Modification of Physico-Chemical Properties of Acryl-Coated Polypropylene Foils for Food Packaging by Reactive Particles from Oxygen Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
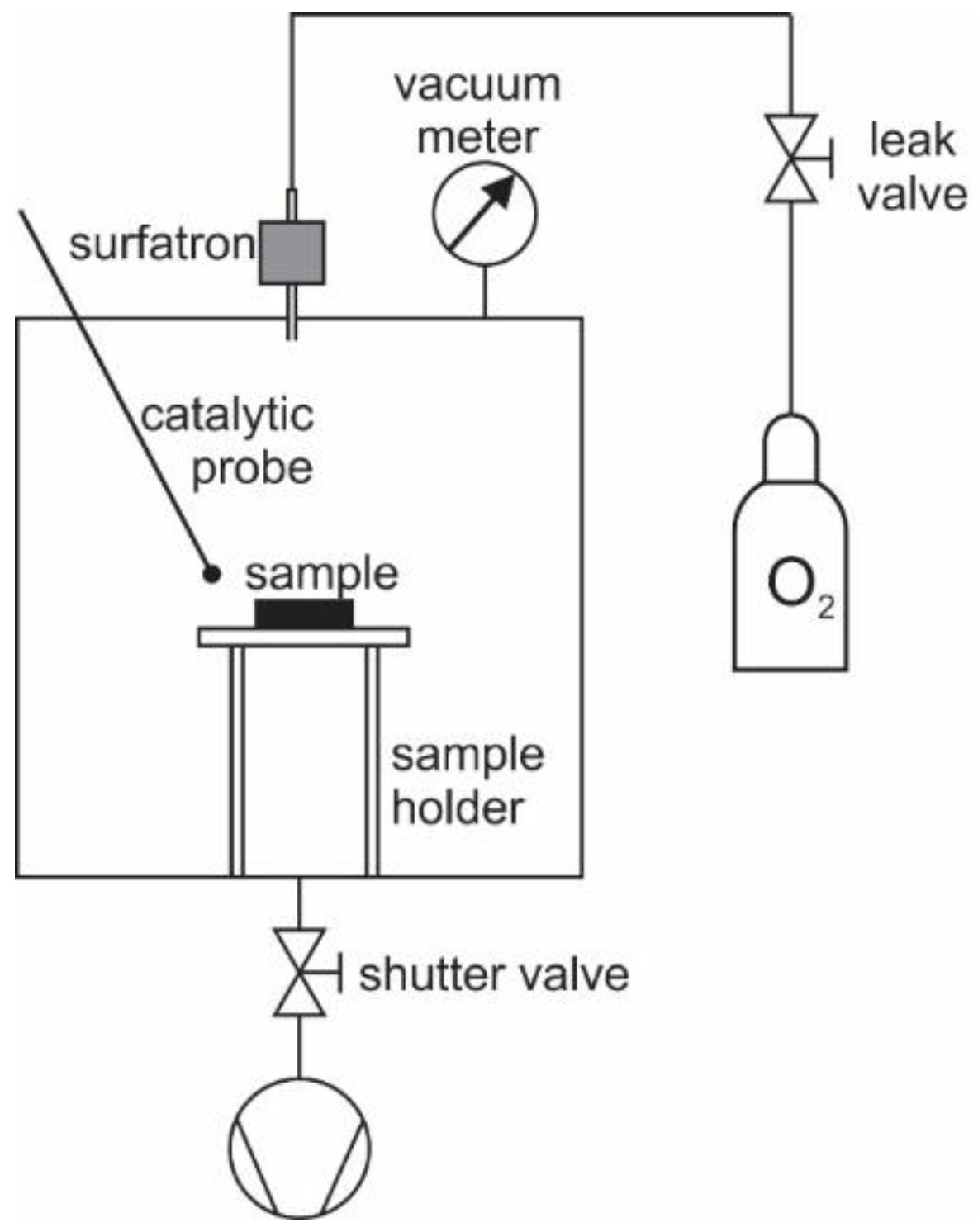
2.2. Plasma Afterglow Treatment
2.3. X-ray Photoelectron Spectroscopy (XPS) Characterization
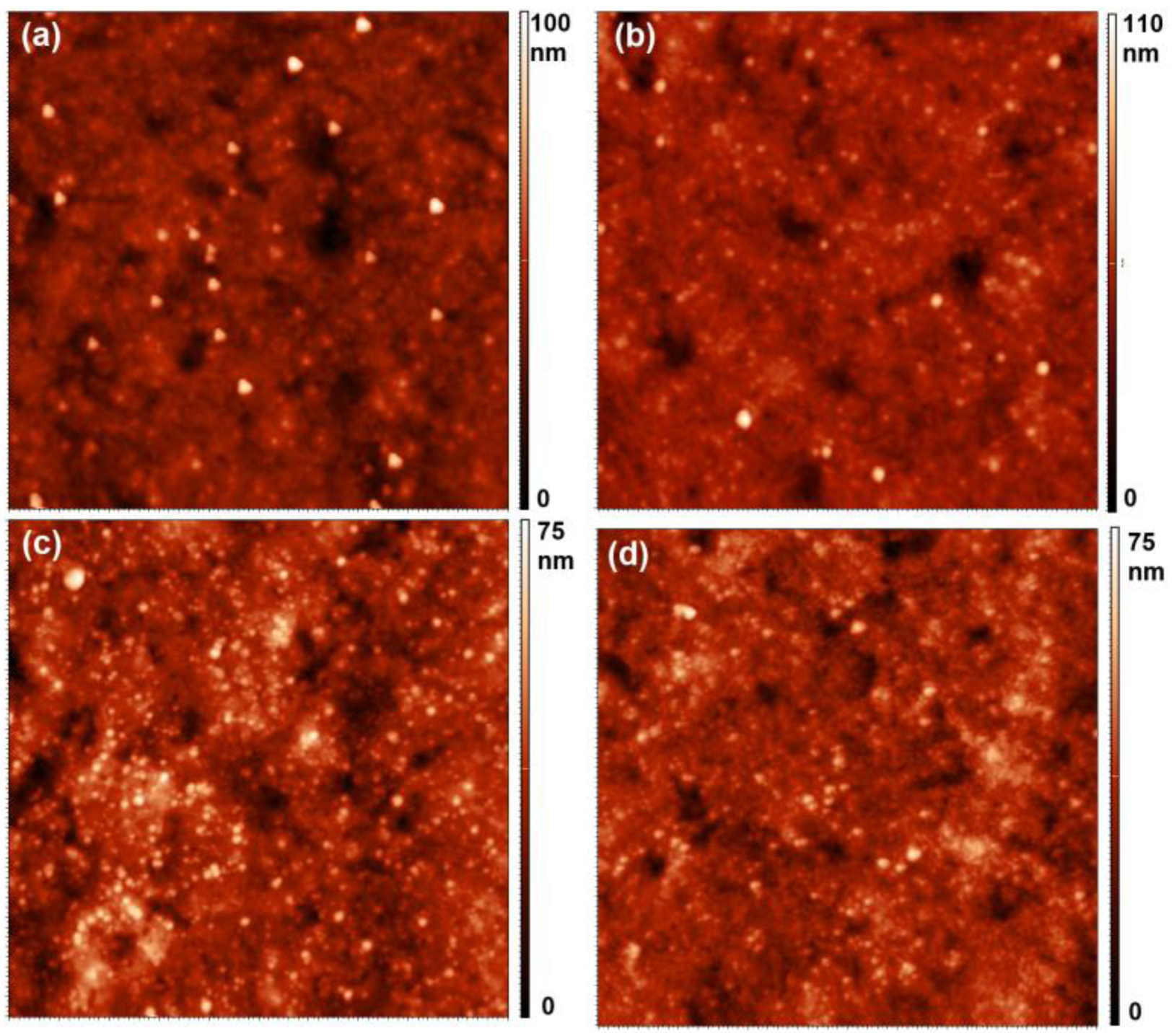
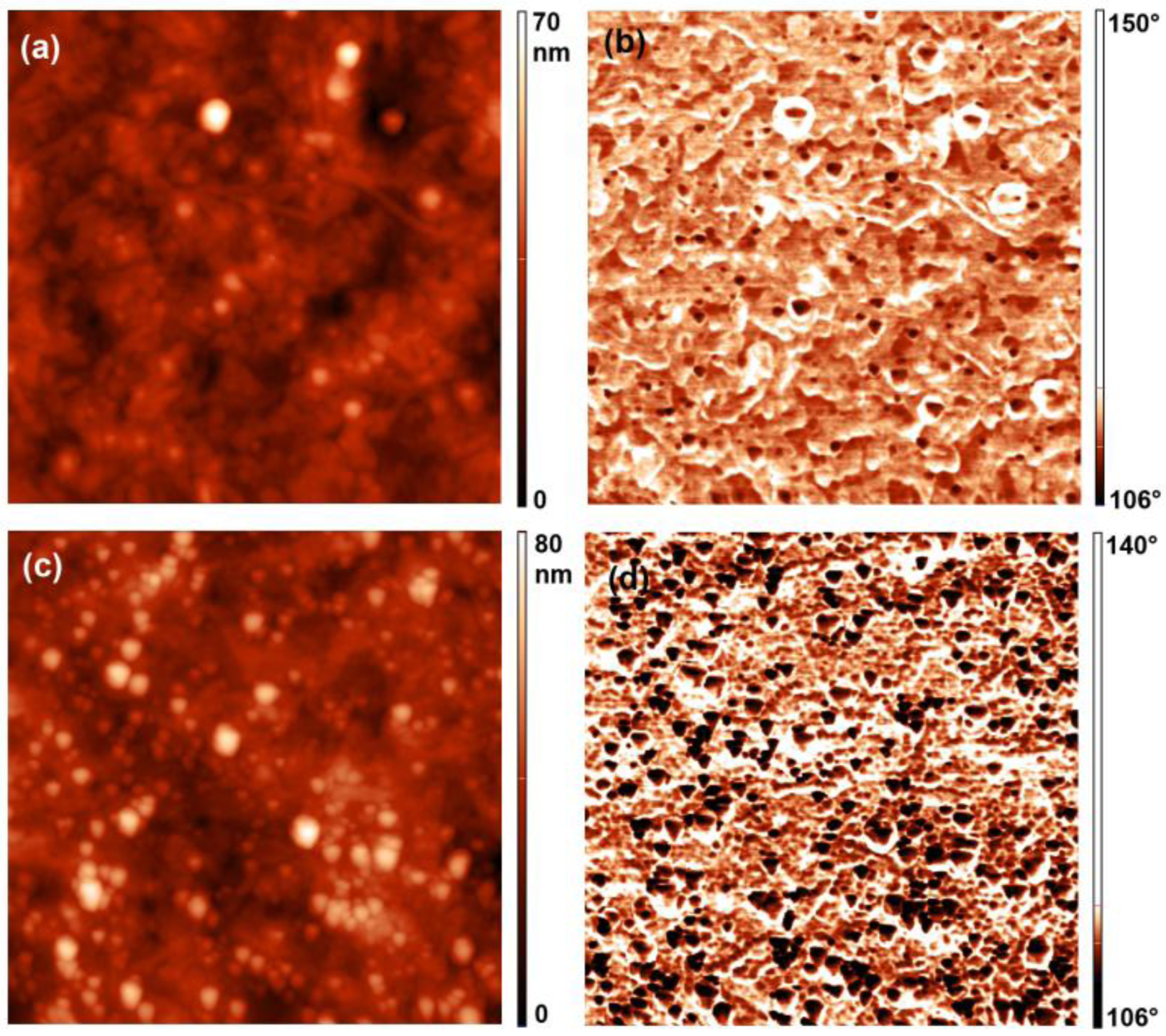
2.4. Atomic Force Microscopy (AFM) Measurements
2.5. Contact Angle Measurements
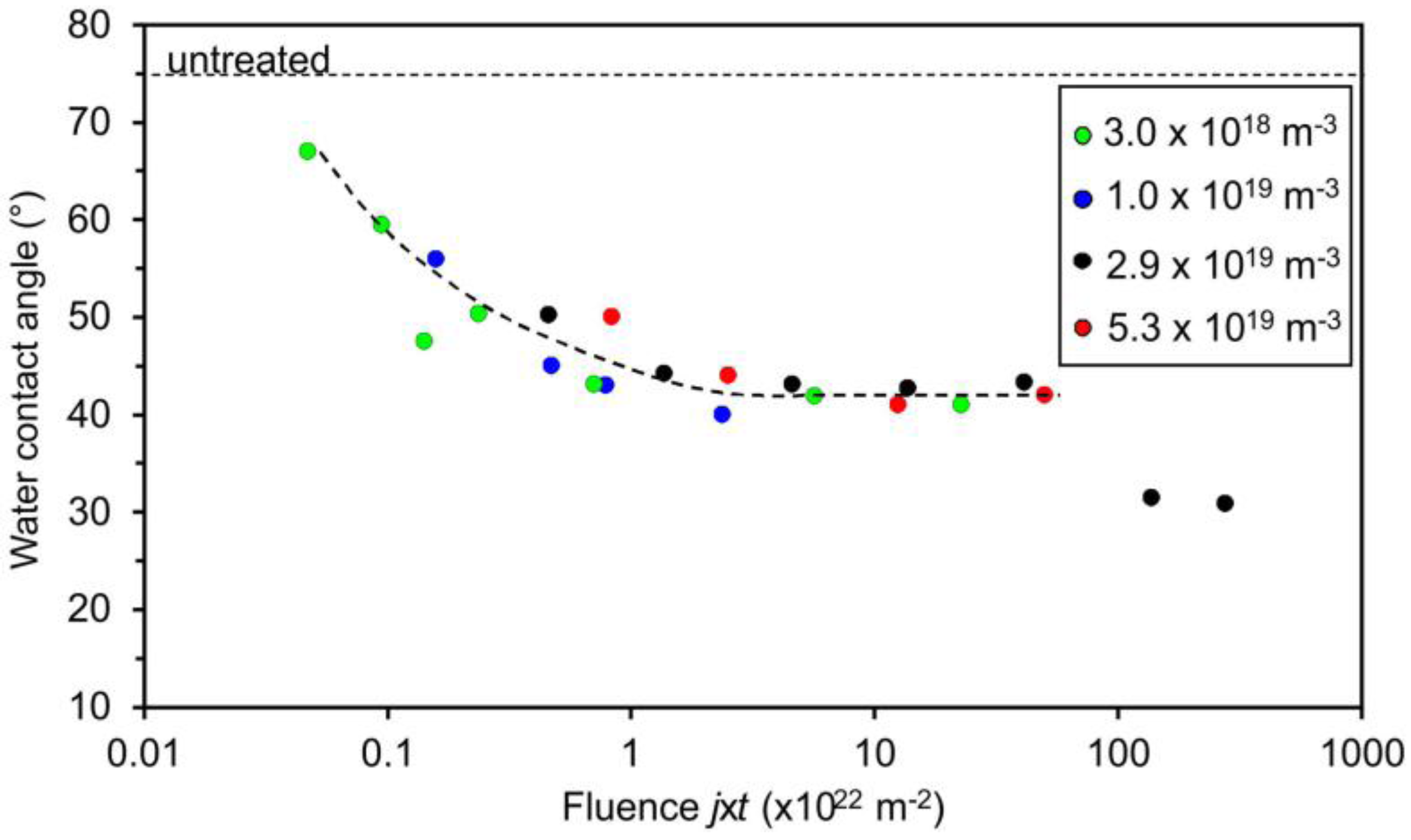
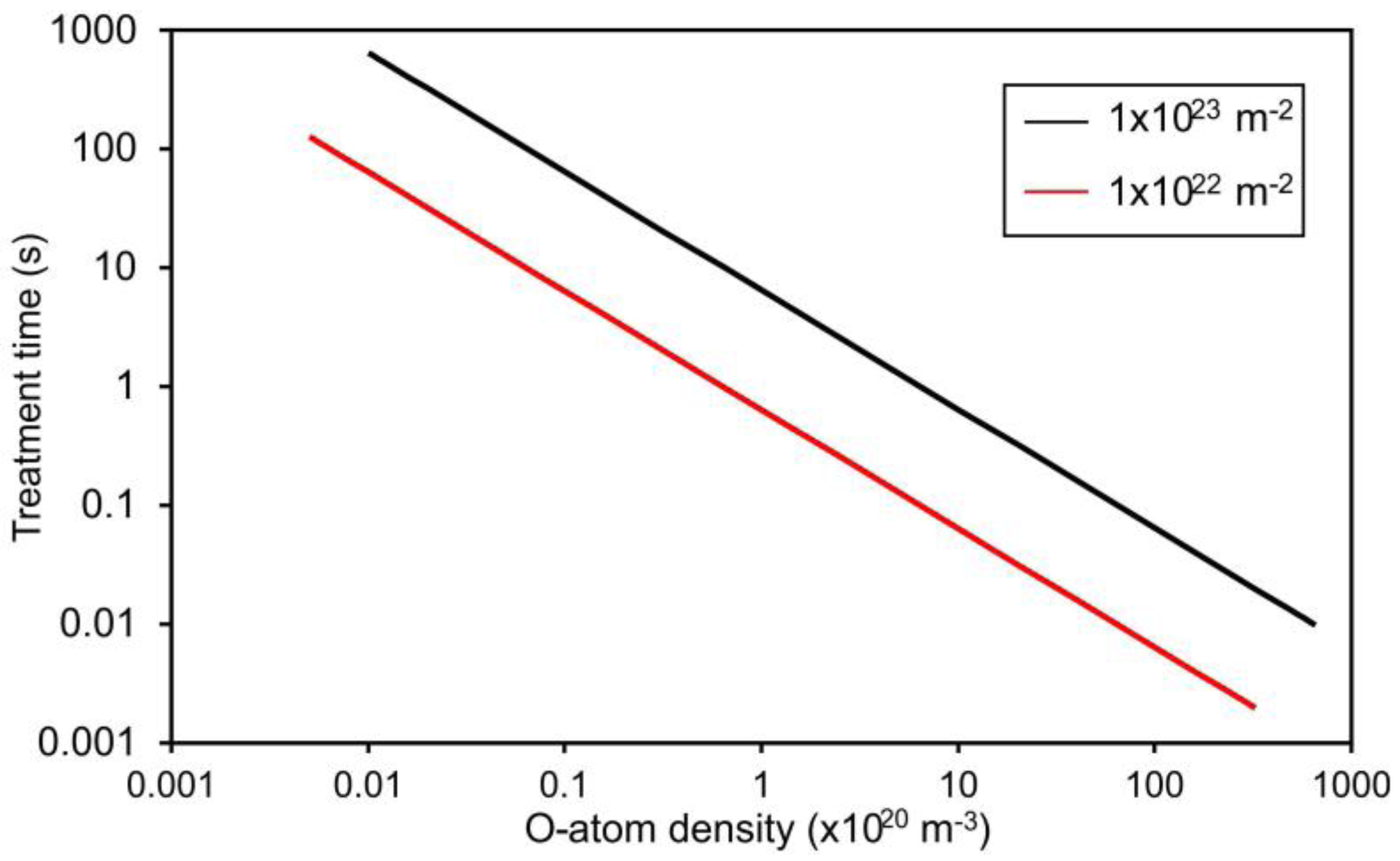
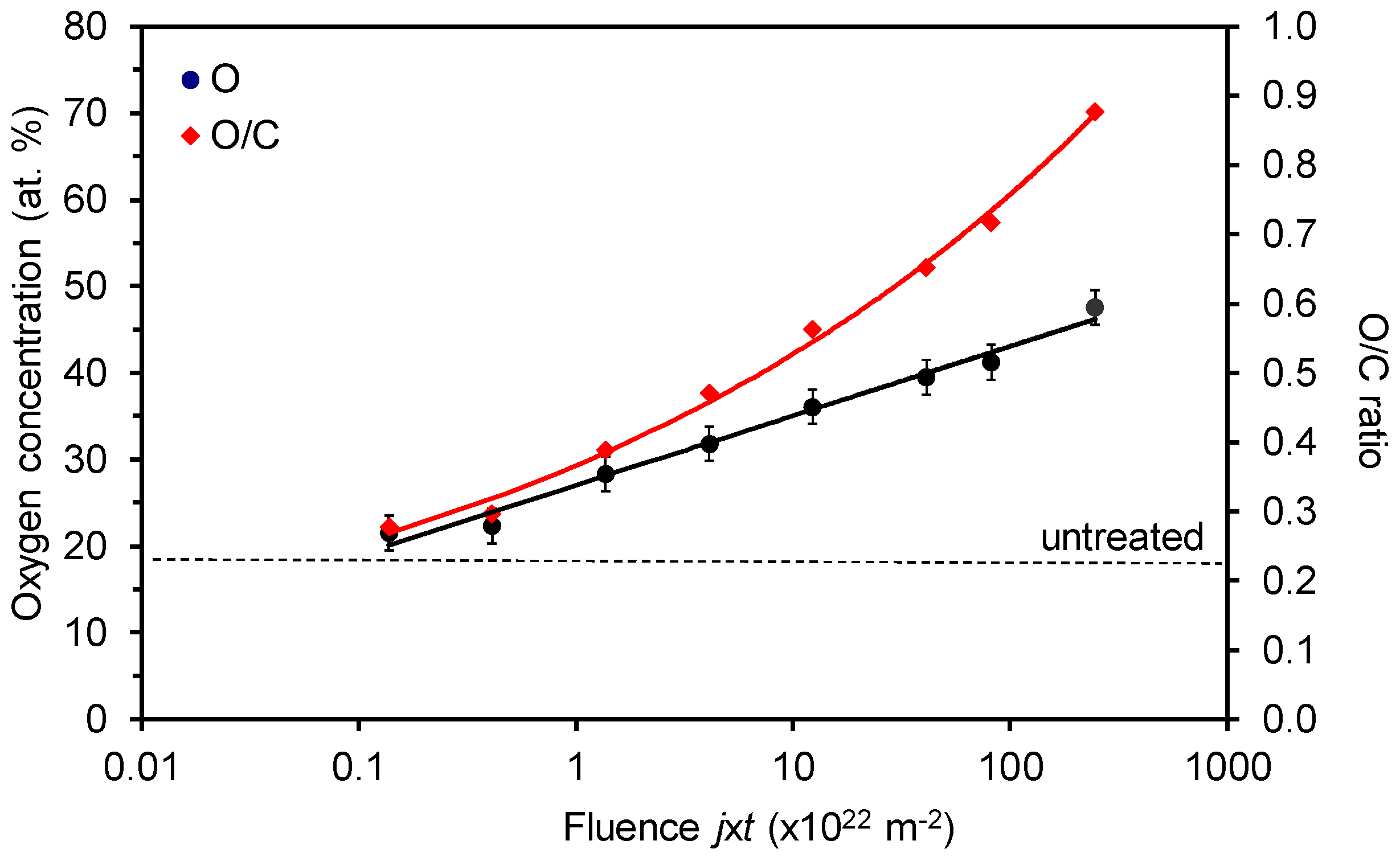
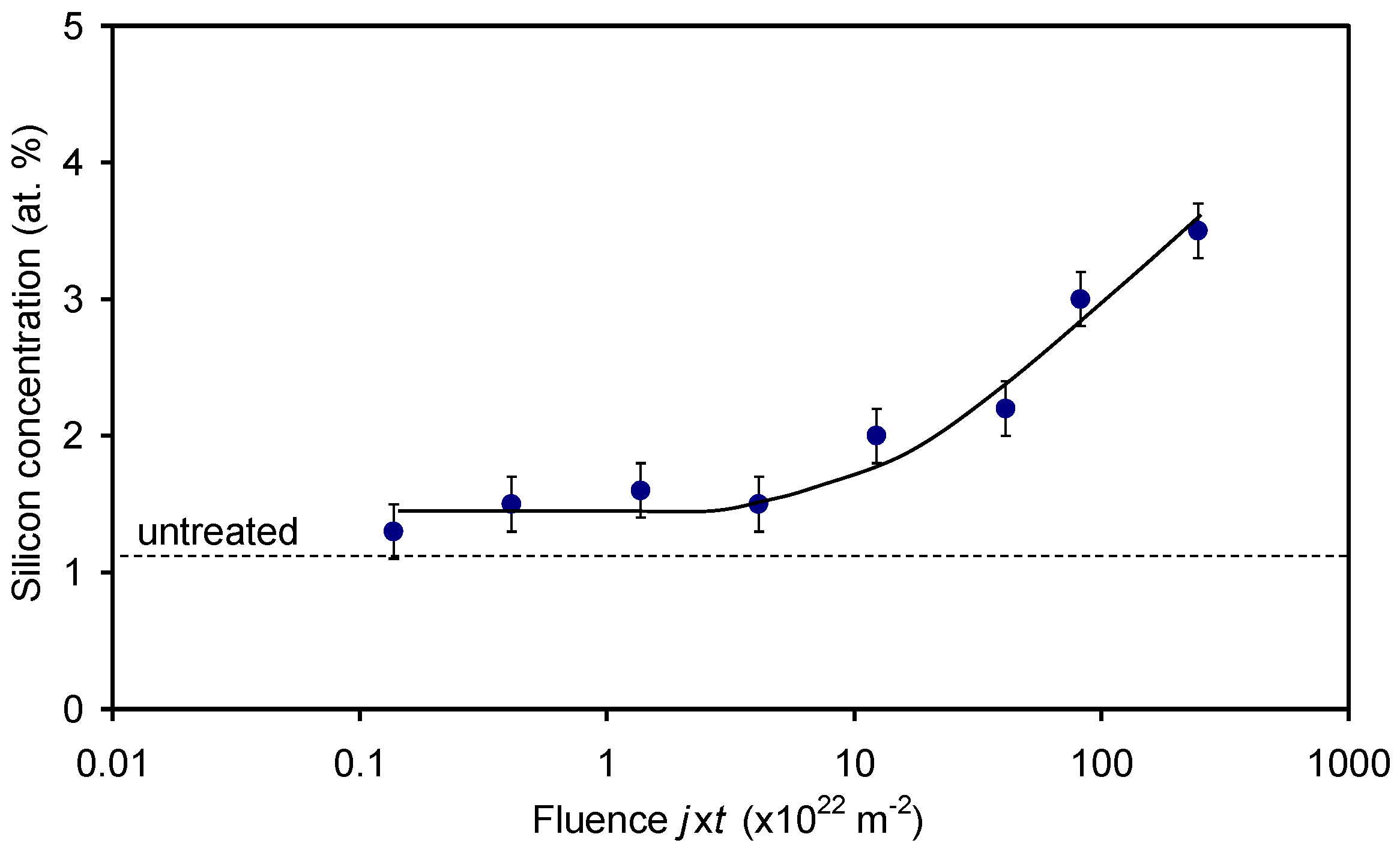
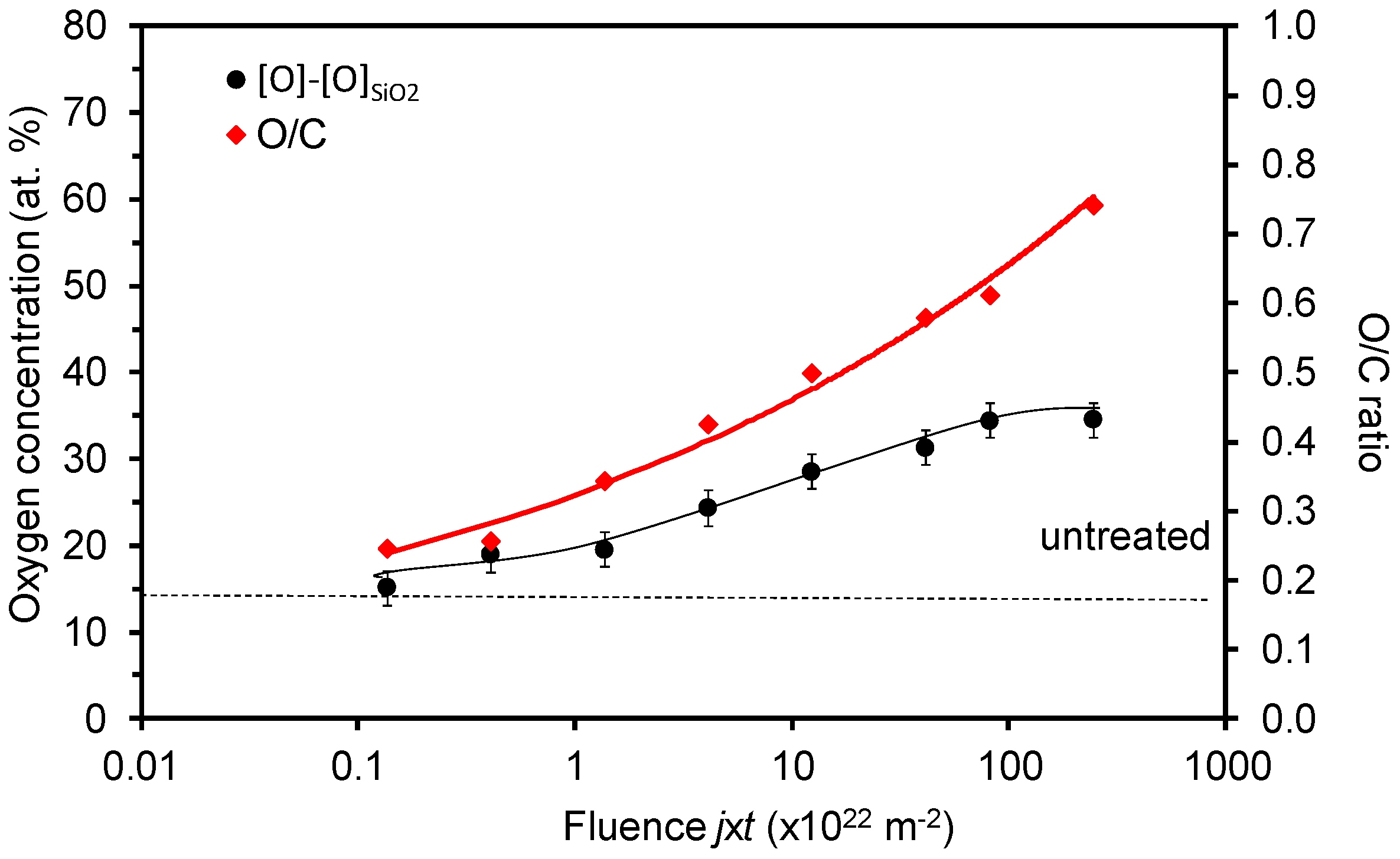
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljević, V.; O’Donnell, C.P.; Bourke, P.; Keener, K.M.; Cullen, P.J. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Gorjanc, M.; Savic, A.; Topalic-Trivunovic, L.; Mozetic, M.; Zaplotnik, R.; Vesel, A.; Grujic, D. Dyeing of plasma treated cotton and bamboo rayon with fallopia japonica extract. Cellulose 2016, 23, 2221–2228. [Google Scholar] [CrossRef]
- Gorjanc, M.; Jazbec, K.; Sala, M.; Zaplotnik, R.; Vesel, A.; Mozetic, M. Creating cellulose fibres with excellent uv protective properties using moist cf4 plasma and zno nanoparticles. Cellulose 2014, 21, 3007–3021. [Google Scholar] [CrossRef]
- Lopez-Garcia, J.; Primc, G.; Junkar, I.; Lehocky, M.; Mozetic, M. On the hydrophilicity and water resistance effect of styrene-acrylonitrile copolymer treated by cf4 and o2 plasmas. Plasma Process. Polym. 2015, 12, 1075–1084. [Google Scholar] [CrossRef]
- Lopez-Garcia, J.; Lehocky, M.; Humpolicek, P.; Novak, I. On the correlation of surface charge and energy in non-thermal plasma-treated polyethylene. Surf. Interface Anal. 2014, 46, 625–629. [Google Scholar] [CrossRef]
- Lehocky, M.; St’ahel, P.; Koutny, M.; Cech, J.; Institoris, J.; Mracek, A. Adhesion of rhodococcus sp. S3E2 and rhodococcus sp. S3E3 to plasma prepared teflon-like and organosilicon surfaces. J. Mater. Process. Technol. 2009, 209, 2871–2875. [Google Scholar] [CrossRef]
