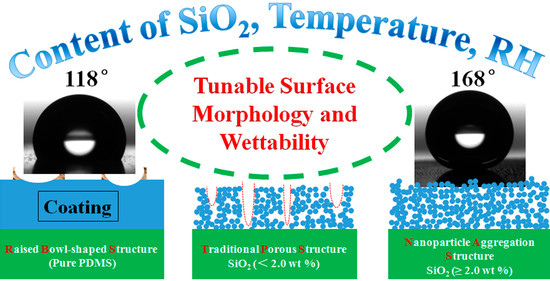
Patternable Poly(chloro-p-xylylene) Film with Tunable Surface Wettability Prepared by Temperature and Humidity Treatment on a Polydimethylsiloxane/Silica Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
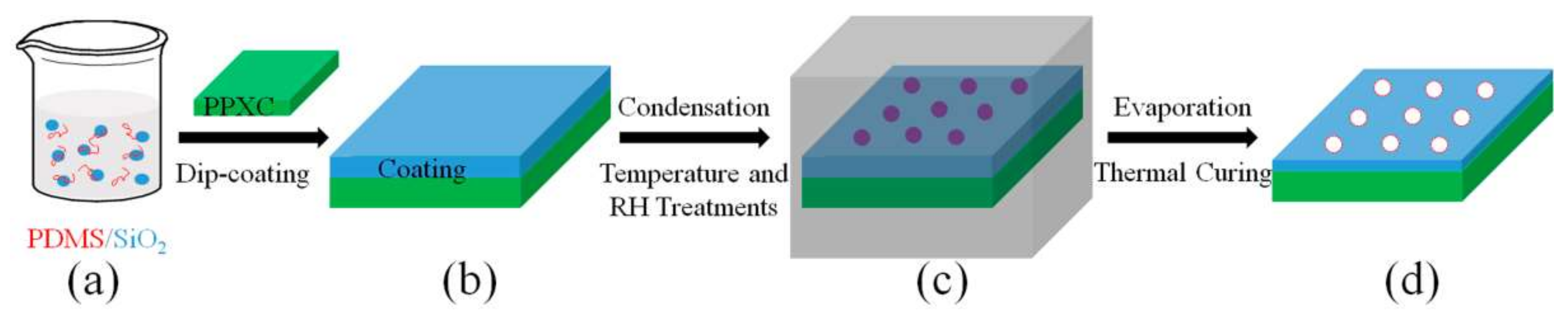
2.2. Dip Coating PDMS/SiO2 on PPXC Film
2.3. Characterization
2.3.1. Differential Scanning Calorimetry (DSC) Measurements
2.3.2. Fourier Transform Infrared (FTIR) Measurements
2.3.3. Surface Morphology Observation
2.3.4. Contact Angle Measurements
3. Results and Discussion
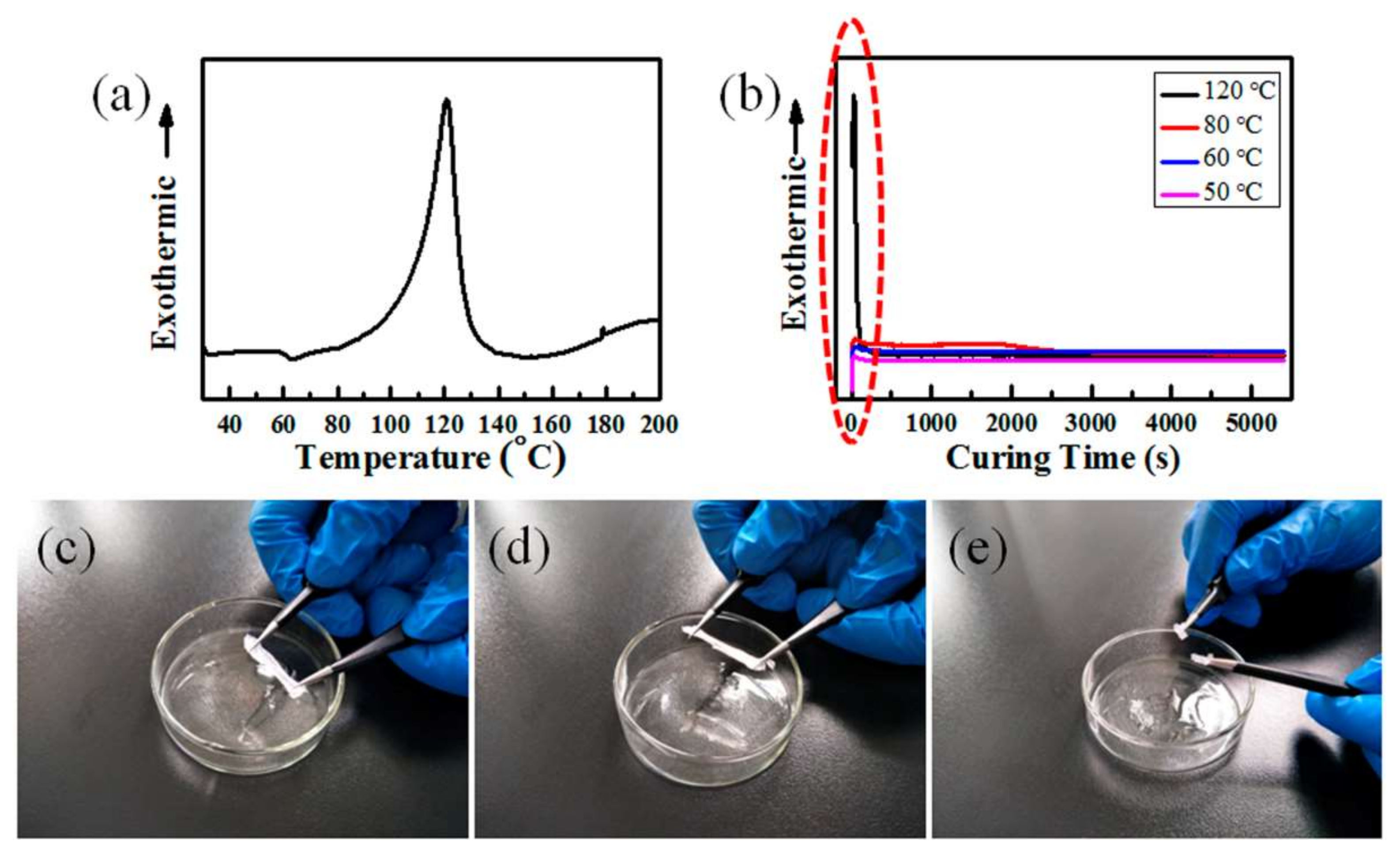
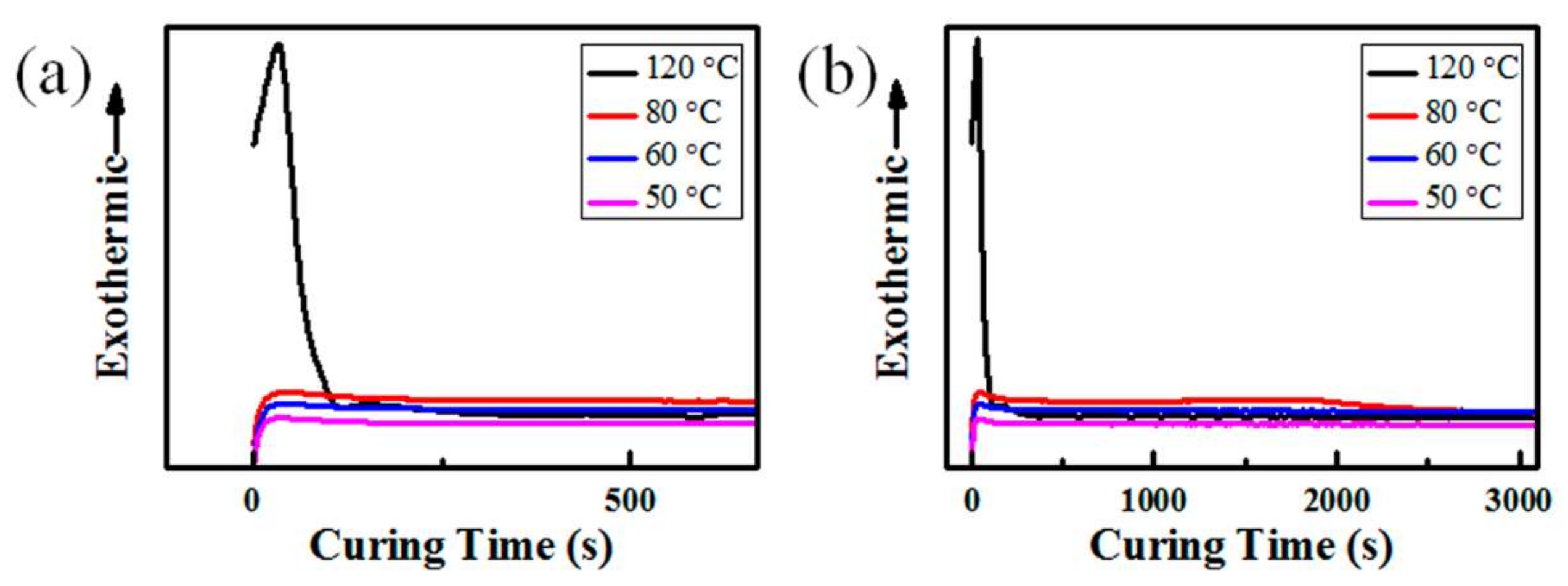
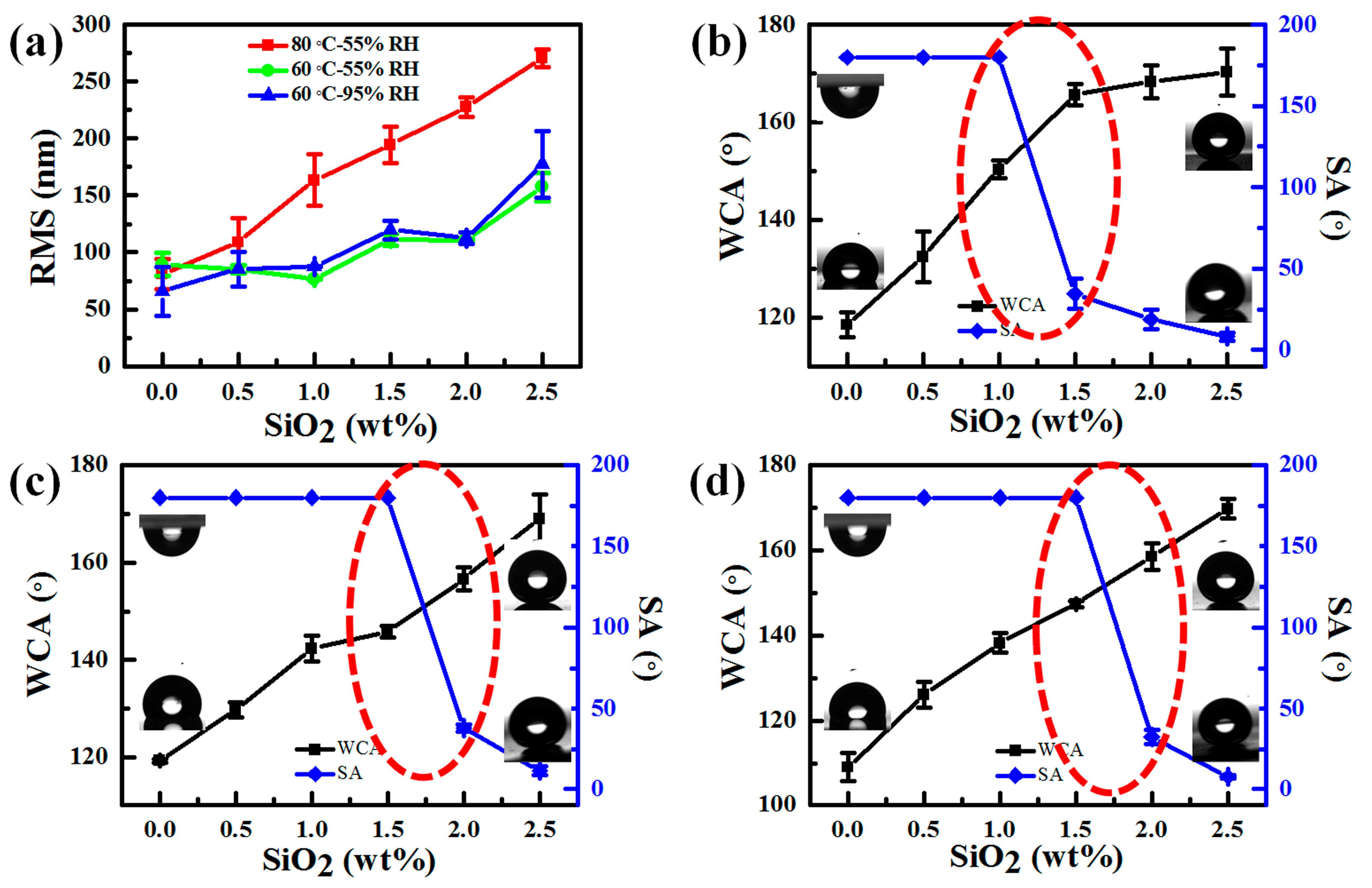
3.1. Effects of Temperature, RH, and Content of SiO2 on the Curing Behavior of PDMS
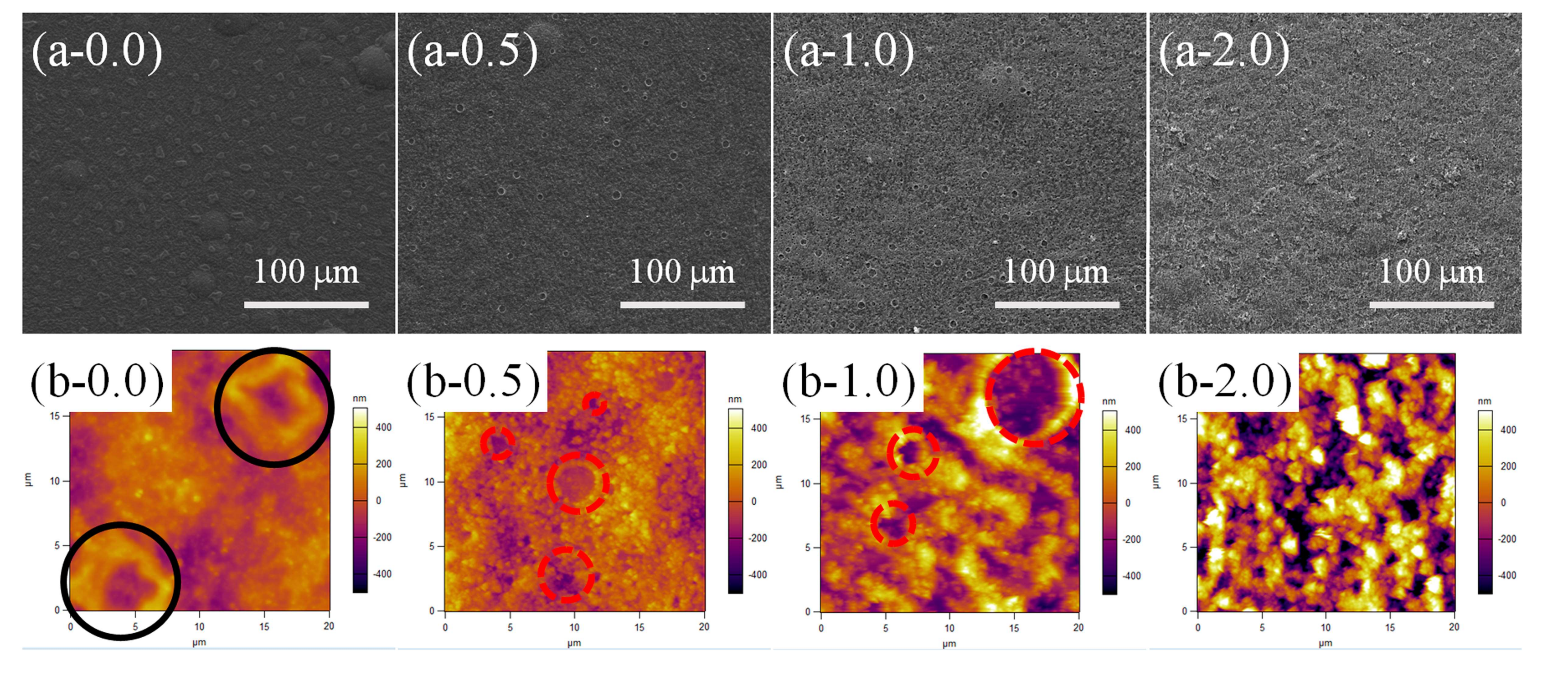
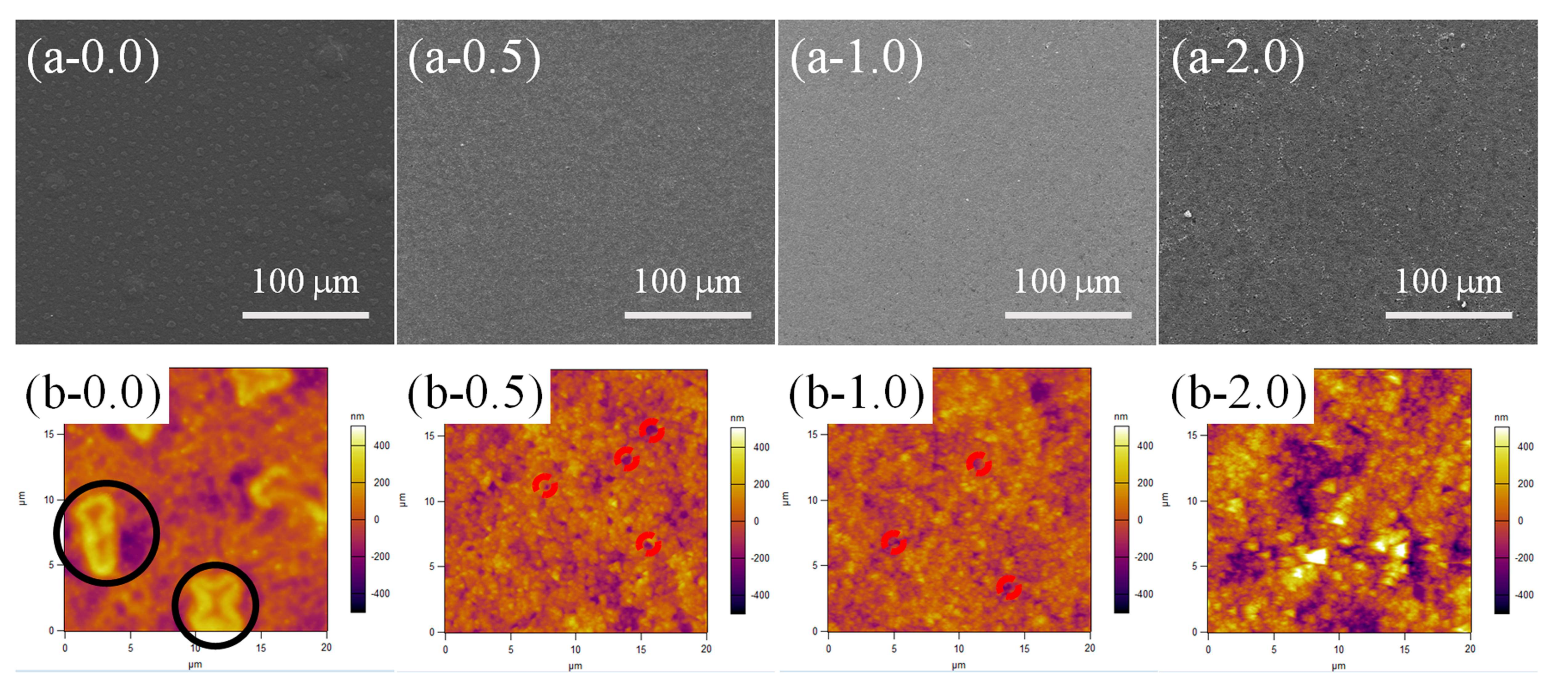
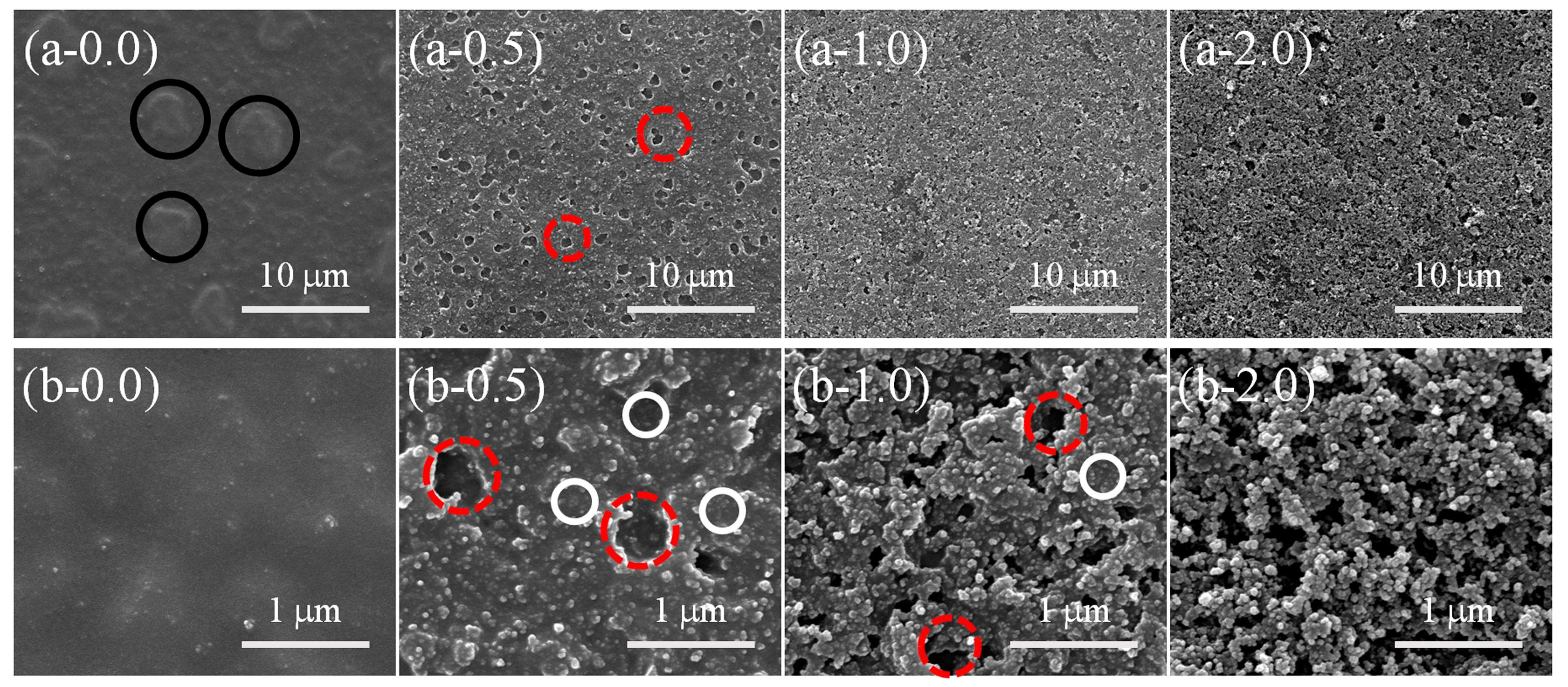
3.2. Surface Morphology Evolutions of PDMS/SiO2 Coating on PPXC Film under Different Treatment Conditions
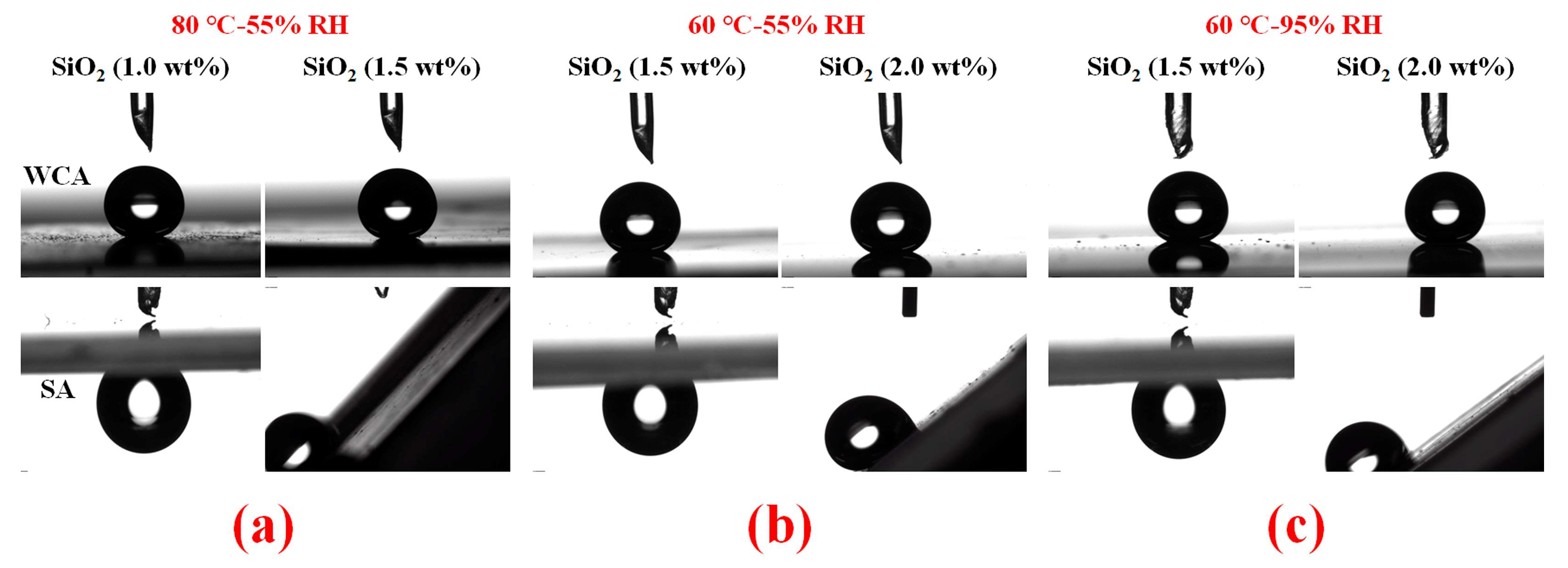
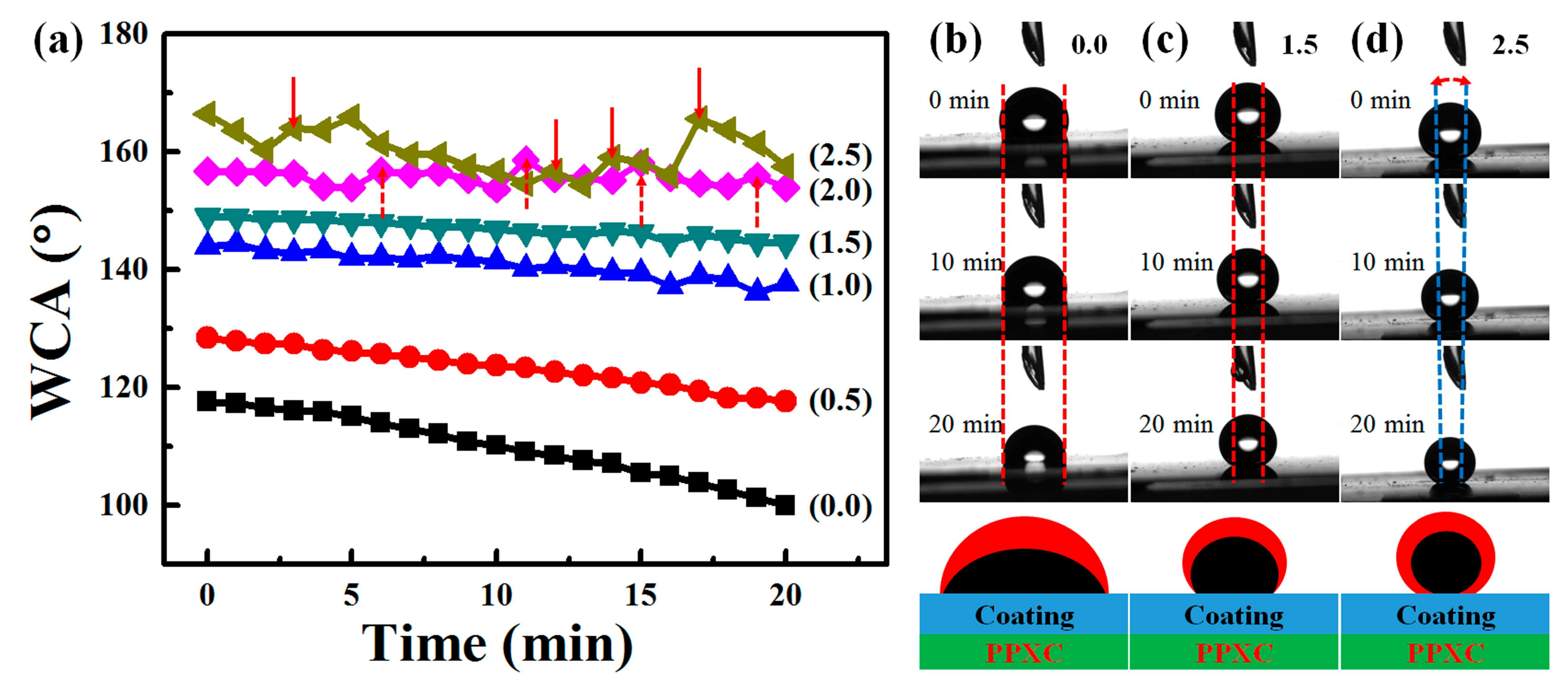
3.3. Surface Wettability of PDMS/SiO2 Coating on PPXC Film under Different Treatment Conditions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galeotti, F.; Hartmann, L.; Botta, C. Robust surface patterning by