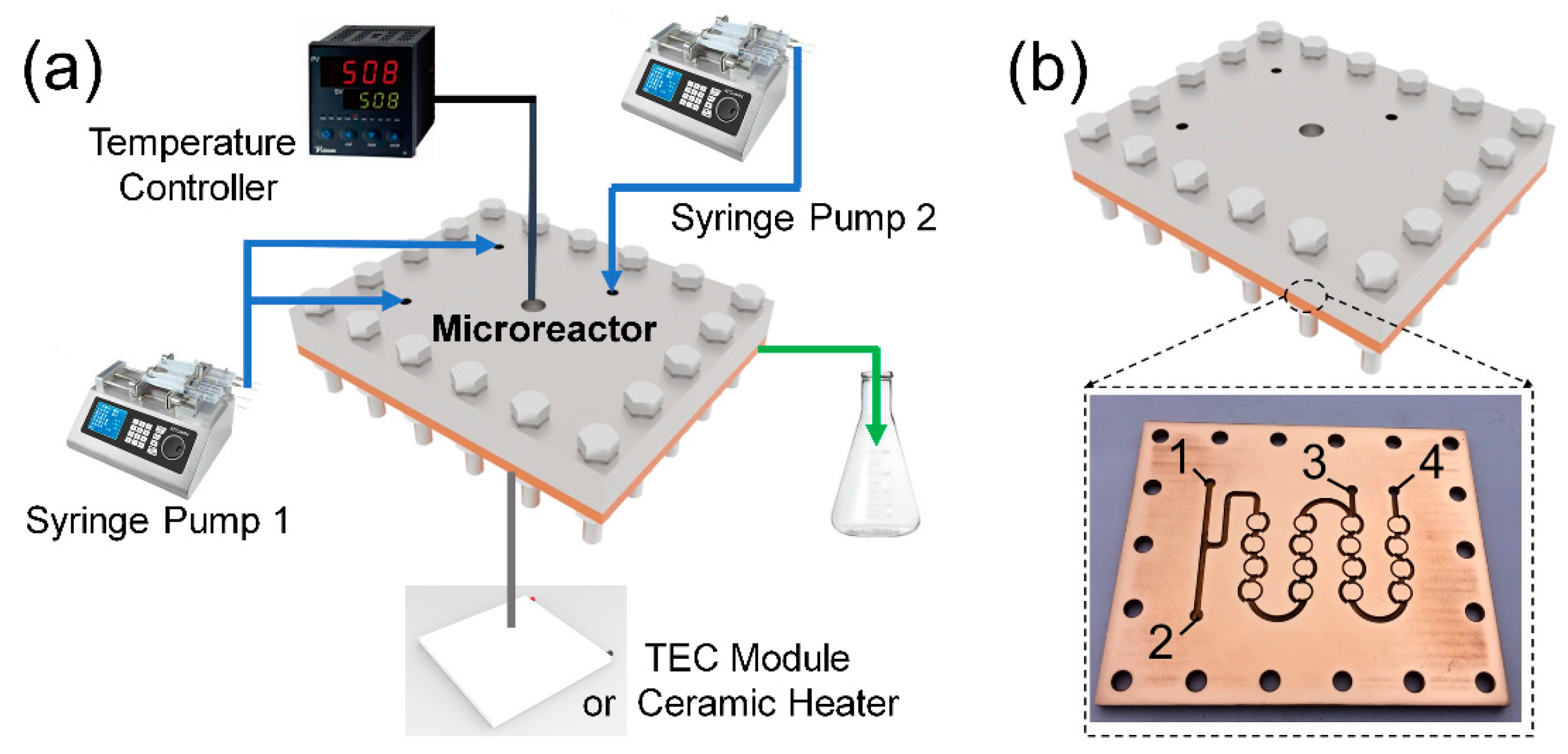
2.2. Synthesis of CsPbBr3 QDs with Tunable Spectrum
2.3. Characterization
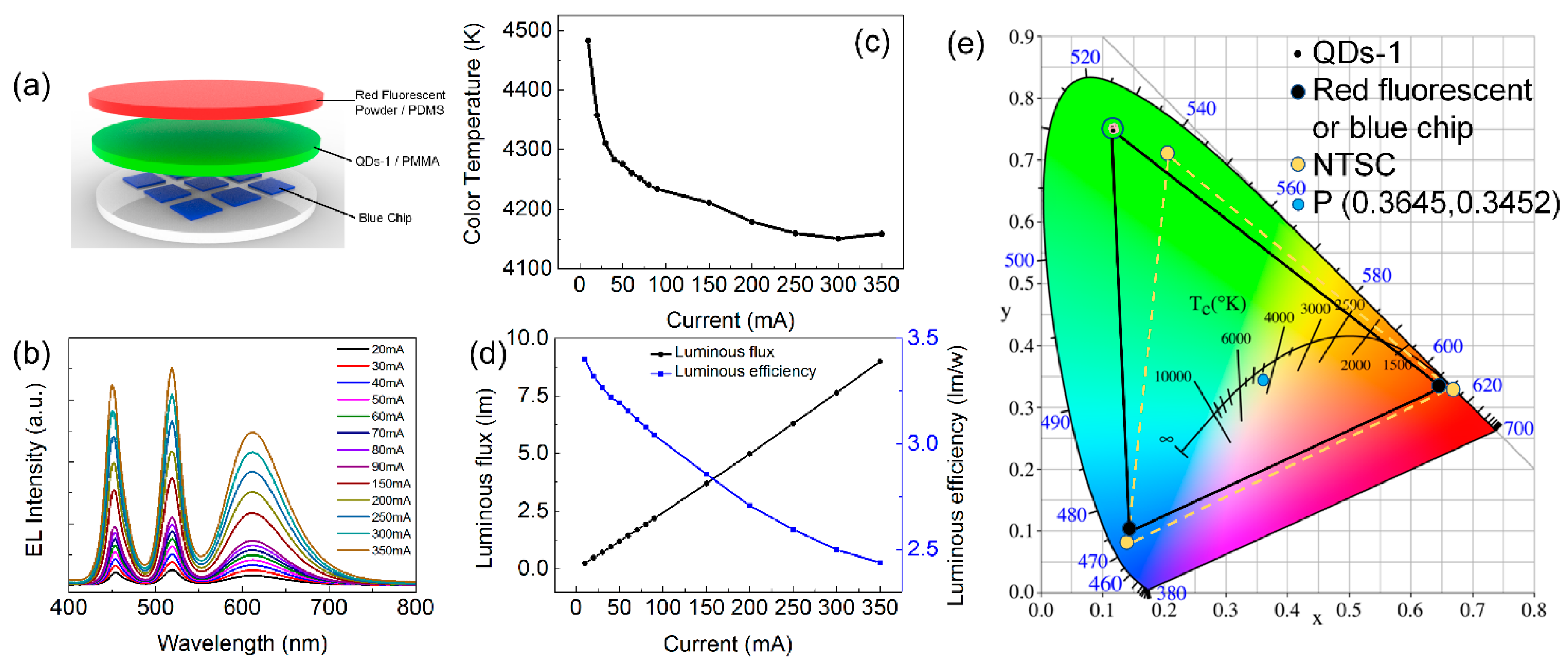
3. Results and Dicussion
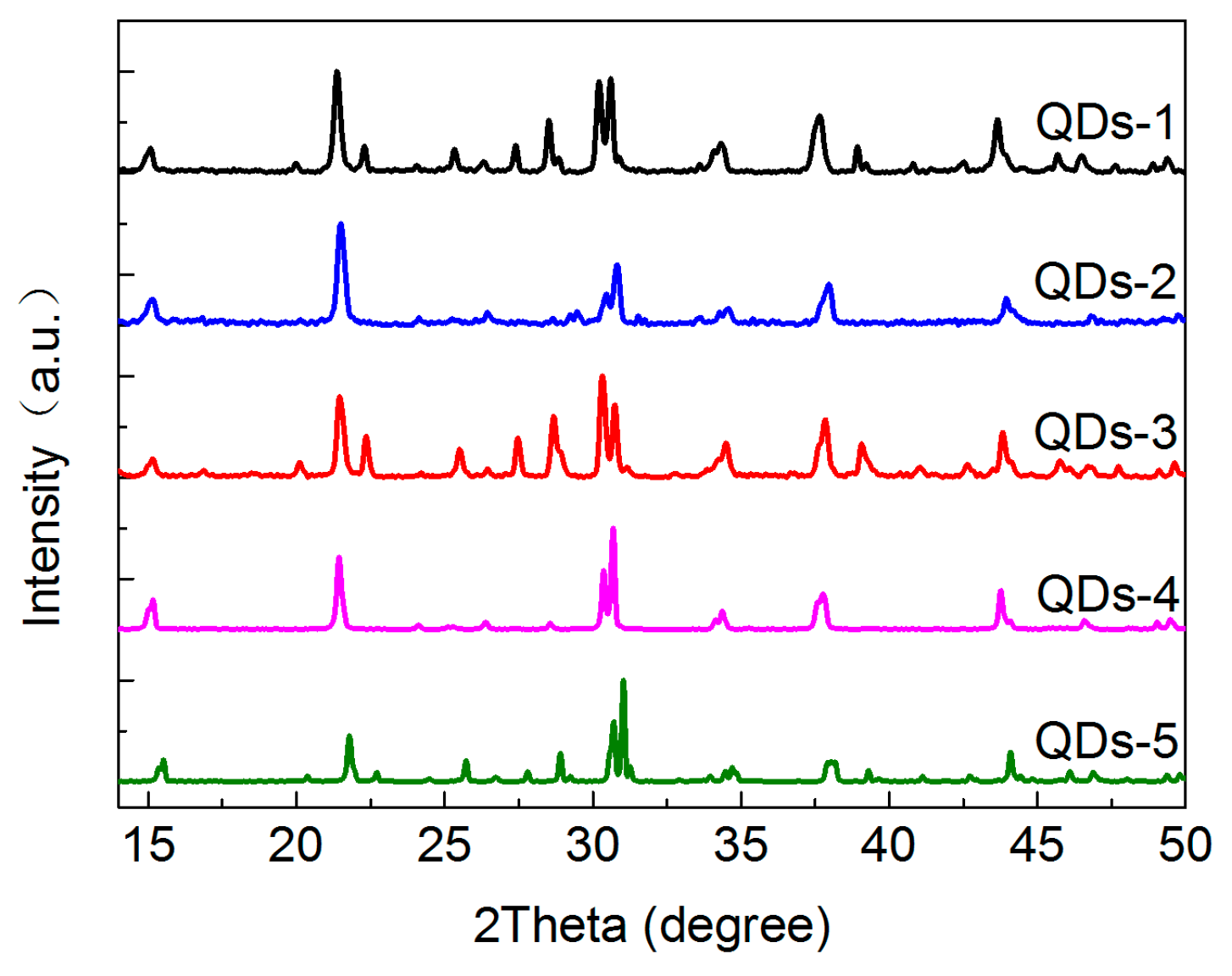
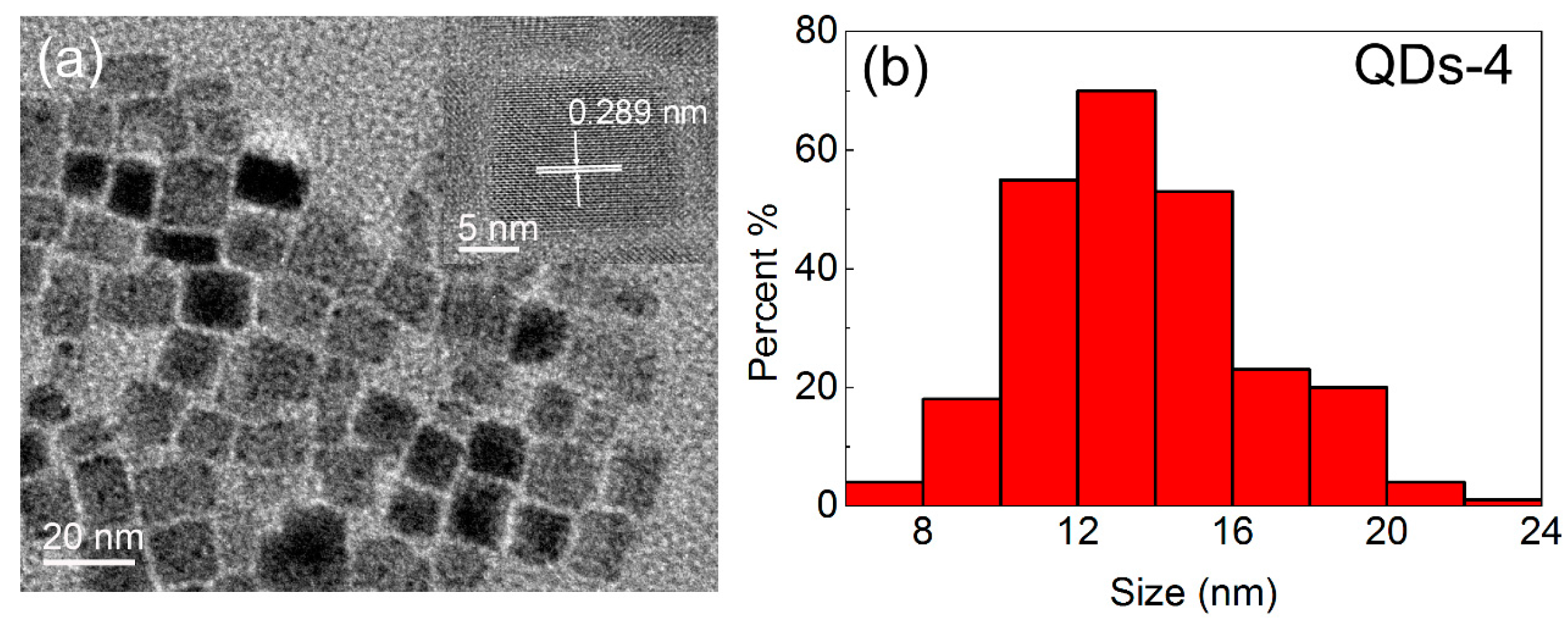
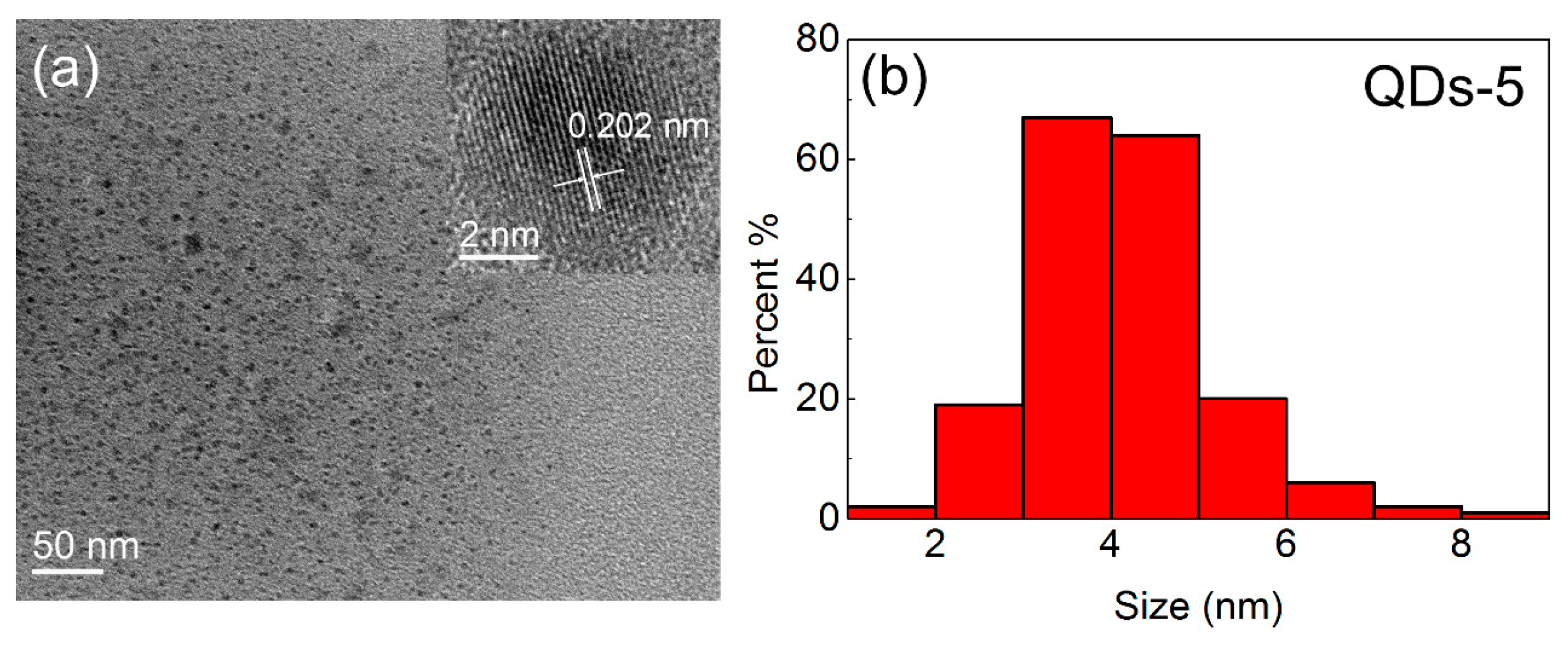
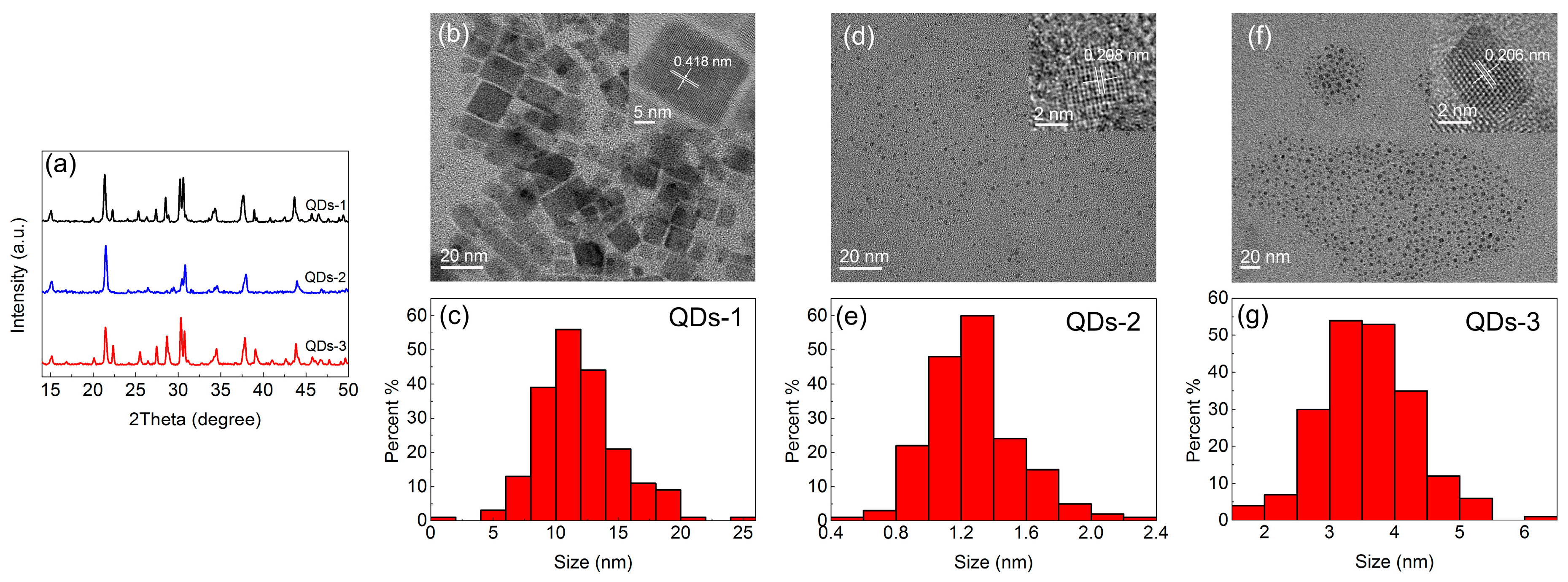
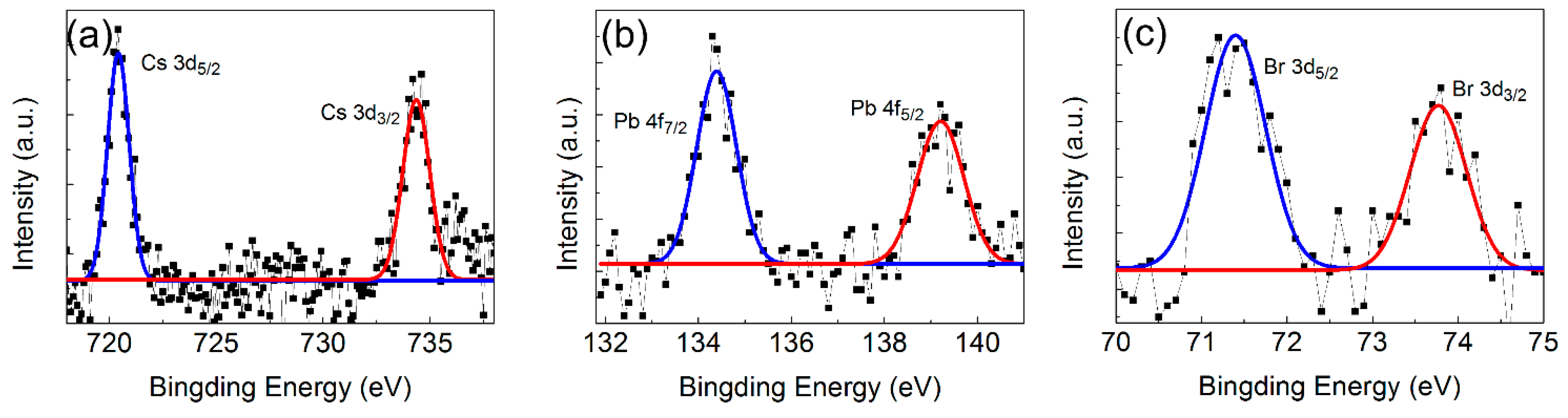
3.1. The Structure and Morphology of CsPbBr3 QDs
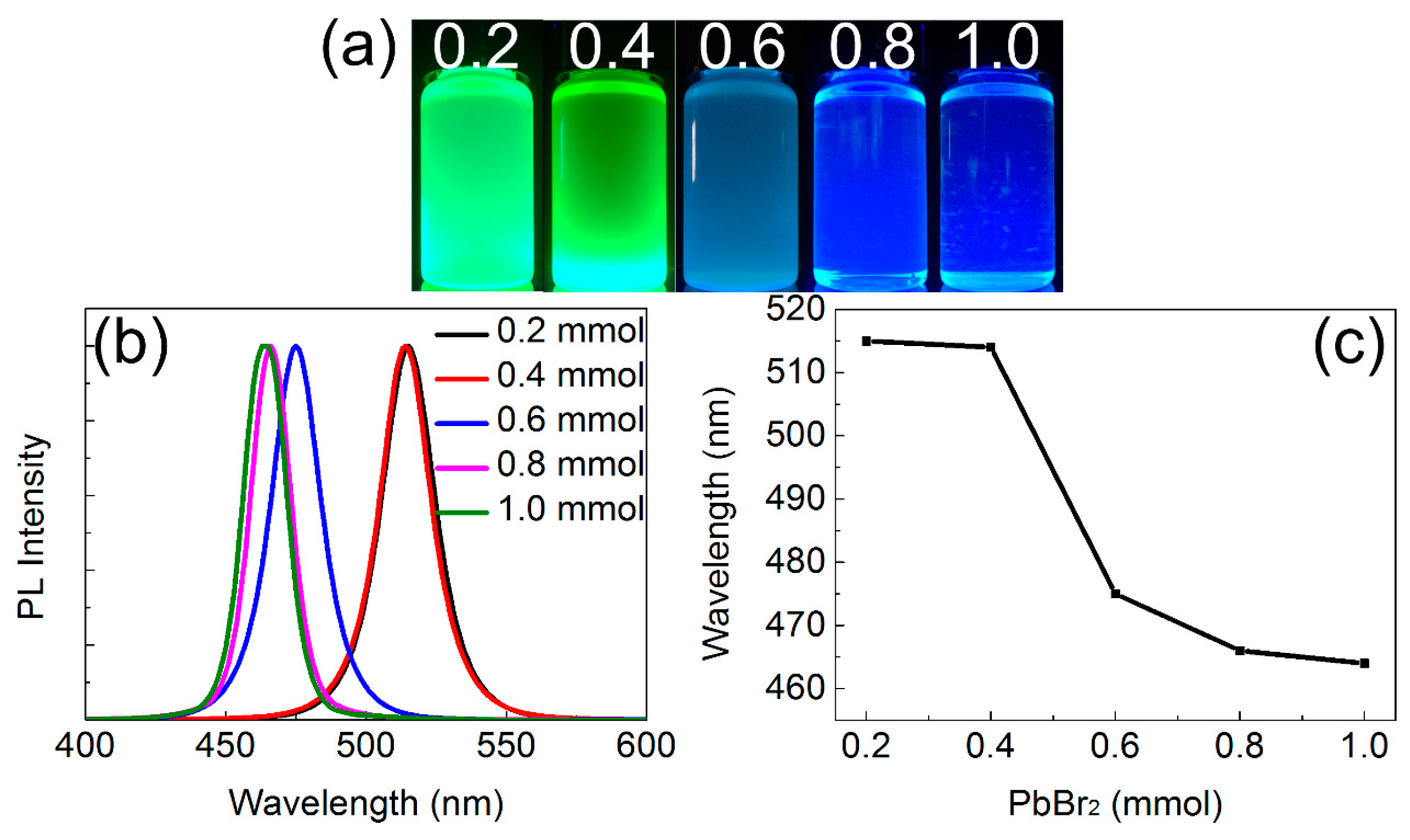
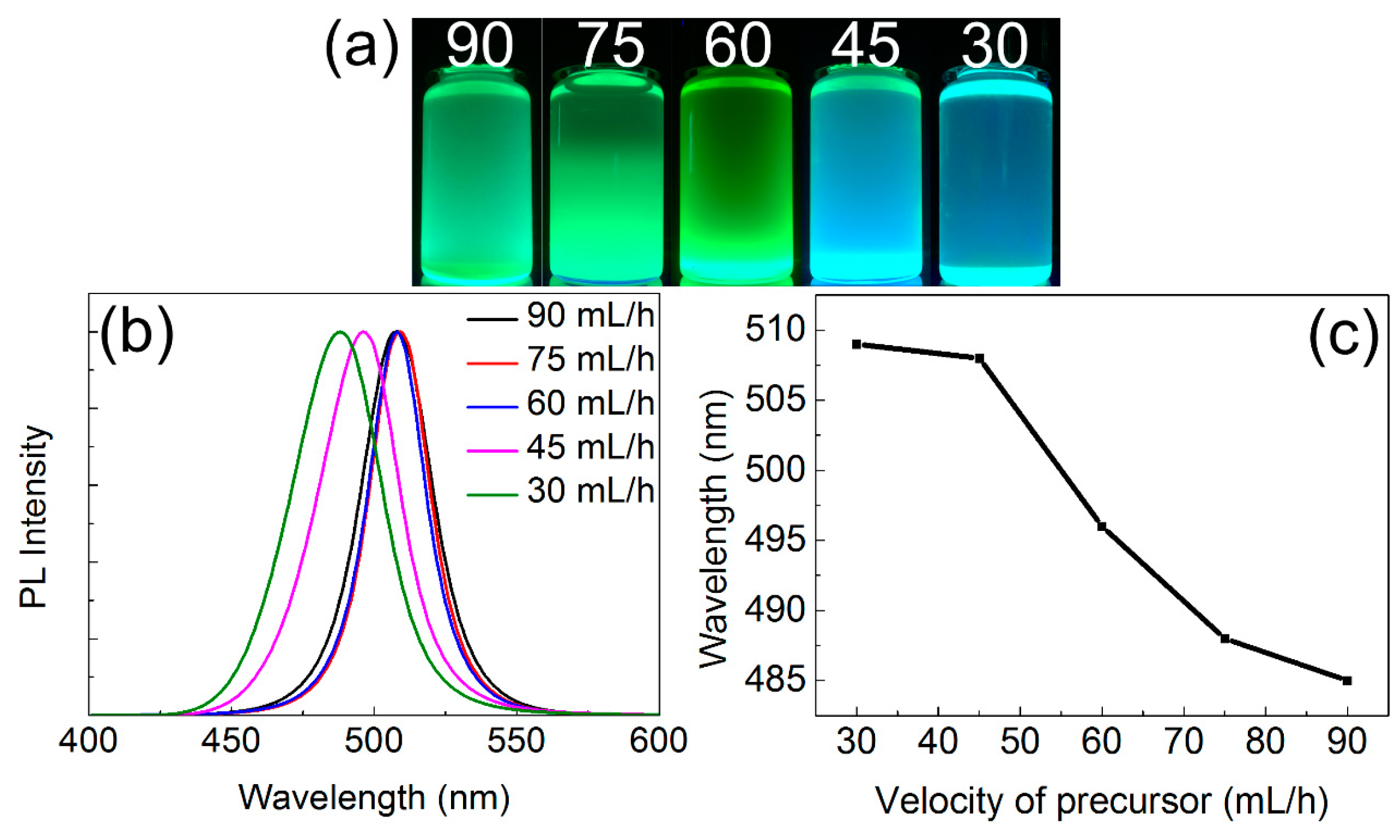
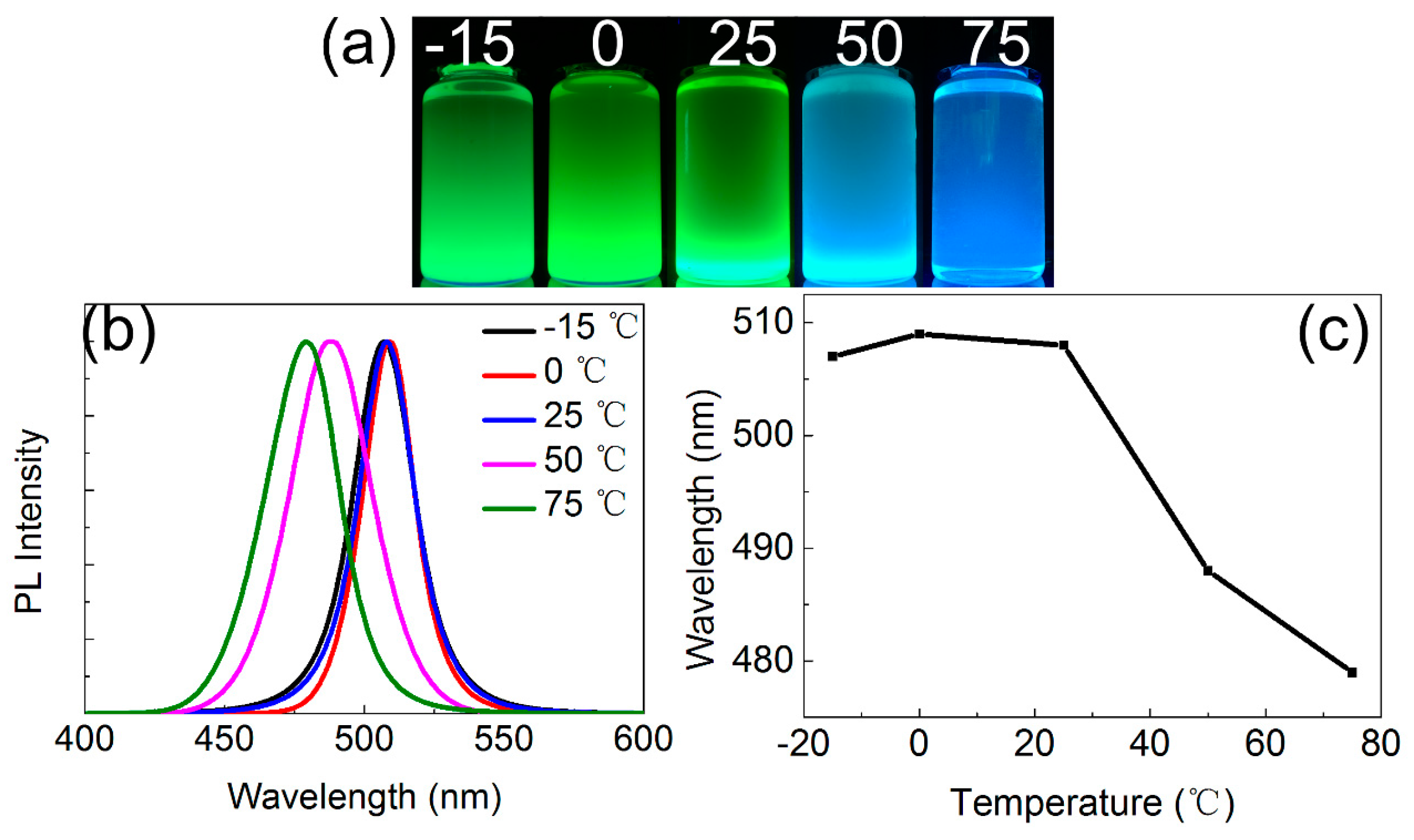
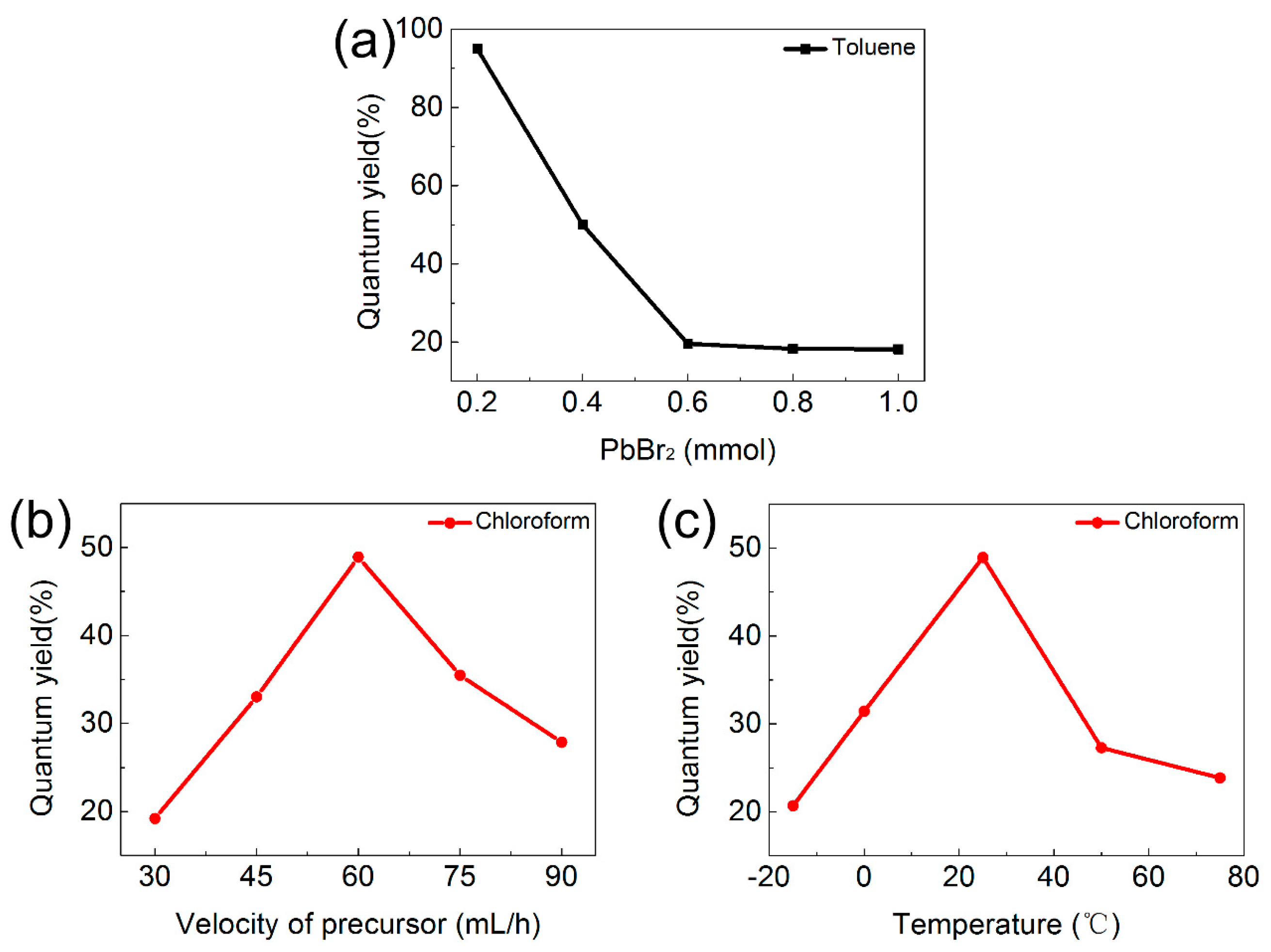
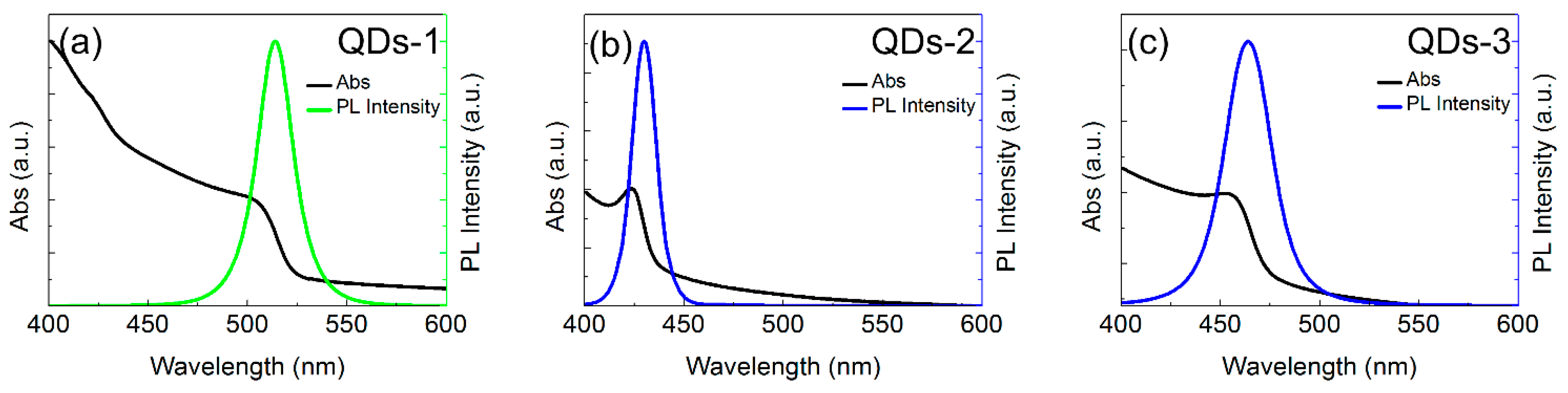
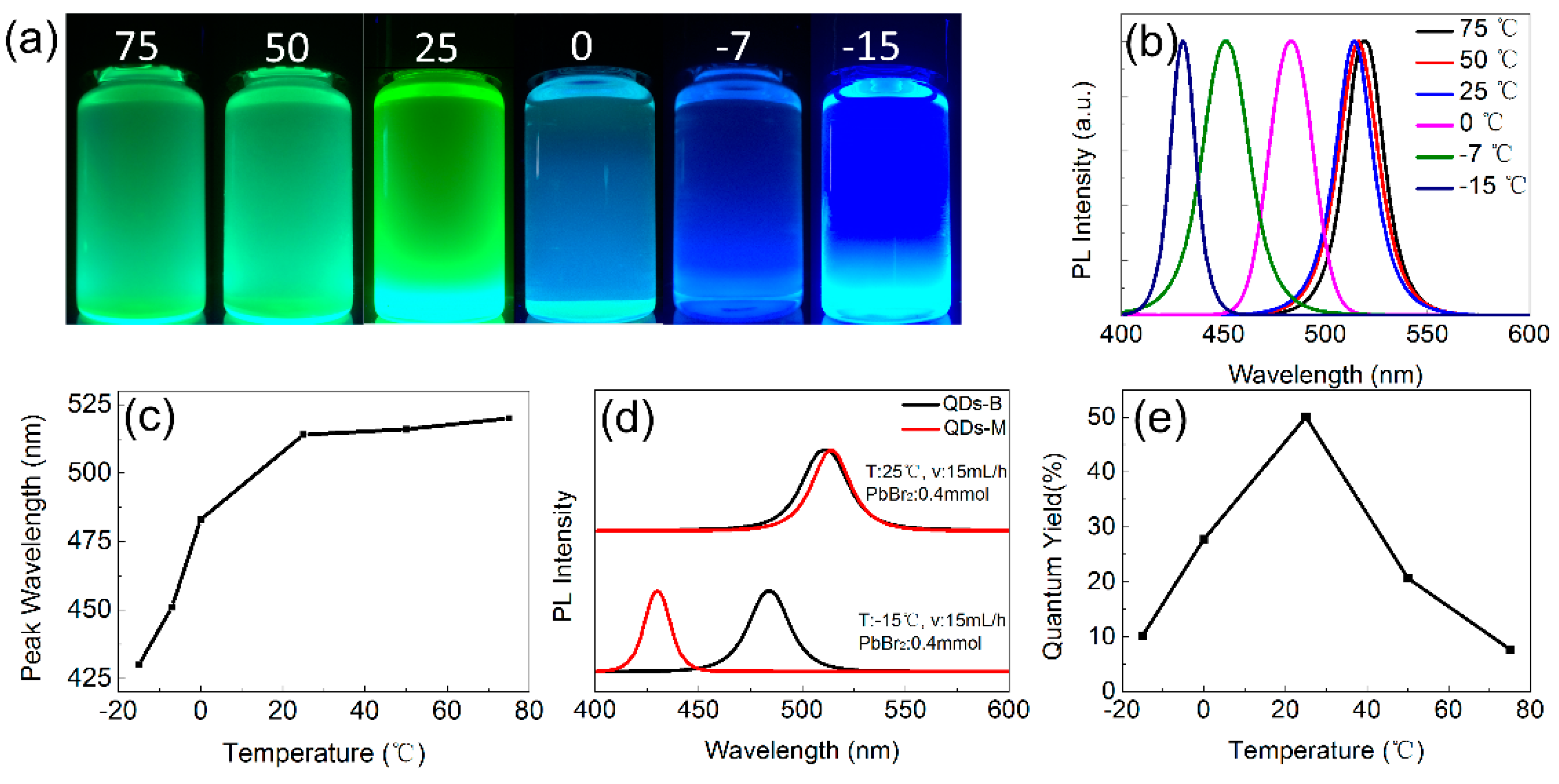
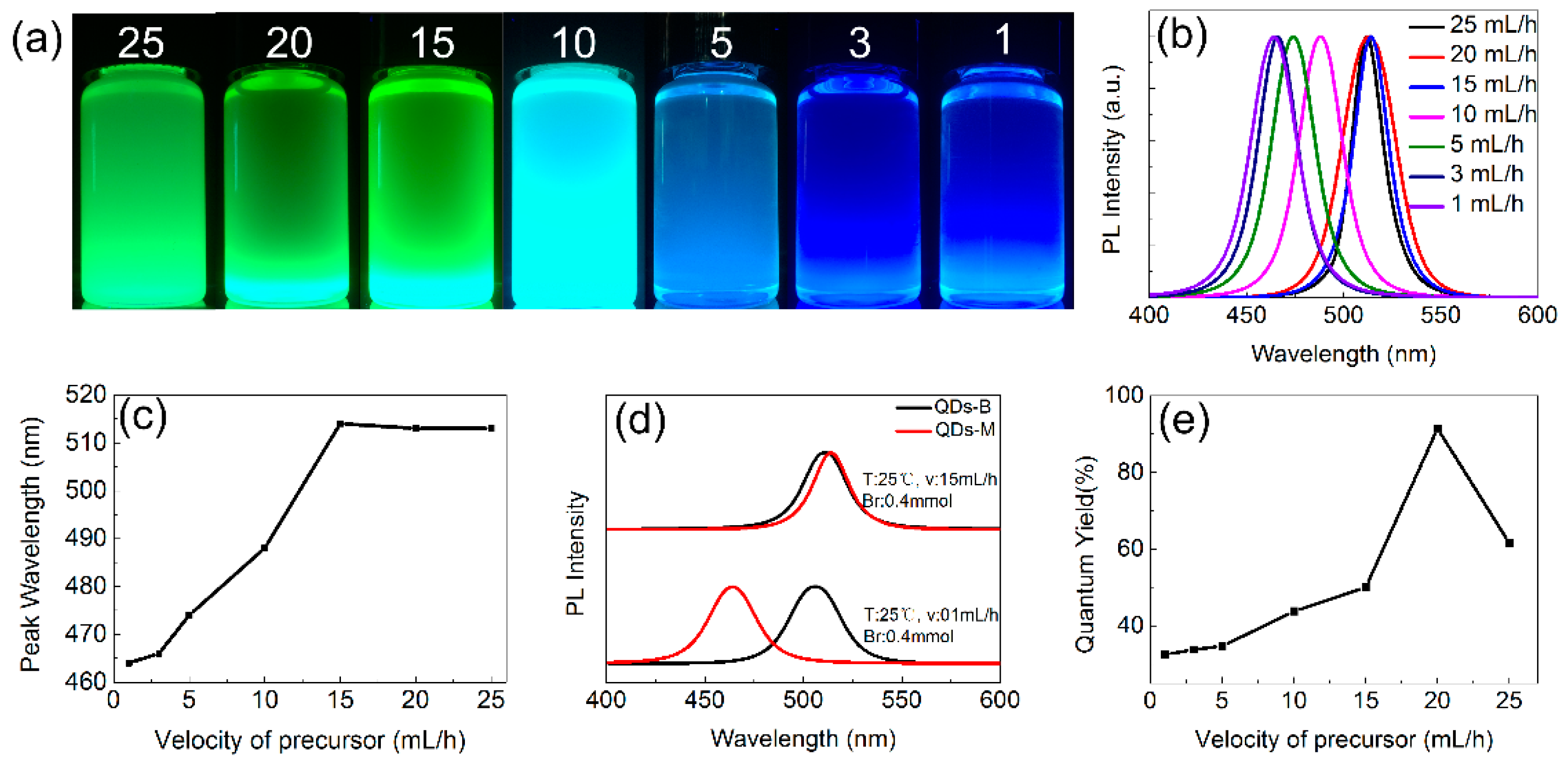
3.2. Optical Properties of CsPbBr3 QDs
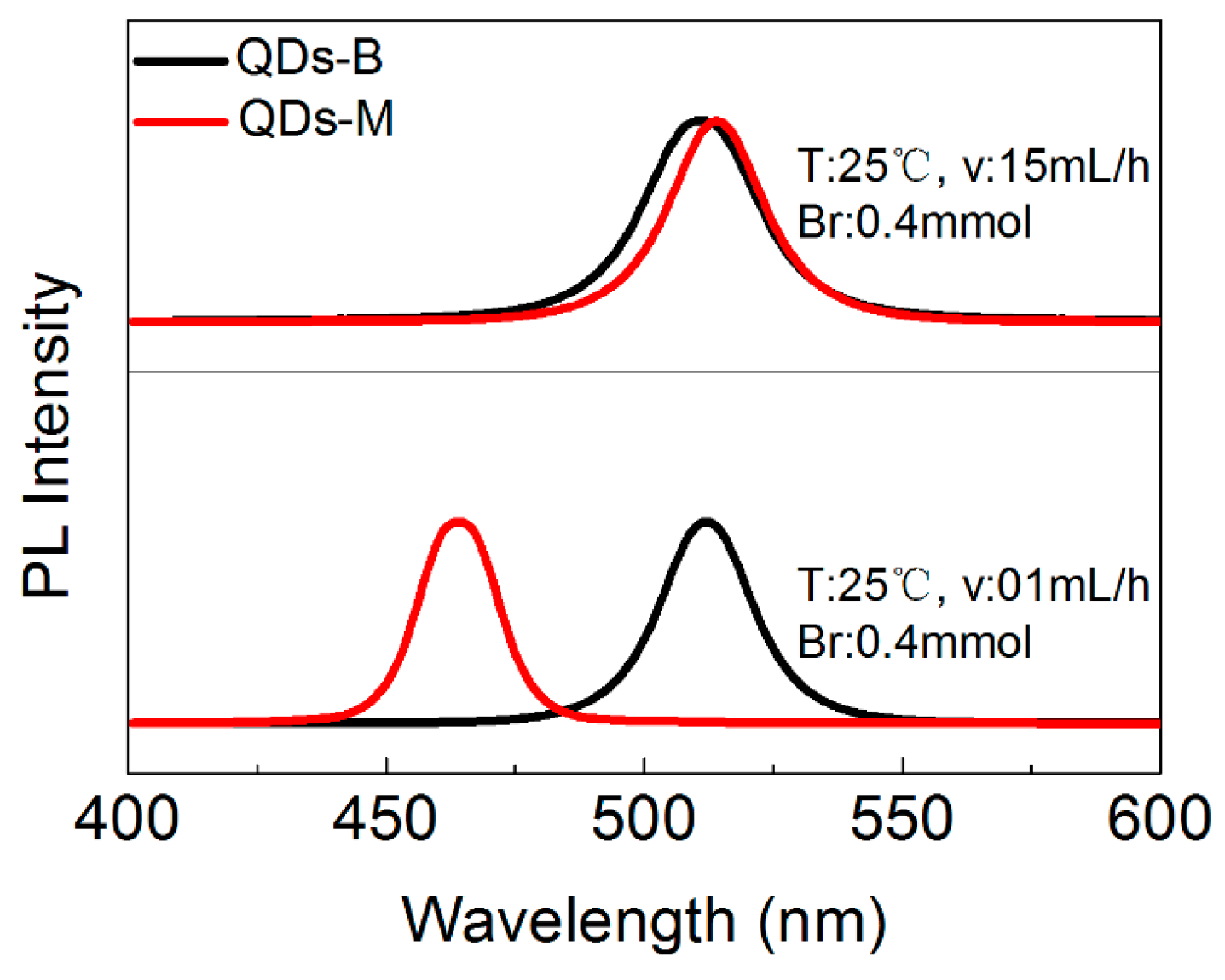
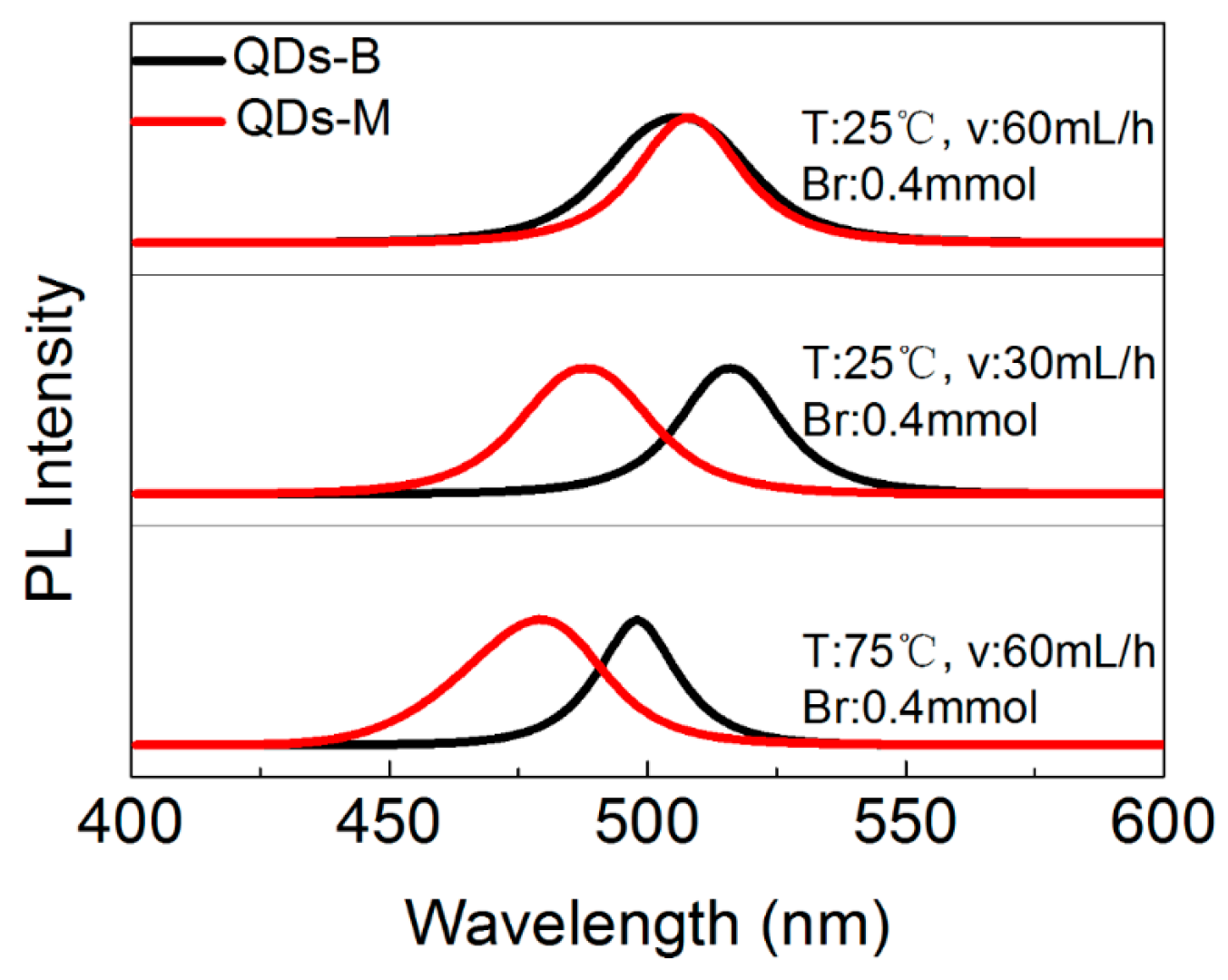
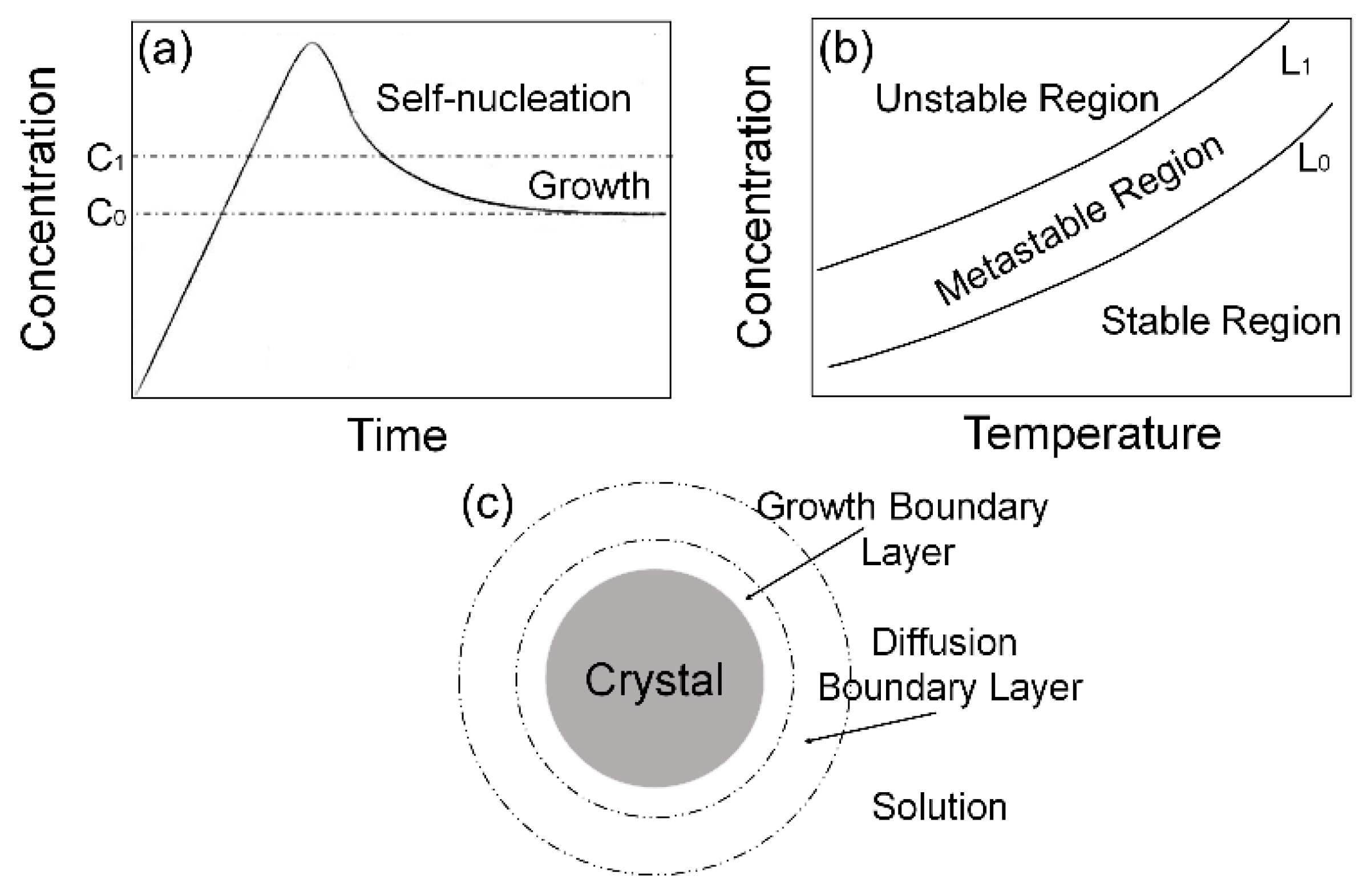
3.3. The PL Spectral Shift Mechanism of CsPbBr3 QDs’ Changeable Wavelength
3.4. The Application of CsPbBr3 QDs in LEDs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Group | Nonpolar Solvent | PbBr2 (mmol) | Precursor Flow Velocity (mL/h) | Temperature (°C) | Wavelength (nm) |
|---|---|---|---|---|---|
| QDs-1 | Toluene | 0.4 | 15 | 25 | 515 |
| QDs-2 | Toluene | 0.4 | 15 | −15 | 430 |
