Silica Modified with Polyaniline as a Potential Sorbent for Matrix Solid Phase Dispersion (MSPD) and Dispersive Solid Phase Extraction (d-SPE) of Plant Samples
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials and Reagents
2.2. Methodology
2.2.1. Synthesis and Characteristic of Si-PANI Sorbent
2.2.2. Extraction Experiments
Dispersive Solid Phase Extraction (d-SPE)
2.2.3. Matrix Solid Phase Dispersion (MSPD)
2.2.4. HPLC Analysis
3. Results and Discussion
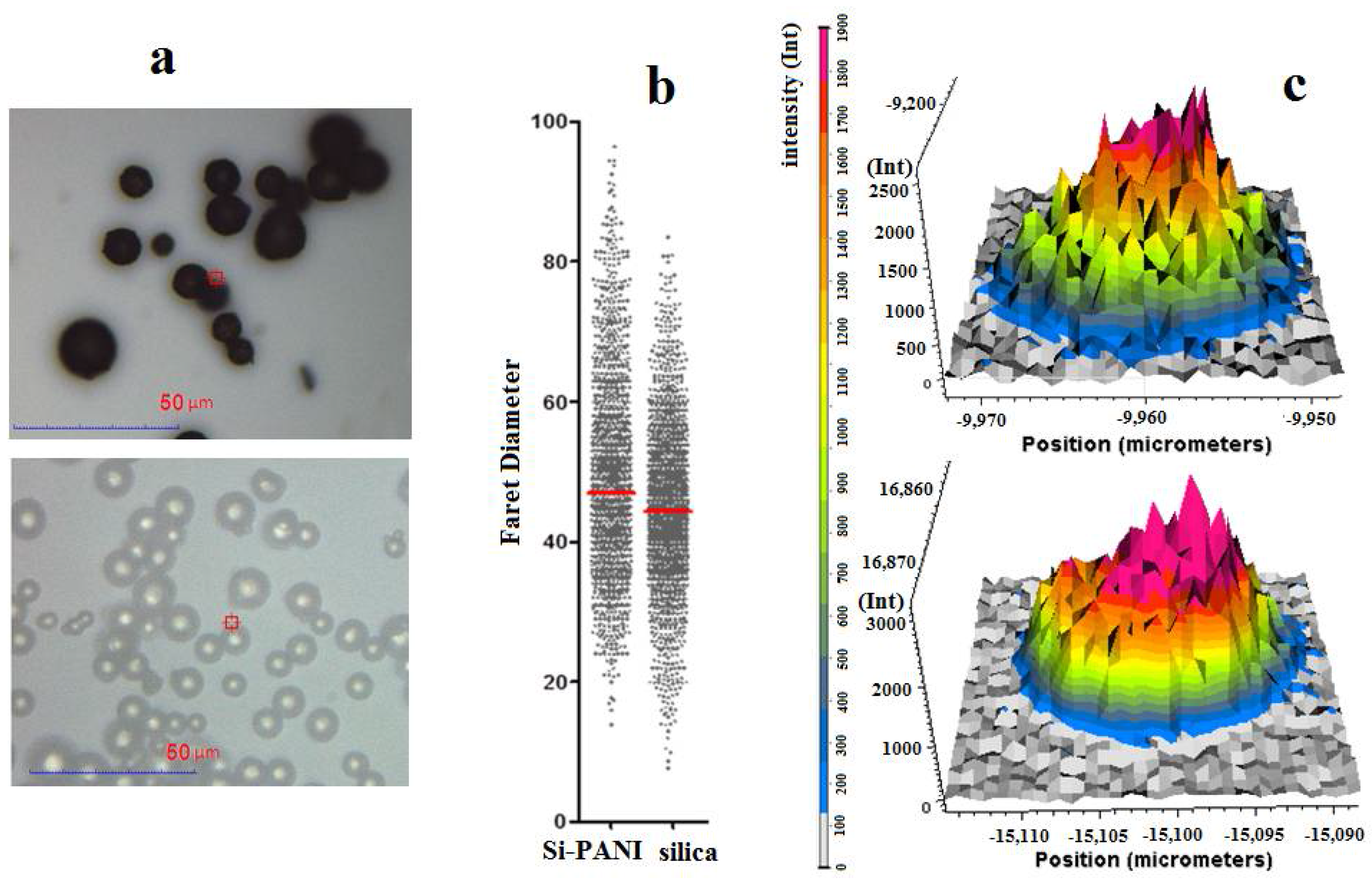
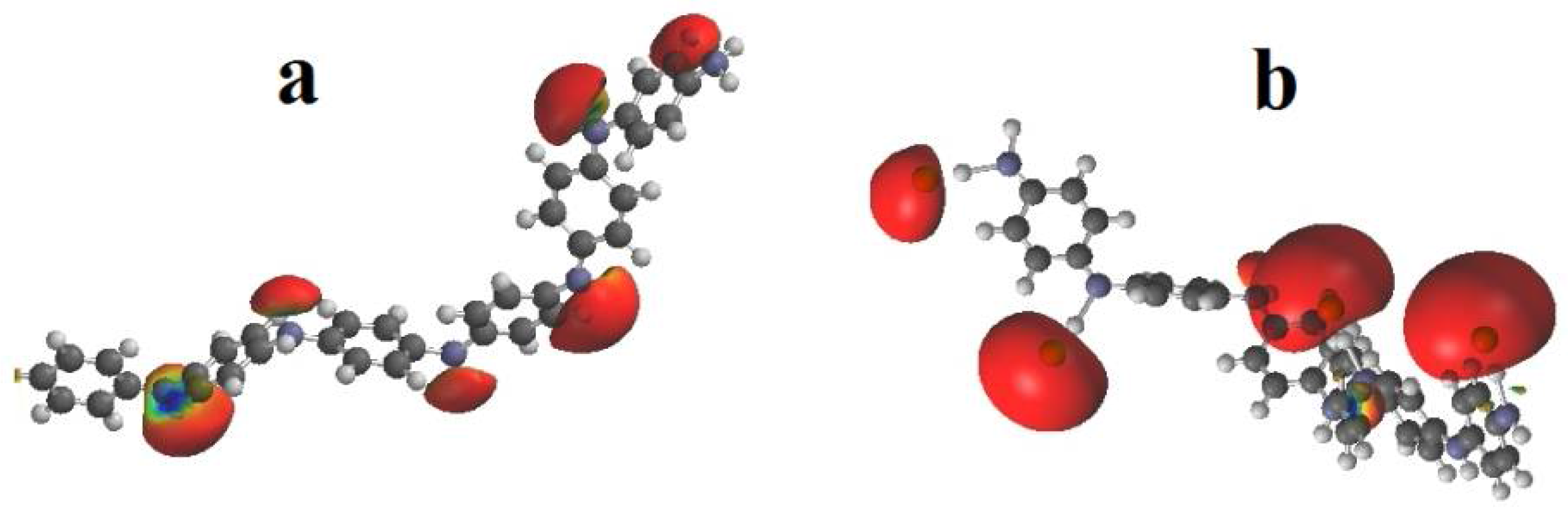
3.1. Characteristics of Si-PANI
3.2. Optimization of d-SPE Parameters
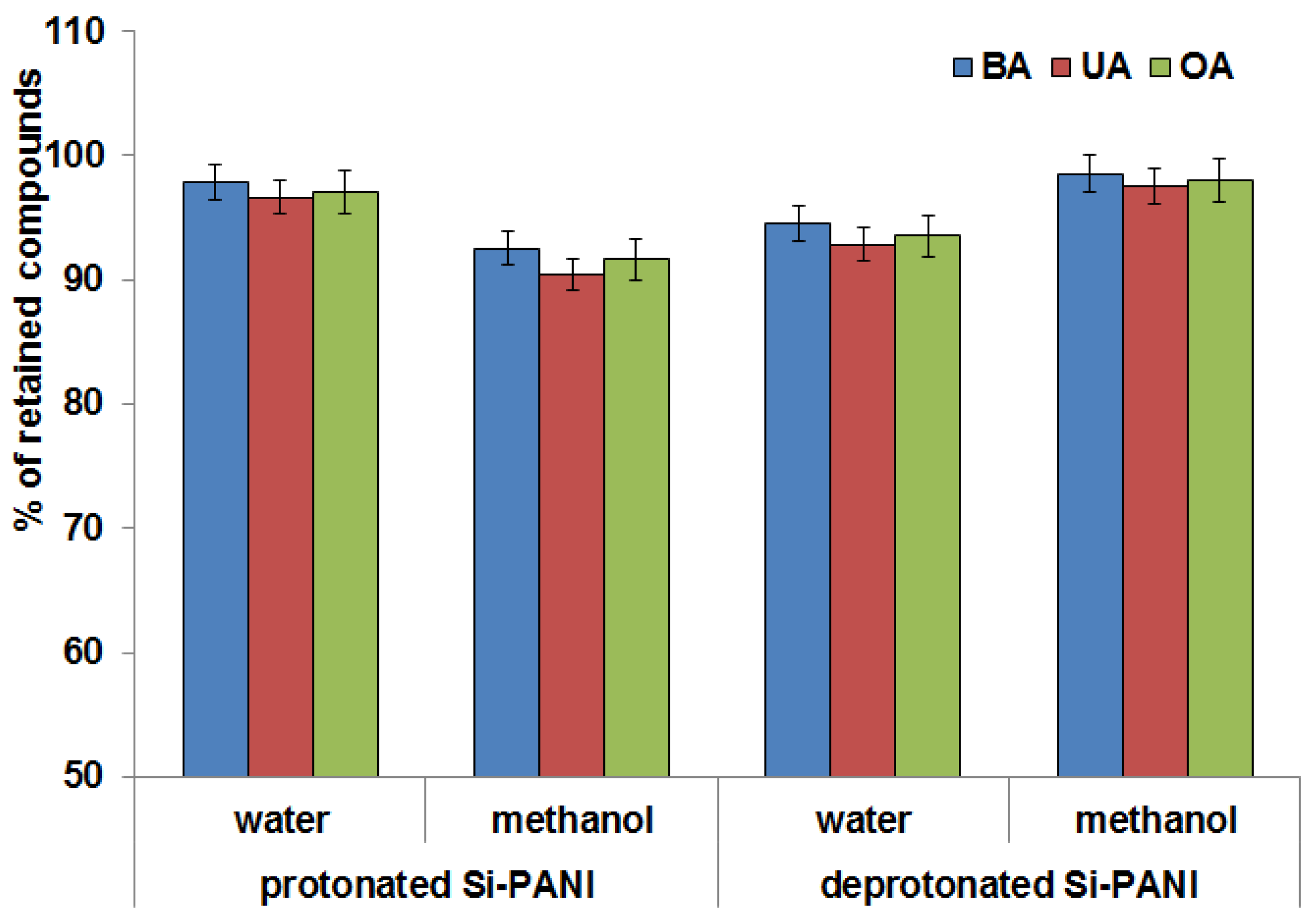
3.2.1. Form of PANI and Impregnation Solution
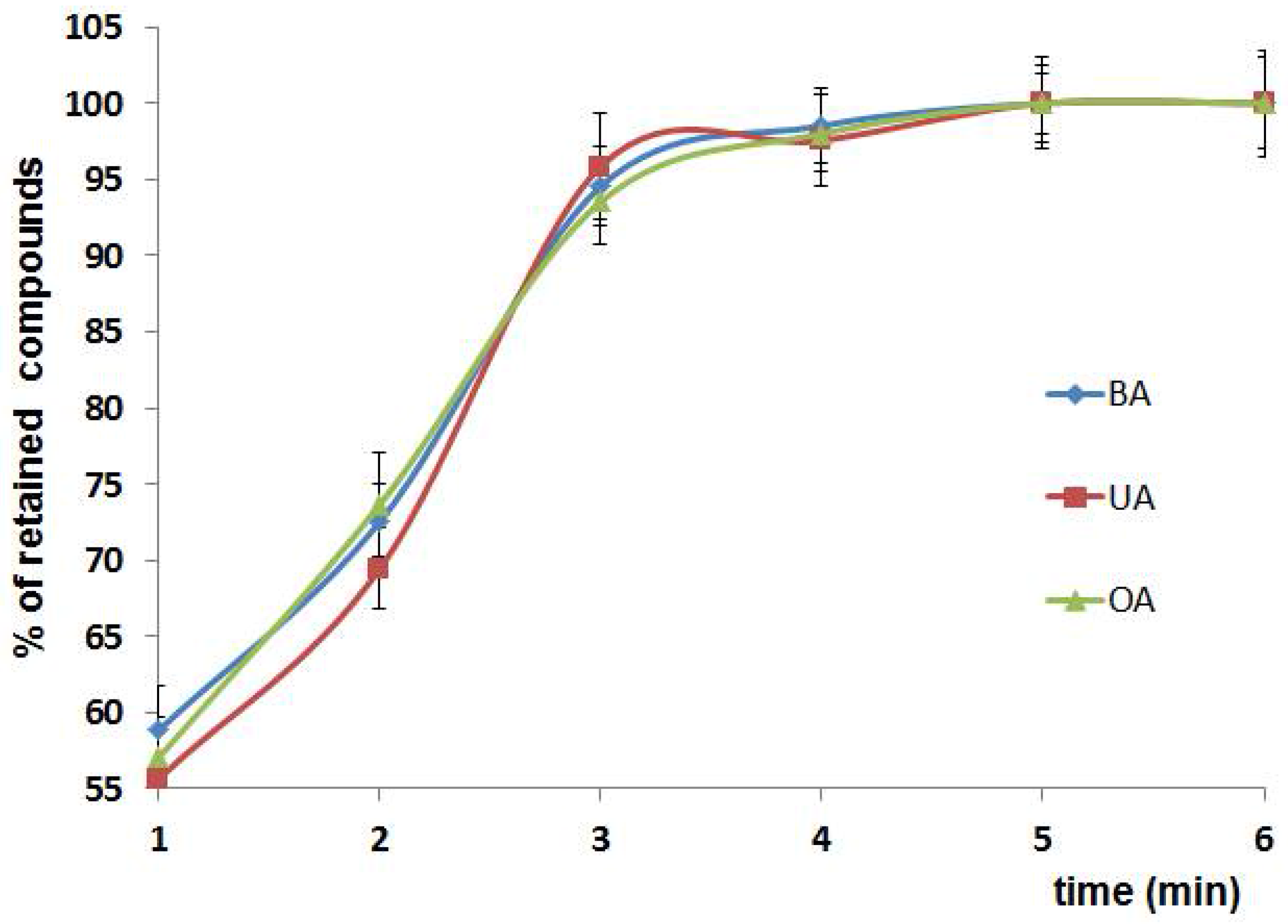
3.2.2. Time of Extraction
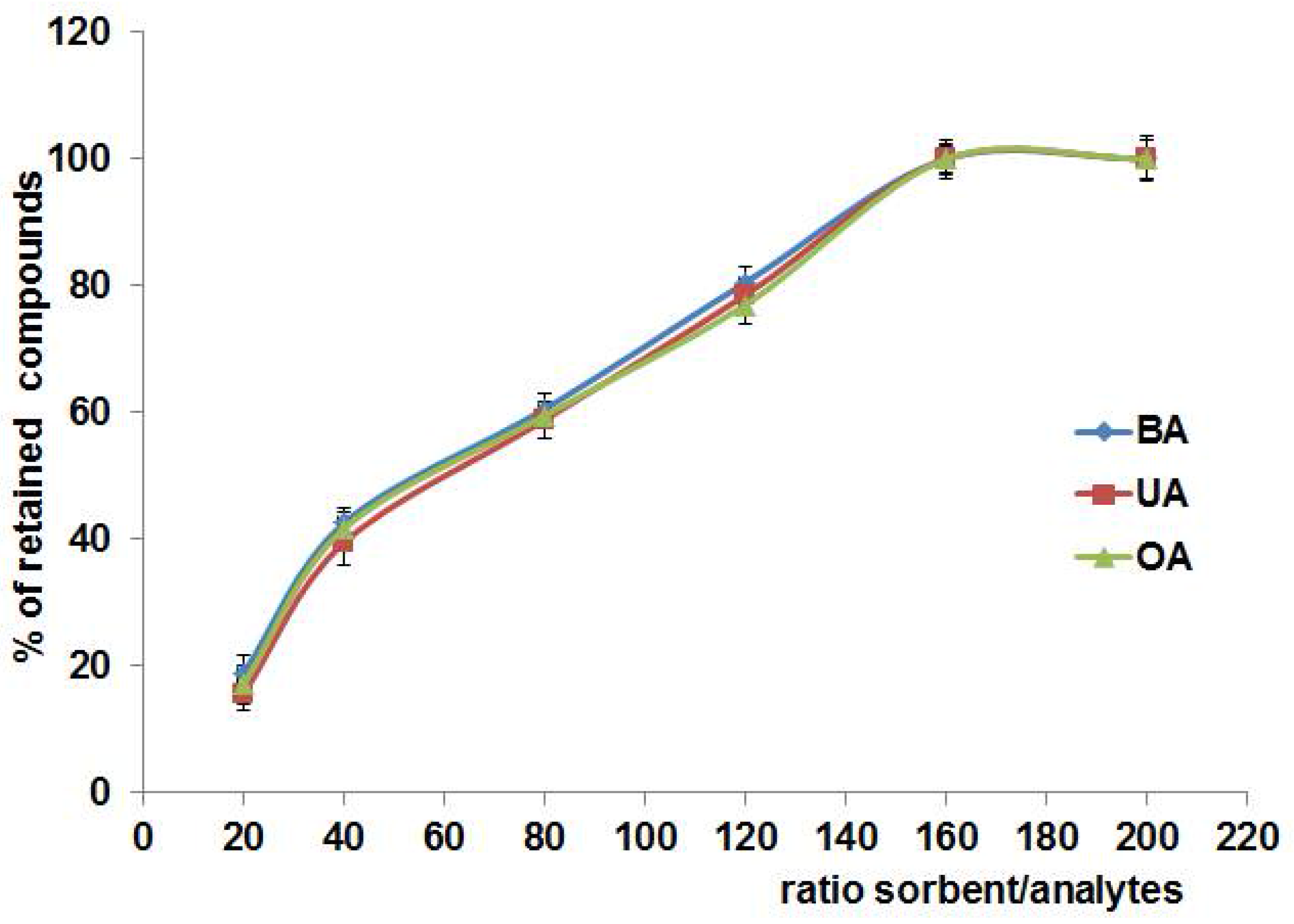
3.2.3. Ratio of Sorbent to Analyte
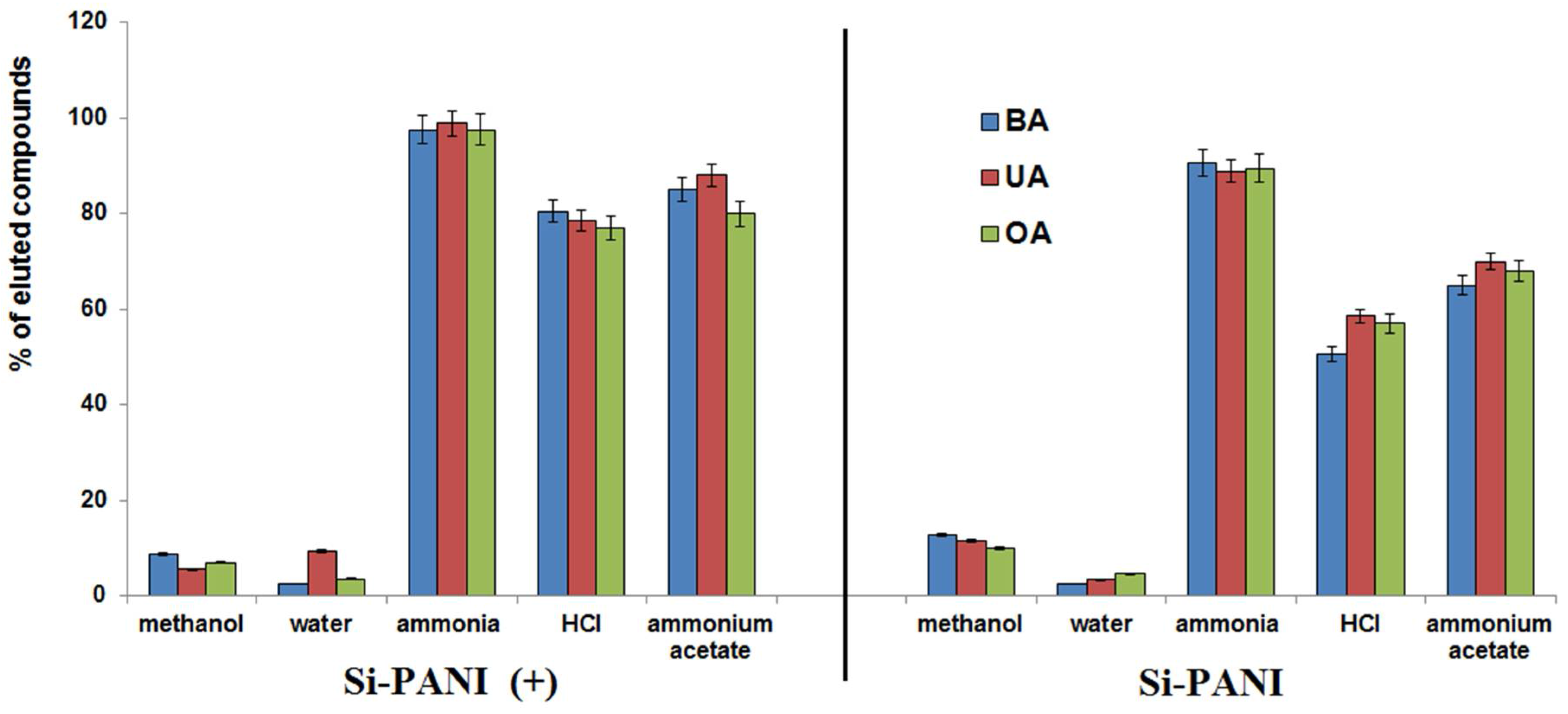
3.2.4. Elution Solvent
3.2.5. Desorption Time
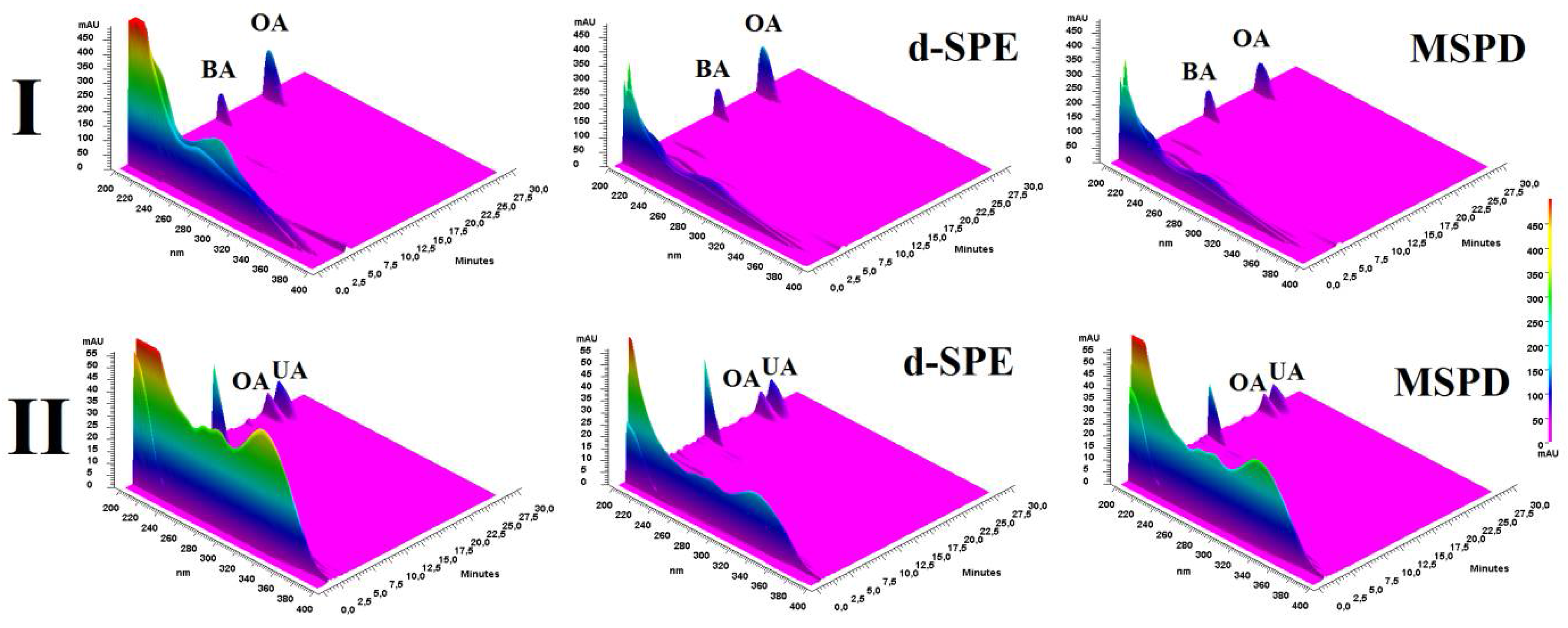
3.3. Application of Si-PANI Sorbent for Pretreatment of Plant Material
