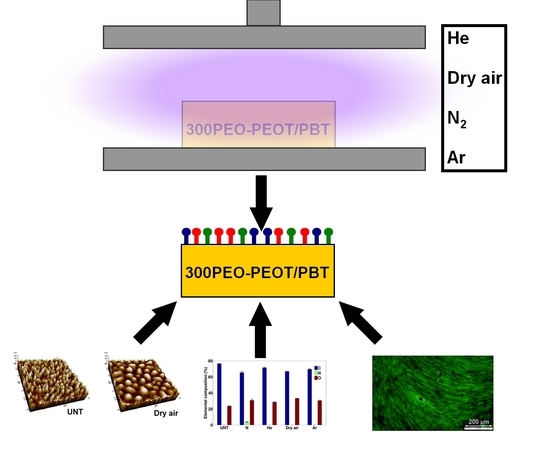
Surface Treatment of PEOT/PBT (55/45) with a Dielectric Barrier Discharge in Air, Helium, Argon and Nitrogen at Medium Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spin Coating of PEOT/PBT Thin Films
2.3. DBD System Description and Plasma Treatment Procedure
2.4. Contact Angle Goniometry
2.5. Atomic Force Microscopy
2.6. X-ray Photoelectron Spectroscopy
2.7. In Vitro Analysis
3. Results and Discussion
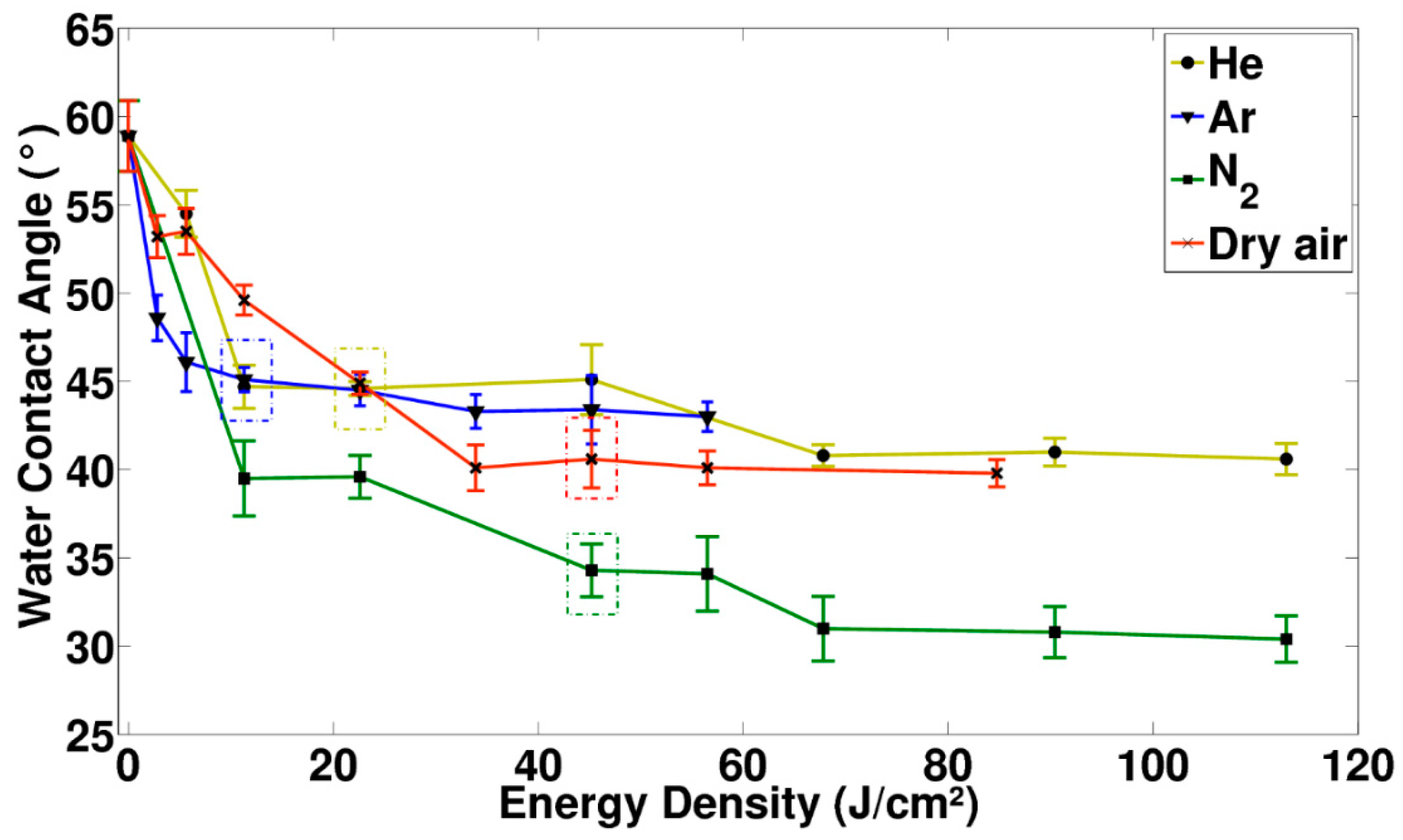
3.1. Analysis of the Wettability
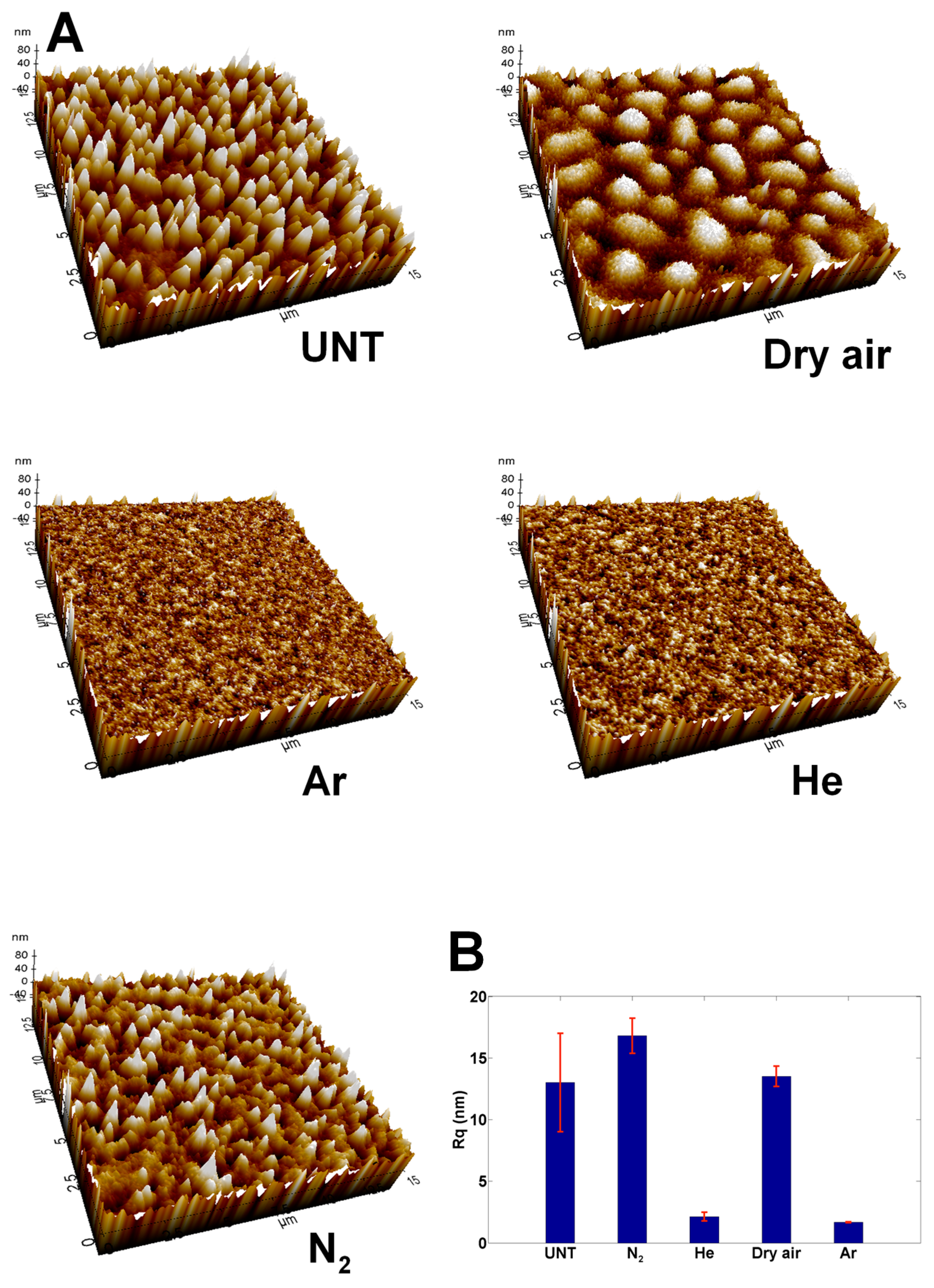
3.2. Changes in Surface Topography
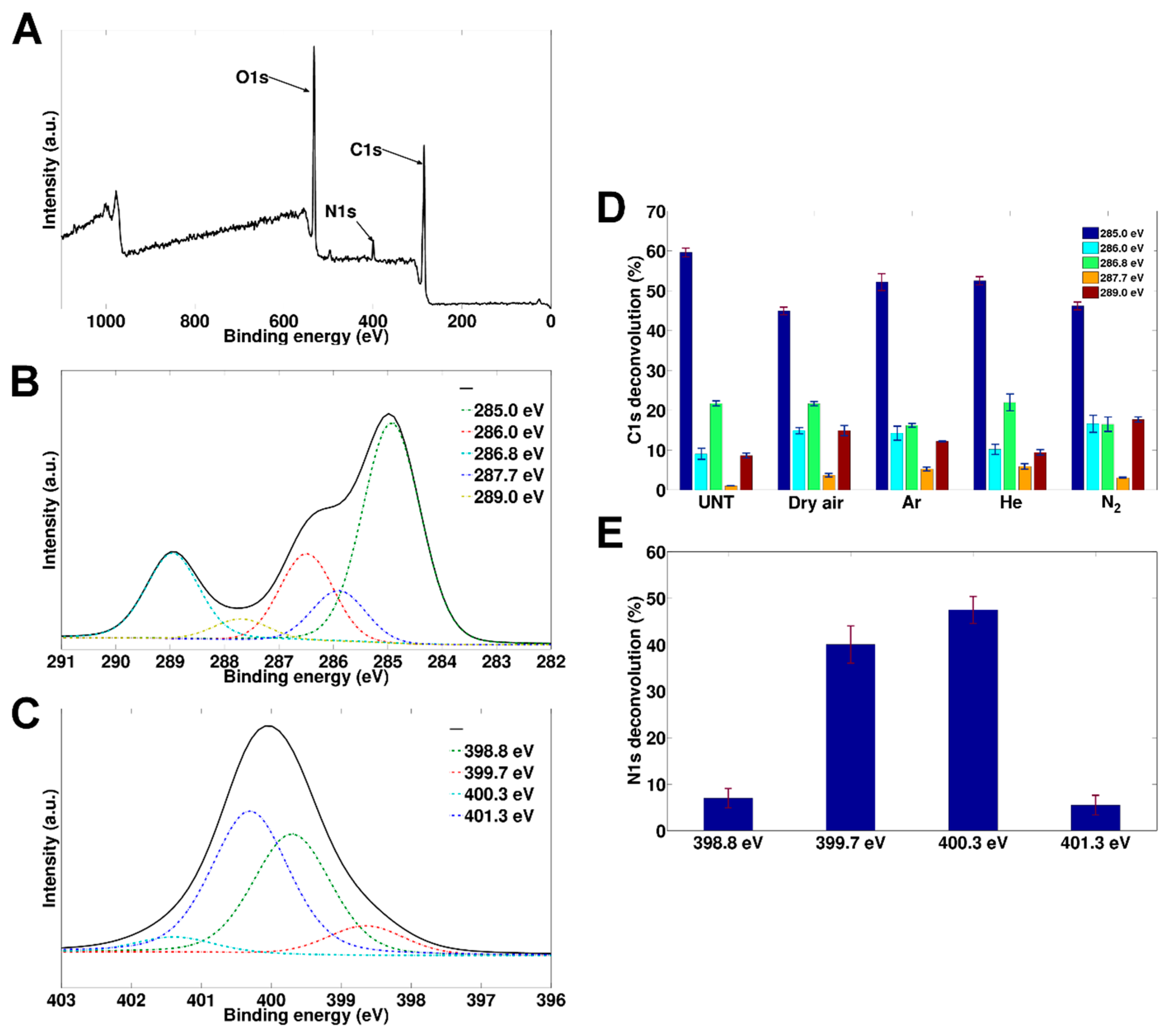
3.3. Changes in Surface Chemical Composition
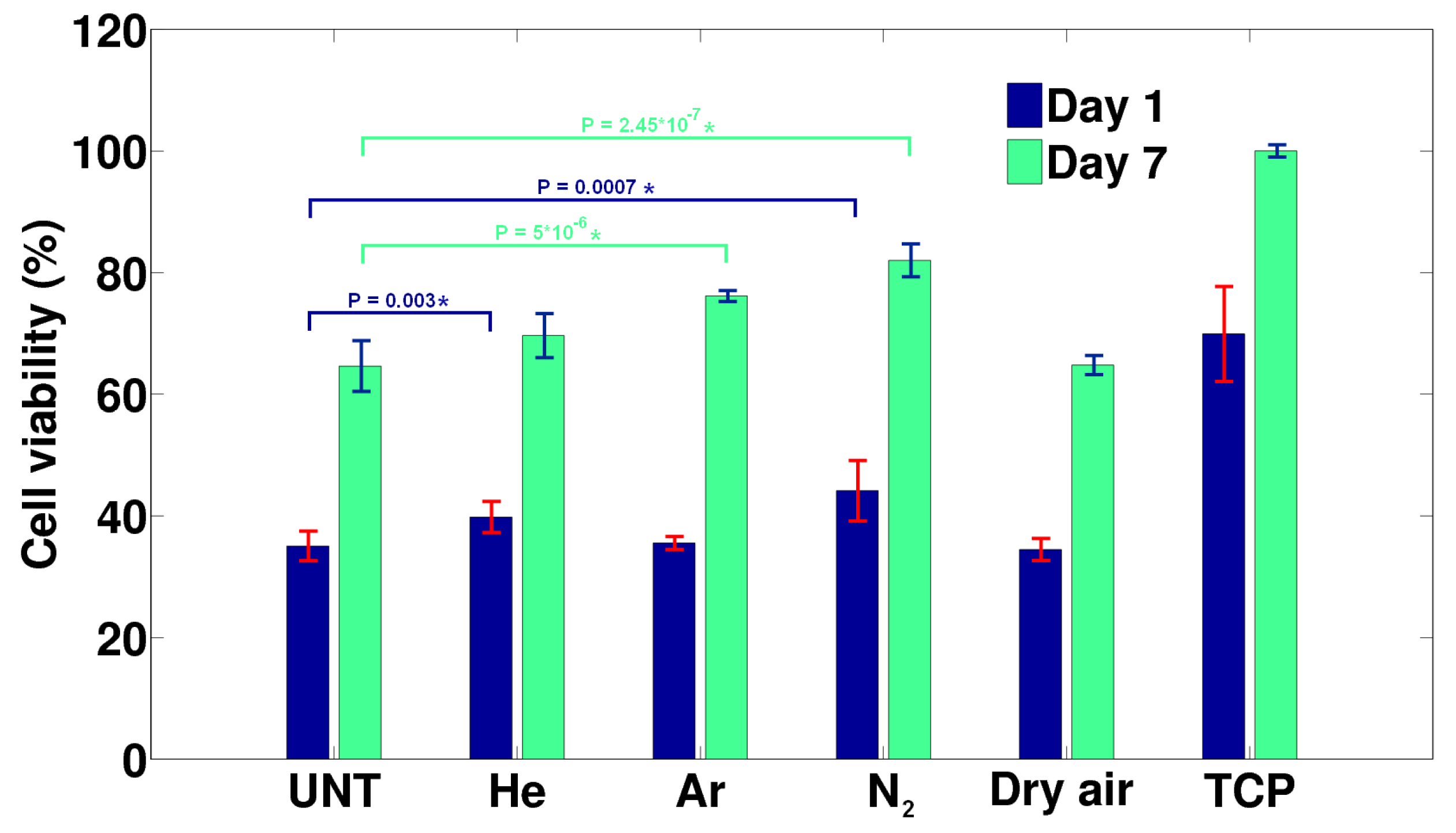
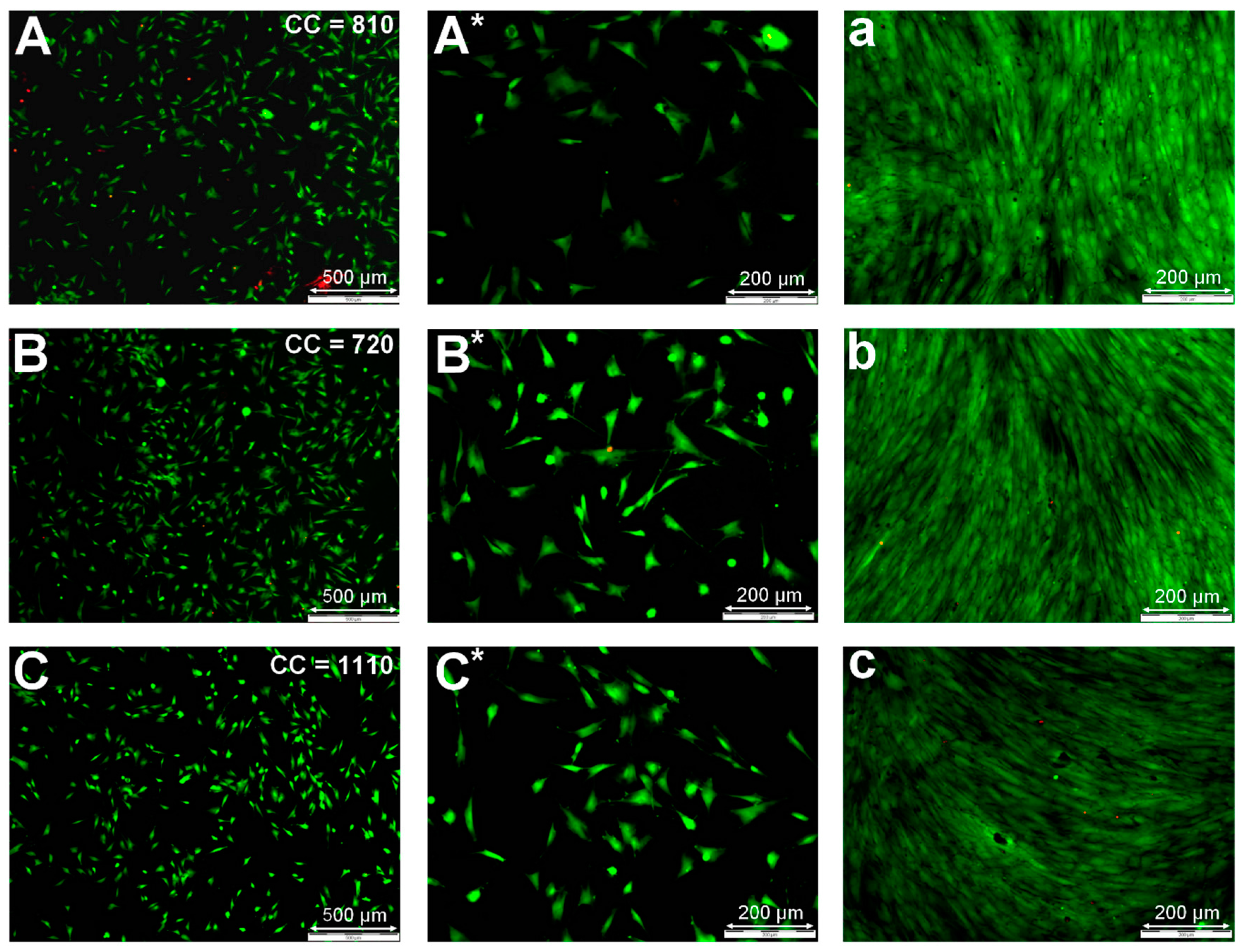
3.4. Improvement of Cell-Surface Interactions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Gilding, D.; Reed, A. Biodegradable polymers for use in surgery—polyglycolic/poly (actic acid) homo-and copolymers: 1. Polymer 1979, 20, 1459–1464. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Raimo, M. Review Properties of blends and composites based on poly (3-hydroxy) butyrate (PHB) and poly (3-hydroxybutyrate-hydroxyvalerate) (PHBV) copolymers. J. Mater. Sci. 2000, 35, 523–545. [Google Scholar] [CrossRef]
- Cools, P.; Ghobeira, R.; Van Vrekhem, S.; De Geyter, N.; Morent, R. Non-thermal Plasma Technology for the Improvement of Scaffolds for Tissue Engineering and Regenerative Medicine—A Review. In Plasma Science and Technology—Progress in Physical States and Chemical Reactions; Mieno, P.T., Ed.; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, T.M.; Samstein, R.M.; Harness, C.C.; Saltzman, W.M. Surface modification of biodegradable polyesters with fatty acid conjugates for improved drug targeting. Biomaterials 2005, 26, 5727–5736. [Google Scholar] [CrossRef] [PubMed]