parylene-reinforced breath figures: An enabling tool for liquid crystal microcell arrays. J. Coll. Interface Sci. 2016, 465, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Salazar, F.; Deng, H.; Peljo, P.; Pereira, C.M.; Kontturi, K.; Girault, H.H. Parylene C coated microelectrodes for scanning electrochemical microscopy. Electrochim. Acta. 2013, 110, 22–29. [Google Scholar] [CrossRef]
- Davis, E.M.; Benetatos, N.M.; Regnault, W.F.; Winey, K.I.; Elabd, Y.A. The influence of thermal history on structure and water transport in Parylene C coatings. Polymer 2011, 52, 5378–5386. [Google Scholar] [CrossRef]
- Golda, M.; Brzychczy-Wloch, M.; Faryna, M.; Engvall, K.; Kotarba, A. Oxygen plasma functionalization of parylene C coating for implants surface: Nanotopography and active sites for drug anchoring. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4221–4227. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; He, Z.; Xu, K.; Hu, X.; Zhou, Y.; Tang, C.; Mei, J.; Shuai, M.; Lau, W.-M.; Hui, D. Preparation of stable wetting surface by hyperthermal hydrogen induced cross-linking of poly(acrylic acid) on poly(chloro-p-xylylene) film. J. Phys. Chem. C 2016, 120, 28598–28606. [Google Scholar] [CrossRef]