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Progress Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Wang, G.; Vivek, R.; Wang, J.-Y. Polyaniline nanoparticles: Synthesis, dispersion and biomedical applications. Mini-Rev. Organ. Chem. 2017, 14, 56–64. [Google Scholar] [CrossRef]
- Liu, S.; Liu, L.; Meng, F.; Li, Y.; Wang, F. Protective performance of polyaniline-sulfosalicylic acid/epoxy coating for 5083 aluminum. Materials 2018, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Silakhori, M.; Naghavi, M.S.; Metselaar, H.S.C.; Mahlia, T.M.I.; Fauzi, H.; Mehrali, M. Accelerated thermal cycling test of microencapsulated paraffin wax/polyaniline made by simple preparation method for solar thermal energy storage. Materials 2013, 6, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Zu, L.; Cui, X.; Jiang, Y.; Hu, Z.; Lian, H.; Liu, Y.; Jin, Y.; Li, Y.; Wang, X. Preparation and electrochemical characterization of mesoporous polyaniline-silica nanocomposites as an electrode material for pseudocapacitors. Materials 2015, 8, 1369–1383. [Google Scholar] [CrossRef] [PubMed]
- Sowa, I.; Wójciak-Kosior, M.; Drączkowski, P.; Szwerc, W.; Tylus, J.; Pawlikowski, A.; Kocjan, R. Evaluation of pH and thermal stability of sorbent based on silica modified with polyaniline using high-resolution continuum source graphite furnace atomic absorption spectrometry and Raman spectroscopy. Microchem. J. 2015, 118, 88–94. [Google Scholar] [CrossRef]