- Poncin-Epaillard, F.; Brosse, J.C.; Falher, T. Reactivity of surface groups formed onto a plasma treated poly(propylene) film. Macromol. Chem. Phys. 1999, 200, 989–996. [Google Scholar] [CrossRef]
- Izdebska, J.; Sabu, T. Printing on Polymers: Fundamentals and Applications; Elsevier Science Publishing Co Inc: Waltham, MA, USA, 2016. [Google Scholar]
- Gorjanc, M.; Mozetic, M. Modification of Fibrous Polymers by Gaseous Plasma: Principles, Techniques and Applications; Lambert Academic Publishing: Saarbrücken, Germany, 2014. [Google Scholar]
- Primc, G.; Tomsic, B.; Vesel, A.; Mozetic, M.; Razic, S.E.; Gorjanc, M. Biodegradability of oxygen-plasma treated cellulose textile functionalized with zno nanoparticles as antibacterial treatment. J. Phys. D Appl. Phys. 2016, 49, 324002. [Google Scholar] [CrossRef]
- Gorjanc, M.; Sala, M. Durable antibacterial and uv protective properties of cellulose fabric functionalized with ag/tio2 nanocomposite during dyeing with reactive dyes. Cellulose 2016, 23, 2199–2209. [Google Scholar] [CrossRef]
- Popelka, A.; Novak, I.; Lehocky, M.; Bilek, F.; Kleinova, A.; Mozetic, M.; Spirkova, M.; Chodak, I. Antibacterial treatment of ldpe with halogen derivatives via cold plasma. Express Polym. Lett. 2015, 9, 402–411. [Google Scholar] [CrossRef]
- Asadinezhad, A.; Novak, I.; Lehocky, M.; Sedlarik, V.; Vesel, A.; Junkar, I.; Saha, P.; Chodak, I. A physicochemical approach to render antibacterial surfaces on plasma-treated medical-grade pvc: Irgasan coating. Plasma Process. Polym. 2010, 7, 504–514. [Google Scholar] [CrossRef]
- Sulovska, K.; Lehocky, M. Characterization of plasma treated surfaces for food safety by terahertz spectroscopy. Proc. SPIE 2014, 9252, 925209. [Google Scholar]
- Lin, Y.J.; Dias, P.; Chen, H.Y.; Chum, S.; Hiltner, A.; Baer, E. Oxygen permeability of biaxially oriented polypropylene films. Polym. Eng. Sci. 2008, 48, 642–648. [Google Scholar] [CrossRef]