- Desmet, T.; Morent, R.; Geyter, N.D.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Teoh, S.-H. Surface modification of ultra thin poly (ε-caprolactone) films using acrylic acid and collagen. Biomaterials 2004, 25, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, C.; Liu, X.; Shen, J. Surface modification of polycaprolactone membrane via aminolysis and biomacromolecule immobilization for promoting cytocompatibility of human endothelial cells. Biomacromolecules 2002, 3, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Tiaw, K.; Goh, S.; Hong, M.; Wang, Z.; Lan, B.; Teoh, S. Laser surface modification of poly (ε-caprolactone) (PCL) membrane for tissue engineering applications. Biomaterials 2005, 26, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Gao, C.; Gong, Y.; Shen, J. Cartilage tissue engineering PLLA scaffold with surface immobilized collagen and basic fibroblast growth factor. Biomaterials 2005, 26, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.-P.; Cui, F.-Z. Surface modification of polyester biomaterials for tissue engineering. Biomed. Mater. 2007, 2, R24–R37. [Google Scholar] [CrossRef] [PubMed]
- Liston, E.; Martinu, L.; Wertheimer, M. Plasma surface modification of polymers for improved adhesion: A critical review. J. Adhes. Sci. Technol. 1993, 7, 1091–1127. [Google Scholar] [CrossRef]
- Grace, J.M.; Gerenser, L.J. Plasma treatment of polymers. J. Dispers. Sci. Technol. 2003, 24, 305–341. [Google Scholar] [CrossRef]
- Kogelschatz, U. Atmospheric-pressure plasma technology. Plasma Phys. Control. Fusion 2004, 46, B63–B75. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Leys, C.; Gengembre, L.; Payen, E. Treatment of polymer films with a dielectric barrier discharge in air, helium and argon at medium pressure. Surf. Coat. Technol. 2007, 201, 7066–7075. [Google Scholar] [CrossRef]
- Cools, P.; De Geyter, N.; Morent, R. PLA Enhanced via Plasma Technology: A Review. In New Developments in Polylactic Acid Research; Winthrop, C., Ed.; Nova Science: New York, NY, USA, 2014; p. 218. [Google Scholar]