- Sowa, I.; Wójciak-Kosior, M.; Drączkowski, P.; Strzemski, M.; Kocjan, R. Synthesis and properties of a newly obtained sorbent based on silica gel coated with a polyaniline film as the stationary phase for non-suppressed ion chromatography. Anal. Chim. Acta 2013, 787, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Mehdinia, A.; Khani, H.; Mozaffari, S. Fibers coated with a graphene-polyaniline nanocomposite for the headspace solid-phase microextraction of organochlorine pesticides from seawater samples. Microchim. Acta 2014, 181, 89–95. [Google Scholar] [CrossRef]
- Abolghasemi, M.M.; Parastari, S.; Yousefi, V. Microextraction of phenolic compounds using a fiber coated with a polyaniline-montmorillonite nanocomposite. Microchim. Acta 2015, 182, 273–280. [Google Scholar] [CrossRef]
- Lei, Y.; He, M.; Chen, B.; Hu, B. Polyaniline/cyclodextrin composite coated stir bar sorptive extraction combined with high performance liquid chromatography-ultraviolet detection for the analysis of trace polychlorinated biphenyls in environmental waters. Talanta 2016, 150, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Pirsa, S.; Faraji, N. Determination of lemon juice adulteration by analysis of gas chromatography profile of volatile organic compounds extracted with nano-sized polyester-polyaniline fiber. Food Anal. Methods 2017, 10, 2092–2101. [Google Scholar] [CrossRef]
- Bagheri, H.; Saraji, M. New polymeric sorbent for the solid-phase extraction of chlorophenols from water samples followed by gas chromatography-electron-capture detection. J. Chromatogr. A 2001, 910, 87–93. [Google Scholar] [CrossRef]
- Bagheri, H.; Saraji, M. Conductive polymers as new media for solid-phase extraction: Isolation of chlorophenols from water sample. J. Chromatogr. A 2003, 986, 111–119. [Google Scholar] [CrossRef]
- Bagheri, H.; Saraji, M.; Barceló, D. Evaluation of polyaniline as a sorbent for SPE of a variety of polar pesticides from water followed by CD-MEKC-DAD. Chromatographia 2004, 59, 283–289. [Google Scholar]
- Sowa, I.; Pizoń, M.; Świeboda, R.; Kocjan, R.; Zajdel, D. Properties of chelating sorbent prepared by modification of silica gel with polyaniline and Acid Alizarin Violet N. Sep. Sci. Technol. 2012, 47, 1194–1198. [Google Scholar] [CrossRef]