- Decker, C.; Biry, S. Light stabilisation of polymers by radiation-cured acrylic coatings. Prog. Org. Coat. 1996, 29, 81–87. [Google Scholar] [CrossRef]
- Vujković, I.; Galić, K.; Vereš, M. Ambalaža za Pakiranje Namirnica; Tectus: Zagreb, Croatia, 2007. [Google Scholar]
- Tkavc, T.; Petrinič, I.; Luxbacher, T.; Vesel, A.; Ristić, T.; Zemljič, L.F. Influence of O2 and CO2 plasma treatment on the deposition of chitosan onto polyethylene terephthalate (pet) surfaces. Int. J. Adhes. Adhes. 2014, 48, 168–176. [Google Scholar] [CrossRef]
- Pandiyaraj Navaneetha, K.; Selvarajan, V.; Deshmukh, R.R.; Gao, C. Adhesive properties of polypropylene (pp) and polyethylene terephthalate (pet) film surfaces treated by dc glow discharge plasma. Vacuum 2008, 83, 332–339. [Google Scholar] [CrossRef]
- Choi, H.S.; Rybkin, V.V.; Titov, V.A.; Shikova, T.G.; Ageeva, T.A. Comparative actions of a low pressure oxygen plasma and an atmospheric pressure glow discharge on the surface modification of polypropylene. Surf. Coat. Technol. 2006, 200, 4479–4488. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengernbre, L.; Payen, E. Comparison between xps- and ftir-analysis of plasma-treated polypropylene film surfaces. Surf. Interface Anal. 2008, 40, 597–600. [Google Scholar] [CrossRef]
- Leroux, F.; Campagne, C.; Perwuelz, A.; Gengembre, L. Polypropylene film chemical and physical modifications by dielectric barrier discharge plasma treatment at atmospheric pressure. J. Colloid Interface Sci. 2008, 328, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.H.L.; Oliveira, E.C.; Cruz, S.A. Modification of clarified polypropylene by oxygen plasma to improve the adhesion of thin amorphous hydrogenated carbon films deposited by plasma enhanced chemical vapor deposition. Polym. Eng. Sci. 2013, 53, 1065–1072. [Google Scholar] [CrossRef]