- Martins, A.; Pinho, E.D.; Faria, S.; Pashkuleva, I.; Marques, A.P.; Reis, R.L.; Neves, N.M. Surface modification of electrospun polycaprolactone nanofiber meshes by plasma treatment to enhance biological performance. Small 2009, 5, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.; Intranuovo, F.; Gloria, A.; Gristina, R.; Ambrosio, L.; Bártolo, P.; Favia, P. Improved osteoblast cell affinity on plasma-modified 3-D extruded PCL scaffolds. Acta Biomater. 2013, 9, 5997–6005. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.; Mirzadeh, H.; Irani, S. Plasma surface modification of poly (L-lactic acid) and poly (lactic-co-glycolic acid) films for improvement of nerve cells adhesion. Radiat. Phys. Chem. 2008, 77, 280–287. [Google Scholar] [CrossRef]
- Ferreira, B.; Pinheiro, L.; Nascente, P.; Ferreira, M.; Duek, E. Plasma surface treatments of poly (l-lactic acid) (PLLA) and poly (hydroxybutyrate-co-hydroxyvalerate) (PHBV). Mater. Sci. Eng. C 2009, 29, 806–813. [Google Scholar] [CrossRef]
- Wan, Y.; Tu, C.; Yang, J.; Bei, J.; Wang, S. Influences of ammonia plasma treatment on modifying depth and degradation of poly (L-lactide) scaffolds. Biomaterials 2006, 27, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- Chim, H.; Ong, J.L.; Schantz, J.T.; Hutmacher, D.W.; Agrawal, C. Efficacy of glow discharge gas plasma treatment as a surface modification process for three-dimensional poly (D, L-lactide) scaffolds. J. Biomed. Mater. Res. A 2003, 65, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Keidar, M.; Robert, E. Preface to Special Topic: Plasmas for Medical Applications; AIP Publishing: Melville, NY, USA, 2015. [Google Scholar]
- Deschamps, A.A.; Grijpma, D.W.; Feijen, J. Poly (ethylene oxide)/poly (butylene terephthalate) segmented block copolymers: The effect of copolymer composition on physical properties and degradation behavior. Polymer 2001, 42, 9335–9345. [Google Scholar] [CrossRef]
- Deschamps, A.; Van Apeldoorn, A.; De Bruijn, J.; Grijpma, D.; Feijen, J. Poly (ether ester amide) s for tissue engineering. Biomaterials 2003, 24, 2643–2652. [Google Scholar] [CrossRef]
- Moroni, L.; De Wijn, J.; Van Blitterswijk, C. Three-dimensional fiber-deposited PEOT/PBT copolymer scaffolds for tissue engineering: Influence of porosity, molecular network mesh size, and swelling in aqueous media on dynamic mechanical properties. J. Biomed. Mater. Res. A 2005, 75, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, A.; Van Apeldoorn, A.; Hayen, H.; De Bruijn, J.; Karst, U.; Grijpma, D.; Feijen, J. In vivo and in vitro degradation of poly (ether ester) block copolymers based on poly (ethylene glycol) and poly (butylene terephthalate). Biomaterials 2004, 25, 247–258. [Google Scholar] [CrossRef]