| QDs-3 | Toluene | 0.4 | 1 | 25 | 464 |
| QDs-4 | Chloroform | 0.4 | 60 | 25 | 508 |
| QDs-5 | Chloroform | 0.4 | 60 | 75 | 479 |
References
- Dou, L.; Yang, Y.M.; You, J.; Hong, Z.; Chang, W.H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhu, H.; Stoumpos, C.C.; Ding, Q.; Wang, J.; Kanatzidis, M.G.; Zhu, X.; Jin, S. Broad Wavelength Tunable Robust Lasing from Single-Crystal Nanowires of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). ACS Nano 2016, 10, 7963–7972. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.M.; Tai, T.Y.; Chen, Y.Y.; Lin, P.Y.; Fu, Y.S. Fabrication of CH(3)NH(3)PbI(3)/PVP Composite Fibers via Electrospinning and Deposition. Materials 2015, 8, 5467–5478. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Daoud, W.A. Crystal Structure Formation of CH(3)NH(3)PbI3-xClx Perovskite. Materials 2016, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, J.; Zhang, C.; Chang, J.; Lin, Z.; Chen, D.; Xi, H.; Hao, Y. Effects of Annealing Conditions on Mixed Lead Halide Perovskite Solar Cells and Their Thermal Stability Investigation. Materials 2017, 10, 837. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 Quantum Dots for Lighting and Displays: Room-Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- He, X.; Qiu, Y.; Yang, S. Fully-Inorganic Trihalide Perovskite Nanocrystals: A New Research Frontier of Optoelectronic Materials. Adv. Mater. 2017, 29, 29. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wu, G.; Cheng, J.; Dang, H.; Ma, K.; Zhang, Y.-W.; Tan, P.-F.; Chen, S. High-quality CsPbBr3 perovskite nanocrystals for quantum dot light-emitting diodes. RSC Adv. 2017, 7, 10391–10396. [Google Scholar] [CrossRef]
- Ramasamy, P.; Lim, D.H.; Kim, B.; Lee, S.H.; Lee, M.S.; Lee, J.S. All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chem. Commun. 2016, 52, 2067–2070. [Google Scholar] [CrossRef] [PubMed]
- Amgar, D.; Stern, A.; Rotem, D.; Porath, D.; Etgar, L. Tunable Length and Optical Properties of CsPbX3 (X = Cl, Br, I) Nanowires with a Few Unit Cells. Nano Lett. 2017, 17, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Lignos, I.; Stavrakis, S.; Nedelcu, G.; Protesescu, L.; de Mello, A.J.; Kovalenko, M.V. Synthesis of Cesium Lead Halide Perovskite Nanocrystals in a Droplet-Based Microfluidic Platform: Fast Parametric Space Mapping. Nano Lett. 2016, 16, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Marre, S.; Jensen, K.F. Synthesis of micro and nanostructures in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1183–1202. [Google Scholar] [CrossRef] [PubMed]