- Vishnuvarthanan, M.; Rajeswari, N. Effect of mechanical, barrier and adhesion properties on oxygen plasma surface modified pp. Innov. Food Sci. Emerg. Technol. 2015, 30, 119–126. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Arabi, H.; Salem, A.; Asiaban, S. Effect of low-pressure o2 and ar plasma treatments on the wettability and morphology of biaxial-oriented polypropylene (bopp) film. Prog. Org. Coat. 2007, 60, 105–111. [Google Scholar] [CrossRef]
- Wanke, C.H.; Feijo, J.L.; Barbosa, L.G.; Campo, L.F.; de Oliveira, R.V.B.; Horowitz, F. Tuning of polypropylene wettability by plasma and polyhedral oligomeric silsesquioxane modifications. Polymer 2011, 52, 1797–1802. [Google Scholar] [CrossRef]
- Strobel, M.; Jones, V.; Lyons, C.S.; Ulsh, M.; Kushner, M.J.; Dorai, R.; Branch, M.C. A comparison of corona-treated and flame-treated polypropylene films. Plasmas Polym. 2003, 8, 61–95. [Google Scholar] [CrossRef]
- Beke, S.; Anjum, F.; Tsushima, H.; Ceseracciu, L.; Chieregatti, E.; Diaspro, A.; Athanassiou, A.; Brandi, F. Towards excimer-laser-based stereolithography: A rapid process to fabricate rigid biodegradable photopolymer scaffolds. J. R. Soc. Interface 2012, 9, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Vesel, A.; Mozetic, M. New developments in surface functionalization of polymers using controlled plasma treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Doliska, A.; Vesel, A.; Kolar, M.; Stana-Kleinschek, K.; Mozetic, M. Interaction between model poly(ethylene terephthalate) thin films and weakly ionised oxygen plasma. Surf. Interface Anal. 2012, 44, 56–61. [Google Scholar] [CrossRef]
- Vesel, A.; Kolar, M.; Doliska, A.; Stana-Kleinschek, K.; Mozetic, M. Etching of polyethylene terephthalate thin films by neutral oxygen atoms in the late flowing afterglow of oxygen plasma. Surf. Interface Anal. 2012, 44, 1565–1571. [Google Scholar] [CrossRef]
- Chashmejahanbin, M.R.; Salimi, A.; Ershad Langroudi, A. The study of the coating adhesion on pp surface modified in different plasma/acrylic acid solution. Int. J. Adhes. Adhes. 2014, 49, 44–50. [Google Scholar] [CrossRef]