- Gupta, S.; He, W.-D.; Tai, N.-H. A comparative study on superhydrophobic sponges and their application as fluid channel for continuous separation of oils and organic solvents from water. Compos. Part. B Eng. 2016, 101, 99–106. [Google Scholar] [CrossRef]
- Tan, H.; Diddens, C.; Versluis, M.; Butt, H.-J.; Lohse, D.; Zhang, X. Self-wrapping of an ouzo drop induced by evaporation on a superamphiphobic surface. Soft Matter 2017, 13, 2749–2759. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, W.; Tsui, C.P.; Wang, G.; Tang, C.Y.; Zhong, L. Ultrasonic atomization based fabrication of bio-inspired micro-nano-binary particles for superhydrophobic composite coatings with lotus/petal effect. Compos. Part. B Eng. 2017, 121, 92–98. [Google Scholar] [CrossRef]
- Nouri, N.M.; Saadat-Bakhsh, M. Fabrication method of large-scale and mechanically durable superhydrophobic silicon rubber/aerogel coating on fibrous substrates. J. Coat. Technol. Res. 2017, 14, 477–488. [Google Scholar] [CrossRef]
- Zang, D.; Zhu, R.; Zhang, W.; Yu, X.; Lin, L.; Guo, X.; Liu, M.; Jiang, L. Corrosion-resistant superhydrophobic coatings on mg alloy surfaces inspired by lotus seedpod. Adv. Funct. Mater. 2017, 27, 1605446. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, H.; Wang, L.; Li, X. Comparison of barrier properties for a superhydrophobic epoxy coating under different simulated corrosion environments. Corros. Sci. 2016, 103, 230–241. [Google Scholar] [CrossRef]