- Epps, R.W.; Felton, K.C.; Coley, C.W.; Abolhasani, M. Automated microfluidic platform for systematic studies of colloidal perovskite nanocrystals: Towards continuous nano-manufacturing. Lab Chip 2017, 17, 4040–4047. [Google Scholar] [CrossRef] [PubMed]
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Hogan, J. Lab on a chip: A little goes a long way. Nature 2006, 442, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Jahnisch, K.; Hessel, V.; Lowe, H.; Baerns, M. Chemistry in microstructured reactors. Angew. Chem. 2004, 43, 406–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Yang, R.J. Electroosmotic flow mixing in zigzag microchannels. Electrophoresis 2007, 28, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.K.; Shih, T.R. A rhombic micromixer with asymmetrical flow for enhancing mixing. J. Micromech. Microeng. 2007, 17, 2495–2504. [Google Scholar] [CrossRef]
- Gobby, D.; Angeli, P.; Gavriilidis, A. Mixing characteristics of T-type microfluidic mixers. J. Micromech. Microeng. 2001, 11, 126–132. [Google Scholar] [CrossRef]
- Sudarsan, A.P.; Ugaz, V.M. Fluid mixing in planar spiral microchannels. Lab Chip 2006, 6, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Kim, K.-Y.; Anwar, K.; Kim, S.M. A novel passive micromixer based on unbalanced splits and collisions of fluid streams. J. Micromech. Microeng. 2010, 20, 055007. [Google Scholar] [CrossRef]
- Bol, A.A.; Meijerink, A. Luminescence Quantum Efficiency of Nanocrystalline ZnS:Mn2+. 2. Enhancement by UV Irradiation. J. Phys. Chem. B 2001, 105, 10203–10209. [Google Scholar] [CrossRef]
- Pradhan, N.; Goorskey, D.; Thessing, J.; Peng, X. An alternative of CdSe nanocrystal emitters: Pure and tunable impurity emissions in ZnSe nanocrystals. J. Am. Chem. Soc. 2005, 127, 17586–17587. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Tang, Y.; Li, Z.; Ding, X.; Liang, G.; Lu, H.; Yan, C.; Tang, K.; Yu, B. Efficient synthesis of highly fluorescent carbon dots by microreactor method and their application in Fe3+ ion detection. Mater. Sci. Eng. C Mate. Biol. Appl. 2017, 81, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Tang, A.; Yang, C.; Teng, F. Size-controlled synthesis of highly luminescent organometal halide perovskite quantum dots. J. Alloys Compd. 2016, 687, 506–513. [Google Scholar] [CrossRef]