- Masaeli, E.; Morshed, M.; Tavanai, H. Study of the wettability properties of polypropylene nonwoven mats by low-pressure oxygen plasma treatment. Surf. Interface Anal. 2007, 39, 770–774. [Google Scholar] [CrossRef]
- Yun, Y.I.; Kim, K.S.; Uhm, S.-J.; Khatua, B.B.; Cho, K.; Kim, J.K.; Park, C.E. Aging behavior of oxygen plasma-treated polypropylene with different crystallinities. J. Adhes. Sci. Technol. 2004, 18, 1279–1291. [Google Scholar] [CrossRef]
- Chen, R.; Bayon, Y.; Hunt, J.A. Preliminary study on the effects of ageing cold oxygen plasma treated pet/pp with respect to protein adsorption. Colloid. Surface B 2012, 96, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Zaplotnik, R.; Vesel, A.; Mozetic, M. A fiber optic catalytic sensor for neutral atom measurements in oxygen plasma. Sensors 2012, 12, 3857–3867. [Google Scholar] [CrossRef] [PubMed]
- Šorli, I.; Ročak, R. Determination of atomic oxygen density with a nickel catalytic probe. J. Vac. Sci. Technol. A 2000, 18, 338–342. [Google Scholar] [CrossRef]
- Primc, G.; Vesel, A.; Dolanc, G.; Vrančić, D.; Mozetič, M. Recombination of oxygen atoms along a glass tube loaded with a copper sample. Vacuum 2017, 138, 224–229. [Google Scholar] [CrossRef]
- Mozetic, M.; Primc, G.; Vesel, A.; Zaplotnik, R.; Modic, M.; Junkar, I.; Recek, N.; Klanjsek-Gunde, M.; Guhy, L.; Sunkara, M.K.; et al. Application of extremely non-equilibrium plasmas in the processing of nano and biomedical materials. Plasma Sour. Sci. Technol. 2015, 24, 015026. [Google Scholar] [CrossRef]
- Junkar, I.; Cvelbar, U.; Vesel, A.; Hauptman, N.; Mozetič, M. The role of crystallinity on polymer interaction with oxygen plasma. Plasma Process. Polym. 2009, 6, 667–675. [Google Scholar] [CrossRef]
- Pike, S.; Dewison, M.G.; Spears, D.A. Sources of error in low temperature plasma ashing procedures for quantitative mineral analysis of coal ash. Fuel 1989, 68, 664–668. [Google Scholar] [CrossRef]