- Sowa, I.; Wójciak-Kosior, M.; Kocjan, R. The content of some trace elements in selected medicinal plants collected in the province of Lublin. Acta Sci. Pol. Hortorum Cultus 2012, 11, 15–22. [Google Scholar]
- Sowa, I.; Wójciak-Kosior, M.; Kocjan, R. Application of SPE technique using a newly obtained sorbent based on silica gel covered with polyaniline to simultaneous determination of nitrate (III) and nitrate (V) anions in water samples. Pol. J. Environ. Stud. 2013, 22, 881–884. [Google Scholar]
- Sowa, I.; Wójciak-Kosior, M.; Rokicka, K.; Kocjan, R.; Szymczak, G. Application of solid phase extraction with the use of silica modified with polyaniline film for pretreatment of samples from plant material before HPLC determination of triterpenic acids. Talanta 2014, 122, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Arnnok, P.; Patdhanagul, N.; Burakham, R. Dispersive solid-phase extraction using polyaniline-modified zeolite NaY as a new sorbent for multiresidue analysis of pesticides in food and environmental samples. Talanta 2017, 164, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Bladergroen, M.R.; Van Der Burgt, Y.E.M. Solid-phase extraction strategies to surmount body fluid sample complexity in high-throughput mass spectrometry-based proteomics. J. Anal. Methods Chem. 2015, 2015, 250131. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Qiao, Y.; Deng, Z.; Wu, M.; Liu, W. SPE-UHPLC-FLD method for the simultaneous determination of five anthraquinones in human urine using mixed-mode bis(tetraoxacalix[2]arene[2]triazine) modified silica as sorbent. J. Anal. Methods Chem. 2017, 2017, 1963908. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review (Part I). Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easymultiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [PubMed]
- Fontana, A.R.; Camargo, A.; Martinez, L.D.; Altamirano, J.C. Dispersive solid-phase extraction as a simplified clean-up technique for biological sample extracts. Determination of polybrominated diphenyl ethers by gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, Y.; Ma, J.; Jin, Z.; Chai, M.; Xiao, X.; Zhang, L.; Zhang, Y. A polyethyleneimine-modified attapulgite as a novel solid support in matrix solid-phase dispersion for the extraction of cadmium traces in seafood products. Talanta 2018, 180, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wei, Y. Fabrication of a novel hydrophobic/ion-exchange mixed-mode adsorbent for the dispersive solid-phase extraction of chlorophenols from environmental water samples. J. Sep. Sci. 2016, 39, 3186–3194. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, Y.; Ghorbani-Bidkorbeh, F.; Shekarchi, M. Superparamagnetic graphene oxide-based dispersive-solid phase extraction for preconcentration and determination of tamsulosin hydrochloride in human plasma by high performance liquid chromatography-ultraviolet detection. J. Chromatogr. A 2017, 1499, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Sowa, I.; Kocjan, R.; Wójciak-Kosior, M.; Świeboda, R.; Zajdel, D.; Hajnos, M. Physicochemical properties of silica gel coated with a thin layer of polyaniline (PANI) and its application in non-suppressed ion chromatography. Talanta 2013, 115, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ. U.S. National Institutes of Health: Bethesda, Maryland, USA, 1997–2014. Available online: https://imagej.nih.gov/ij/ (accessed on 22 March 2018).