- Bartha, L.; Hamann, D.; Pieper, J.; Péters, F.; Riesle, J.; Vajda, A.; Novak, P.K.; Hangody, L.R.; Vasarhelyi, G.; Bodó, L. A clinical feasibility study to evaluate the safety and efficacy of PEOT/PBT implants for human donor site filling during mosaicplasty. Eur. J. Orthop. Surg. Traumatol. 2013, 23, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kellomäki, M.; Paasimaa, S.; Grijpma, D.; Kolppo, K.; Törmälä, P. In vitro degradation of Polyactive® 1000PEOT70PBT30 devices. Biomaterials 2002, 23, 283–295. [Google Scholar] [CrossRef]
- Danti, S.; Mota, C.; D’alessandro, D.; Trombi, L.; Ricci, C.; Redmond, S.L.; De Vito, A.; Pini, R.; Dilley, R.J.; Moroni, L. Tissue engineering of the tympanic membrane using electrospun PEOT/PBT copolymer scaffolds: A morphological in vitro study. Hear. Balance Commun. 2015, 13, 133–147. [Google Scholar] [CrossRef]
- Mensik, I.; Lamme, E.N.; Riesle, J.; Brychta, P. Effectiveness and Safety of the PEGT/PBT Copolymer Scaffold as Dermal Substitute in Scar Reconstruction Wounds (Feasibility Trial). Cell Tissue Bank. 2002, 3, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Meijer, G.J.; van Dooren, A.; Gaillard, M.L.; Dalmeijer, R.; de Putter, C.; Koole, R.; van Blitterwijk, C.A. Polyactive as a bone-filler in a beagle dog model. Int. J. Oral Maxillofac. Surg. 1996, 25, 210–216. [Google Scholar] [CrossRef]
- Du, C.; Meijer, G.J.; van de Valk, C.; Haan, R.E.; Bezemer, J.M.; Hesseling, S.C.; Cui, F.Z.; de Groot, K.; Layrolle, P. Bone growth in biomimetic apatite coated porous Polyactive 1000PEGT70PBT30 implants. Biomaterials 2002, 23, 4649–4656. [Google Scholar] [CrossRef]
- Papenburg, B.J.; Rodrigues, E.D.; Wessling, M.; Stamatialis, D. Insights into the role of material surface topography and wettability on cell-material interactions. Soft Matter 2010, 6, 4377–4388. [Google Scholar] [CrossRef]