- Alam, A.U.; Howlader, M.M.R.; Deen, M.J. The effects of oxygen plasma and humidity on surface roughness, water contact angle and hardness of silicon, silicon dioxide and glass. J. Micromech. Microeng. 2014, 24, 035010. [Google Scholar] [CrossRef]




| Plasma Treatment | Discharge Parameters | Wettability | Surface Analysis | Reference |
|---|---|---|---|---|
| Low-pressure oxygen plasma |
| WCA 1 was decreasing with increasing power and treatment time. From 74.5° it decreased to approx. 44° at the highest power of 29.6 W and at the longest treatment time 300 s. SFE 2 increased from 56.5 to max. 94.1 mJ/m2. | AFM 3: surface roughness RMS 4 increased from 1.5 to 7.3 nm. XRD 5: higher degree of crystallinity observed after oxygen plasma treatment. Mechanical properties: tensile strength decreased after plasma treatment. Barrier properties: water vapor transmission was increasing with increasing power and time. | [24] |
| Low-pressure oxygen and argon plasma |
| Increase of the surface energy of oxygen plasma-treated sample was higher than for the one treated in Ar. SFE was 70 and over 50 mJ/m2 for O2 and Ar plasma, respectively. | ATR-FTIR 6: carbonyl groups observed as well as C=C which could be a sign of crosslinking. AFM: O2 plasma caused higher roughness than Ar. Adhesion: High improvement of surface adhesion strength, especially for O2 plasma. | [32] |
| Low-pressure oxygen plasma |
| WCA decreased from 110° to 40°. | AFM: surface roughness first decreased with treatment time. At longer treatment times, a significant increase is observed. FTIR: C=O and –OH peaks observed for plasma-treated samples. | [23] |
| Low-pressure air plasma |
| WCA decreased from 94.9° to 60°. Saturation reached after 10 s. | XPS 7: Oxygen concentration increased from 4.3 to 13.7 at %. Nitrogen (0.8 at. %) was also found, the rest being carbon. 8.2% C–O, 2.7% C=O and O–C=O and 86.4% C–C, C–H groups observed on plasma treated sample. ATR-FTIR: peaks attributed to OH and C=O in ketones, aldehydes and carboxylic acids. | [21] |