- Huang, H.; Susha, A.S.; Kershaw, S.V.; Hung, T.F.; Rogach, A.L. Control of Emission Color of High Quantum Yield CH3NH3PbBr3 Perovskite Quantum Dots by Precipitation Temperature. Adv. Sci. 2015, 2, 1500194. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yuan, D.; Xu, Y.; Wang, A.; Deng, Z. Ligand-Mediated Synthesis of Shape-Controlled Cesium Lead Halide Perovskite Nanocrystals via Reprecipitation Process at Room Temperature. ACS Nano 2016, 10, 3648–3657. [Google Scholar] [CrossRef] [PubMed]
- Buyevich, Y.A.; Mansurov, V.V. Kinetics of the intermediate stage of phase transition in batch crystallization. J. Cryst. Growth 1990, 104, 861–867. [Google Scholar] [CrossRef]
- LaMer, V.K.; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Lifshitz, I.M.; Slyozov, V.V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Marqusee, J.A.; Ross, J. Theory of Ostwald ripening: Competitive growth and its dependence on volume fraction. J. Chem. Phys. 1984, 80, 536–543. [Google Scholar] [CrossRef]
- Scheel, H. The Development of Crystal Growth Technology. Cheminform 2010, 36, 3–14. [Google Scholar]
- Walton, D. Nucleation of Vapor Deposits. J. Chem. Phys. 1962, 37, 2182–2188. [Google Scholar] [CrossRef]
- Zinsmeister, G. Theory of thin film condensation. Part B: Solution of the simplified condensation equation. Thin Solid Films 1968, 2, 497–507. [Google Scholar] [CrossRef]