- Buitinga, M.; Truckenmüller, R.; Engelse, M.A.; Moroni, L.; Ten Hoopen, H.W.; van Blitterswijk, C.A.; de Koning, E.J.; van Apeldoorn, A.A.; Karperien, M. Microwell scaffolds for the extrahepatic transplantation of islets of Langerhans. PLoS ONE 2013, 8, e64772. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, L.; Kerkelä, E.; Kujala, K.; Haaparanta, A.; Ahola, N.; Ellä, V.; Poh, T.; Kellomäki, M.; Aalto-Setäläet, K. Analysis of different natural and synthetic biomaterials to support cardiomyocyte growth. J. Clin. Exp. Cardiol. 2011, 4, 2. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Leys, C.; Gengembre, L.; Payen, E.; Van Vlierberghe, S.; Schacht, E. DBD treatment of polyethylene terephthalate: Atmospheric versus medium pressure treatment. Surf. Coat. Technol. 2008, 202, 3000–3010. [Google Scholar] [CrossRef]
- Cools, P.; De Geyter, N.; Vanderleyden, E.; Dubruel, P.; Morent, R. Surface Analysis of Titanium Cleaning and Activation Processes: Non-thermal Plasma Versus Other Techniques. Plasma Chem. Plasma Process. 2014, 34, 917–932. [Google Scholar] [CrossRef]
- Cools, P.; De Geyter, N.; Vanderleyden, E.; Barberis, F.; Dubruel, P.; Morent, R. Adhesion improvement at the PMMA bone cement-titanium implant interface using methyl methacrylate atmospheric pressure plasma polymerization. Surf. Coat. Technol. 2016, 294, 201–209. [Google Scholar] [CrossRef]
- Deschamps, A.A.; Claase, M.B.; Sleijster, W.J.; de Bruijn, J.D.; Grijpma, D.W.; Feijen, J. Design of segmented poly(ether ester) materials and structures for the tissue engineering of bone. J. Control. Release 2002, 78, 175–186. [Google Scholar] [CrossRef]
- Cools, P.; Van Vrekhem, S.; De Geyter, N.; Morent, R. The use of DBD plasma treatment and polymerization for the enhancement of biomedical UHMWPE. Thin Solid Films 2014, 572, 251–259. [Google Scholar] [CrossRef]
- Van Deynse, A.; Cools, P.; Leys, C.; Morent, R.; De Geyter, N. Surface modification of polyethylene in an argon atmospheric pressure plasma jet. Surf. Coat. Technol. 2015, 276, 384–390. [Google Scholar] [CrossRef]