| Atmospheric pressure air plasma |
| WCA decreased from 104° to 64° even at 1.2 kJ/m2. SFE increased from 33.7 to almost 50 mN/m. | XPS: O/C ratio increased over 0.16. Nitrogen (2 at %) was also found. After one month, O/C decreased to 0.12. Groups like C–O (22.5%), C=O or O–C–O (8.4%) and O=C–O (5.3%) were found. Maximum concentration was obtained at the lowest treatment speed. AFM: Ra 8 increased from 5.8 to 12.9 nm. Bumps were observed on the surface. The height and width were increasing with treatment power and reached 60 and 500 nm, respectively. | [22] |
| Low-pressure oxygen and argon plasma |
| WCA was decreasing with increasing power and treatment time. The lowest WCA was 34.4° for O2 and 38.2° for Ar plasma (initially 98.3°). SFE increased to ~45 mN/m. | SEM 9 and AFM: topology and roughness changed significantly, especially for Ar plasma (nodules observed on the surface). RMS roughness increased from 3.6 to 6.9 and 6.1 nm for O2 and Ar plasma, respectively. ATR-FTIR: C=O stretching bond and C=C vibration observed. Some peaks attributed also to carboxylic/ester, aldehydes and ketone groups. | [25] |
| Low-pressure oxygen plasma |
| WCA decreased from 121.5° to 84° on PP nonwoven mats. Ageing for 90 days did not have significant effect on WCA. SFE increased from 13.7 to 29.2 mN/m. | SEM: etching of PP fibers observed. XRD: no significant effect on the crystallinity of the treated fibers. | [33] |
| Low-pressure oxygen plasma |
| The lowest WCA—bellow 10° was observed at 150 W, 3.33 Pa and 60 s. After 30 days of ageing WCA increased to ~50°. | Ageing and crystallinity: Two polymers with different initial crystallinity were used. More crystalline PP was ageing slower—WCA after 30 days was for ~5° lower than for less crystalline one. Degree of crosslinking was increased after the treatment for both polymers. XPS: ~25 at % of oxygen was found on less-crystalline polymer. O concentration on more crystalline polymer was few at % lower. However, after ageing the O concentration changed in favor of more crystalline one. | [34] |