- Zhu, M.; Tang, W.; Huang, L.; Zhang, D.; Du, C.; Yu, G.; Chen, M.; Chowwanonthapunya, T. Preparation of superhydrophobic film on ti substrate and its anticorrosion property. Materials 2017, 10, 628. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Mammen, L.; Zhao, Y.; Lellig, P.; Müllen, K.; Li, C.; Butt, H.-J.; Vollmer, D. Transparent, Thermally stable and mechanically robust superhydrophobic surfaces made from porous silica capsules. Adv. Mater. 2011, 23, 2962–2965. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Zhang, Z.; Men, X.; Zhu, X.; Zhou, X. Sprayed superamphiphobic coatings on copper substrate with enhanced corrosive resistance. Appl. Surf. Sci. 2014, 293, 271–274. [Google Scholar] [CrossRef]
- Martin, S.; Brown, P.S.; Bhushan, B. Fabrication techniques for bioinspired, mechanically-durable, superliquiphobic surfaces for water, oil, and surfactant repellency. Adv. Colloid Interface 2017, 241, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bhushan, B. Wear-resistant and antismudge superoleophobic coating on polyethylene terephthalate substrate using sio2 nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Mammen, L.; Butt, H.J.; Vollmer, D. Candle soot as a template for a transparent robust superamphiphobic coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.S.; Bhushan, B. Mechanically durable, superoleophobic coatings prepared by layer-by-layer technique for anti-smudge and oil-water separation. Sci. Rep. 2015, 5, 8701. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.; Emelyanenko, A.; Pashinin, A.; Gnedenkov, S.; Egorkin, V.; Sinebryukhov, S. Mg alloy treatment for superhydrophobic anticorrosion coating formation. Surf. Innov. 2013, 1, 162–172. [Google Scholar] [CrossRef]
- Qi, Y.; Xiang, B.; Tan, W.; Zhang, J. Hydrophobic surface modification of TiO2 nanoparticles for production of acrylonitrile-styrene-acrylate terpolymer/TiO2 composited cool materials. Appl. Surf. Sci. 2017, 419, 213–223. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Lyu, S. Facile Preparation of a robust and durable superhydrophobic coating using biodegradable lignin-coated cellulose nanocrystal particles. Materials 2017, 10, 1080. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Guo, Z.; Liu, G.; Gyimah, K.G.; Li, X.; Dong, H. A Rapid one-step process for fabrication of biomimetic superhydrophobic surfaces by pulse electrodeposition. Materials 2017, 10, 1229. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Zhang, J. Effects of content and surface hydrophobic modification of BaTiO3 on the cooling properties of ASA (acrylonitrile-styrene-acrylate copolymer). Appl. Surf. Sci. 2018, 427, 654–661. [Google Scholar] [CrossRef]