- Merkus, H.G. Particle Size Measurements: Fundamentals, Practice, Quality; Springer: Dordrecht, The Netherlands, 2009; p. 15. ISBN 978-1-4020-9016-5. [Google Scholar]
- Nascimento, G.M.; Temperini, M.L.A. Studies on the resonance Raman spectra of polyaniline obtained with near-IR excitation. J. Raman Spectrosc. 2008, 39, 772–778. [Google Scholar] [CrossRef]
- Yang, M.; Fazio, S.; Munch, D.; Drumm, P. Impact of methanol and acetonitrile on separations based on π–π interactions with a reversed-phase phenyl column. J. Chromatogr. A 2005, 1097, 124–129. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Oleanolic Acid | Ursolic Acid | Betulinic Acid |
|---|---|---|---|
| Concentration range | 0.05–1.00 mg/mL | 0.005–1.00 mg/mL | 0.002–0.10 mg/mL |
| Correlation coefficient (r) | 0.9994 | 0.9998 | 0.9999 |
| Linear regression equation | y = 80,370,931x − 55,822 | y = 132,468,713x − 13,814 | y =101,240,680x − 28,874 |
| RSD values of peak area | 0.64–1.32% | 0.83–1.22% | 0.41–0.78% |
| LOD (µg/mL) | 0.13 | 0.14 | 0.12 |
| LOQ (µg/mL) | 0.43 | 0.46 | 0.40 |
| Compound | Viscum Album L. | Ocimum Basilicum L. | ||||
|---|---|---|---|---|---|---|
| Without Purification | d-SPE | MSPD | Without Purification | d-SPE | MSPD | |
| BA | 0.82 ± 0.10 | 0.78 ± 0.04 | 0.57 ± 0.04 | - | - | - |
| OA | 6.95 ± 0.41 | 6.55 ± 0.30 | 4.81 ± 0.21 | 0.69 ± 0.09 | 0.64 ± 0.07 | 0.45 ± 0.03 |
| UA | - | - | - | 1.14 ± 0.11 | 1.04 ± 0.10 | 0.77 ± 0.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowa, I.; Wójciak-Kosior, M.; Strzemski, M.; Sawicki, J.; Staniak, M.; Dresler, S.; Szwerc, W.; Mołdoch, J.; Latalski, M. Silica Modified with Polyaniline as a Potential Sorbent for Matrix Solid Phase Dispersion (MSPD) and Dispersive Solid Phase Extraction (d-SPE) of Plant Samples. Materials 2018, 11, 467. https://doi.org/10.3390/ma11040467
Sowa I, Wójciak-Kosior M, Strzemski M, Sawicki J, Staniak M, Dresler S, Szwerc W, Mołdoch J, Latalski M. Silica Modified with Polyaniline as a Potential Sorbent for Matrix Solid Phase Dispersion (MSPD) and Dispersive Solid Phase Extraction (d-SPE) of Plant Samples. Materials. 2018; 11(4):467. https://doi.org/10.3390/ma11040467
Chicago/Turabian StyleSowa, Ireneusz, Magdalena Wójciak-Kosior, Maciej Strzemski, Jan Sawicki, Michał Staniak, Sławomir Dresler, Wojciech Szwerc, Jarosław Mołdoch, and Michał Latalski. 2018. "Silica Modified with Polyaniline as a Potential Sorbent for Matrix Solid Phase Dispersion (MSPD) and Dispersive Solid Phase Extraction (d-SPE) of Plant Samples" Materials 11, no. 4: 467. https://doi.org/10.3390/ma11040467