| Low-pressure oxygen plasma |
| WCA decreased from 98° to 24°. At long treatment times, it increased to 53°. | AFM: roughness RMS increased from ~ 12 nm to ~44 nm. ATR-FTIR: OH, C=O and CO–C=O peaks observed for plasma treated samples. | [26] |
| Low-pressure oxygen plasma |
| WCA decreased from 83° to 60°. SFE increased from 25.7 to 43 mJ/m2. | ATR-FTIR: OH, C=O groups in ester, ketone and carboxyl groups, C=O groups in unsaturated ketones and aldehydes. | [20] |
| Low-pressure oxygen plasma |
| WCA was decreasing with the increasing power and treatment time. Minimal achievable WCA was 55.6° (initially 103°). Ageing in water was faster than in air. After 90 days, the WCA was 81.7° (in water) and 71.2° (in air). | AFM: Roughness RMS increased after treatment from 2.1 nm to ~10 nm (in air) and ~5 nm (in water). Lower roughness of samples stored in water was explained by removing of water-soluble short-chain species. | [35] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukušić, T.; Vesel, A.; Holc, M.; Ščetar, M.; Jambrak, A.R.; Mozetič, M. Modification of Physico-Chemical Properties of Acryl-Coated Polypropylene Foils for Food Packaging by Reactive Particles from Oxygen Plasma. Materials 2018, 11, 372. https://doi.org/10.3390/ma11030372
Vukušić T, Vesel A, Holc M, Ščetar M, Jambrak AR, Mozetič M. Modification of Physico-Chemical Properties of Acryl-Coated Polypropylene Foils for Food Packaging by Reactive Particles from Oxygen Plasma. Materials. 2018; 11(3):372. https://doi.org/10.3390/ma11030372
Chicago/Turabian StyleVukušić, Tomislava, Alenka Vesel, Matej Holc, Mario Ščetar, Anet Režek Jambrak, and Miran Mozetič. 2018. "Modification of Physico-Chemical Properties of Acryl-Coated Polypropylene Foils for Food Packaging by Reactive Particles from Oxygen Plasma" Materials 11, no. 3: 372. https://doi.org/10.3390/ma11030372