- Ge, B.; Zhang, Z.; Zhu, X.; Men, X.; Zhou, X. A superhydrophobic/superoleophilic sponge for the selective absorption oil pollutants from water. Colloid Surf. A 2014, 457, 397–401. [Google Scholar] [CrossRef]
- Men, X.; Shi, X.; Ge, B.; Li, Y.; Zhu, X.; Li, Y.; Zhang, Z. Novel transparent, liquid-repellent smooth surfaces with mechanical durability. Chem. Eng. J. 2016, 296, 458–465. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, K.; Cao, B.; Zhang, Z.; Wu, B.; Liang, L.; Chai, G.; Liu, A. Smart design of wettability-patterned gradients on substrate-independent coated surfaces to control unidirectional spreading of droplets. Soft Matter 2017, 13, 2995–3002. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.S.; Bhushan, B. Bioinspired, roughness-induced, water and oil super-philic and super-phobic coatings prepared by adaptable layer-by-layer technique. Sci. Rep. 2015, 5, 14030. [Google Scholar] [CrossRef] [PubMed]
- Hoshian, S.; Jokinen, V.; Franssila, S. Robust hybrid elastomer/metal-oxide superhydrophobic surfaces. Soft Matter 2016, 12, 6526–6535. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, H.; Niu, H.; Zhao, Y.; Xu, Z.; Lin, T. A Waterborne coating system for preparing robust, self-healing, superamphiphobic surfaces. Adv. Funct. Mater. 2017, 27, 1604261–1604268. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Niu, H.; Gestos, A.; Lin, T. Robust, Self-healing superamphiphobic fabrics prepared by two-step coating of fluoro-containing polymer, fluoroalkyl silane, and modified silica nanoparticles. Adv. Funct. Mater. 2013, 23, 1664–1670. [Google Scholar] [CrossRef]
- Zhang, D.; Li, H.; Chen, X.; Qian, H.; Li, X. Effect of Surface microstructures on hydrophobicity and barrier property of anticorrosive coatings prepared by soft lithography. Adv. Mater. Sci. Eng. 2014, 2014, 7. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Jing, G.; Prieto-López, L.O.; Deng, X.; Cui, J. Controlling the localization of liquid droplets in polymer matrices by evaporative lithography. Angew. Chem. Int. Ed. 2016, 55, 10681–10685. [Google Scholar] [CrossRef] [PubMed]
- Nokes, M.J.; Sharma, H.; Tu, R.; Kim, Y.M.; Chu, M.; Siddiqui, A.; Khine, M. Nanotextured Shrink wrap superhydrophobic surfaces by argon plasma etching. Materials 2016, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, Y.; Yang, J.; Tang, C.; Lv, J.; Liu, Y.; Mei, J.; Lau, W.M.; Hui, D. Fabrication of polydimethylsiloxane films with special surface wettability by 3d printing. Compos. Part. B Eng. 2017, 129, 58–65. [Google Scholar] [CrossRef]