- Jacobs, T.; Declercq, H.; De Geyter, N.; Cornelissen, R.; Dubruel, P.; Leys, C.; Morent, R. Improved cell adhesion to flat and porous plasma-treated poly-ε-caprolactone samples. Surf. Coat. Technol. 2013, 232, 447–455. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma surface modification of biodegradable polymers: A review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Borcia, G.; Anderson, C.A.; Brown, N.M.D. Dielectric barrier discharge for surface treatment: Application to selected polymers in film and fibre form. Plasma Sources Sci. Technol. 2003, 12, 335. [Google Scholar] [CrossRef]
- Van Deynse, A.; Cools, P.; Leys, C.; Morent, R.; De Geyter, N. Influence of ambient conditions on the aging behavior of plasma-treated polyethylene surfaces. Surf. Coat. Technol. 2014, 258, 359–367. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Study of the ageing behaviour of polymer films treated with a dielectric barrier discharge in air, helium and argon at medium pressure. Surf. Coat. Technol. 2007, 201, 7847–7854. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Leys, C. Influence of ambient conditions on the ageing behaviour of plasma-treated PET surfaces. Nucl. Instrum. Methods Phys. Res. Sect. B 2008, 266, 3086–3090. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Trentesaux, M.; Gengembre, L.; Dubruel, P.; Leys, C.; Payen, E. Influence of discharge atmosphere on the ageing behaviour of plasma-treated polylactic acid. Plasma Chem. Plasma Process. 2010, 30, 525–536. [Google Scholar] [CrossRef]
- Wilson, D.; Williams, R.; Pond, R. Plasma modification of PTFE surfaces. Part II: Plasma-treated surfaces following storage in air or PBS. Surf. Interface Anal. 2001, 31, 397–408. [Google Scholar] [CrossRef]
- Murakami, T.; Kuroda, S.-I.; Osawa, Z. Dynamics of polymeric solid surfaces treated with oxygen plasma: Effect of aging media after plasma treatment. J. Colloid Interface Sci. 1998, 202, 37–44. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, S.; Wang, S.; Jiang, L. Superwetting surfaces under different media: Effects of surface topography on wettability. Small 2015, 11, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Pandiyaraj, K.N.; Selvarajan, V.; Deshmukh, R.; Gao, C. Modification of surface properties of polypropylene (PP) film using DC glow discharge air plasma. Appl. Surf. Sci. 2009, 255, 3965–3971. [Google Scholar] [CrossRef]