- Kohl, M.; Puel, F.; Klein, J.P.; Hoff, C.; Monnier, O. Investigation of the growth rate of β-cyclodextrin in water during both flow-cell and batch experiments. J. Cryst. Growth 2004, 270, 633–645. [Google Scholar] [CrossRef]
- Burton, W.K.; Cabrera, N.; Frank, F.C. The Growth of Crystals and the Equilibrium Structure of their Surfaces. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1951, 243, 299–358. [Google Scholar] [CrossRef]
- Chernov, A.A. Stability of faceted shapes. J. Cryst. Growth 1974, 24–25, 11–31. [Google Scholar] [CrossRef]
- Lin, H.; Rosenberger, F.; Alexander, J.I.D.; Nadarajah, A. Convective-diffusive transport in protein crystal growth. J. Cryst. Growth 1995, 151, 153–162. [Google Scholar] [CrossRef]
- Yoo, H.-D.; Wilcox, W.R.; Lal, R.; Trolinger, J.D. Modelling the growth of triglycine sulphate crystals in spacelab 3. J. Cryst. Growth 1988, 92, 101–117. [Google Scholar] [CrossRef]
- Barlow, D.A.; Baird, J.K.; Su, C.-H. Theory of the von Weimarn rules governing the average size of crystals precipitated from a supersaturated solution. J. Cryst. Growth 2004, 264, 417–423. [Google Scholar] [CrossRef]
| Group | Weak Polar Solvent | PbBr2 (mmol) | Precursor Flow Velocity (mL/h) | Temperature (°C) | Wavelength (nm) |
|---|---|---|---|---|---|
| QDs-1 | Toluene | 0.4 | 15 | 25 | 515 |
| QDs-2 | Toluene | 0.4 | 15 | −15 | 430 |
| QDs-3 | Toluene | 0.4 | 1 | 25 | 464 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Lu, H.; Rao, L.; Li, Z.; Ding, X.; Yan, C.; Yu, B. Regulating the Emission Spectrum of CsPbBr3 from Green to Blue via Controlling the Temperature and Velocity of Microchannel Reactor. Materials 2018, 11, 371. https://doi.org/10.3390/ma11030371
Tang Y, Lu H, Rao L, Li Z, Ding X, Yan C, Yu B. Regulating the Emission Spectrum of CsPbBr3 from Green to Blue via Controlling the Temperature and Velocity of Microchannel Reactor. Materials. 2018; 11(3):371. https://doi.org/10.3390/ma11030371
Chicago/Turabian StyleTang, Yong, Hanguang Lu, Longshi Rao, Zongtao Li, Xinrui Ding, Caiman Yan, and Binhai Yu. 2018. "Regulating the Emission Spectrum of CsPbBr3 from Green to Blue via Controlling the Temperature and Velocity of Microchannel Reactor" Materials 11, no. 3: 371. https://doi.org/10.3390/ma11030371
APA StyleTang, Y., Lu, H., Rao, L., Li, Z., Ding, X., Yan, C., & Yu, B. (2018). Regulating the Emission Spectrum of CsPbBr3 from Green to Blue via Controlling the Temperature and Velocity of Microchannel Reactor. Materials, 11(3), 371. https://doi.org/10.3390/ma11030371