- Okuno, T.; Yasuda, T.; Yasuda, H. Effect of crystallinity of PET and nylon 66 fibers on plasma etching and dyeability characteristics. Text. Res. J. 1992, 62, 474–480. [Google Scholar] [CrossRef]
- Riekerink, O.; Claase, M.; Engbers, G.; Grijpma, D.; Feijen, J. Gas plasma etching of PEO/PBT segmented block copolymer films. J. Biomed. Mater. Res. Part A 2003, 65, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Junkar, I.; Vesel, A.; Cvelbar, U.; Mozetič, M.; Strnad, S. Influence of oxygen and nitrogen plasma treatment on polyethylene terephthalate (PET) polymers. Vacuum 2009, 84, 83–85. [Google Scholar] [CrossRef]
- Idage, S.; Badrinarayanan, S. Surface modification of polystyrene using nitrogen plasma. An X-ray photoelectron spectroscopy study. Langmuir 1998, 14, 2780–2785. [Google Scholar] [CrossRef]
- Lai, J.; Sunderland, B.; Xue, J.; Yan, S.; Zhao, W.; Folkard, M.; Michael, B.D.; Wang, Y. Study on hydrophilicity of polymer surfaces improved by plasma treatment. Appl. Surf. Sci. 2006, 252, 3375–3379. [Google Scholar] [CrossRef]
- Foerch, R.; Beamson, G.; Briggs, D. XPS valence band analysis of plasma–treated polymers. Surf. Interface Anal. 1991, 17, 842–846. [Google Scholar] [CrossRef]
- Ghobeira, R.; Philips, C.; Declercq, H.; Cools, P.; De Geyter, N.; Cornelissen, R.; Morent, R. Effects of different sterilization methods on the physico-chemical and bioresponsive properties of plasma-treated polycaprolactone films. Biomed. Mater. 2017, 12, 015017. [Google Scholar] [CrossRef] [PubMed]
- Griesser, H.J.; Chatelier, R.C.; Gengenbach, T.R.; Johnson, G.; Steele, J.G. Growth of human cells on plasma polymers: Putative role of amine and amide groups. J. Biomater. Sci. Polym. Ed. 1994, 5, 531–554. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Myung, S.W.; Jung, S.C.; Ko, Y.M. Plasma Surface modification for immobilization of bone morphogenic protein-2 on polycaprolactone scaffolds. Jpn. J. Appl. Phys. 2013, 52, 11NF01. [Google Scholar] [CrossRef]
- Unalan, I.; Colpankan, O.; Albayrak, A.Z.; Gorgun, C.; Urkmez, A.S. Biocompatibility of plasma-treated poly (3-hydroxybutyrate-co-3-hydroxyvalerate) nanofiber mats modified by silk fibroin for bone tissue regeneration. Mater. Sci. Eng. C 2016, 68, 842–850. [Google Scholar] [CrossRef] [PubMed]

| Discharge Gas | Discharge Power | Treatment Time | Energy Density |
|---|---|---|---|
| Argon | 3 W | 0–240 s | 0–56.5 J/cm2 |
| Helium | 6 W | 0–300 s | 0–113.0 J/cm2 |
| Nitrogen | 6 W | 0–300 s | 0–113.0 J/cm2 |
| Dry air | 3 W | 0–300 s | 0–84.8 J/cm2 |
| Element | Unt | Dry air | Ar | He | N2 |
|---|---|---|---|---|---|
| C | 76.4 ± 0.4 | 66.8 ± 0.2 | 69.5 ± 0.7 | 71.4 ± 0.1 | 65.4 ± 1.5 |
| O | 23.6 ± 0.4 | 33.2 ± 0.2 | 30.5 ± 0.7 | 28.6 ± 0.1 | 30.9 ± 1.3 |
| N | - | - | - | - | 3.7 ± 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cools, P.; Asadian, M.; Nicolaus, W.; Declercq, H.; Morent, R.; De Geyter, N. Surface Treatment of PEOT/PBT (55/45) with a Dielectric Barrier Discharge in Air, Helium, Argon and Nitrogen at Medium Pressure. Materials 2018, 11, 391. https://doi.org/10.3390/ma11030391
Cools P, Asadian M, Nicolaus W, Declercq H, Morent R, De Geyter N. Surface Treatment of PEOT/PBT (55/45) with a Dielectric Barrier Discharge in Air, Helium, Argon and Nitrogen at Medium Pressure. Materials. 2018; 11(3):391. https://doi.org/10.3390/ma11030391
Chicago/Turabian StyleCools, Pieter, Mahtab Asadian, Wannes Nicolaus, Heidi Declercq, Rino Morent, and Nathalie De Geyter. 2018. "Surface Treatment of PEOT/PBT (55/45) with a Dielectric Barrier Discharge in Air, Helium, Argon and Nitrogen at Medium Pressure" Materials 11, no. 3: 391. https://doi.org/10.3390/ma11030391
APA StyleCools, P., Asadian, M., Nicolaus, W., Declercq, H., Morent, R., & De Geyter, N. (2018). Surface Treatment of PEOT/PBT (55/45) with a Dielectric Barrier Discharge in Air, Helium, Argon and Nitrogen at Medium Pressure. Materials, 11(3), 391. https://doi.org/10.3390/ma11030391