- González Lazo, A.M.; Katrantzis, I.; Dalle Vacche, S.; Karasu, F.; Leterrier, Y. A facile in situ and uv printing process for bioinspired self-cleaning surfaces. Materials 2016, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; He, Z.; Wang, Q.; Chen, F.; Fu, Q. Self-assembly of PS-b-PDMS on a tunable PDMS template with nanoscale channels and enhanced anisotropic wetting. Langmuir 2015, 31, 4605–4611. [Google Scholar] [CrossRef] [PubMed]
- Stroj, S.; Kasemann, S.; Domke, M.; Piredda, G.; Zehetner, J.; Matylitskaya, V. Transparent superhydrophobic surfaces with high adhesion generated by the combination of femtosecond laser structuring and wet oxidation. Appl. Surf. Sci. 2017, 420, 550–557. [Google Scholar] [CrossRef]
- Bai, H.; Du, C.; Zhang, A.; Li, L. Breath figure arrays: Unconventional fabrications, functionalizations, and applications. Angew. Chem. Int. Ed. 2013, 52, 12240–12255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Bai, H.; Li, L. Breath figure: A nature-inspired preparation method for ordered porous films. Chem. Rev. 2015, 115, 9801–9868. [Google Scholar] [CrossRef] [PubMed]
- Böker, A.; Lin, Y.; Chiapperini, K.; Horowitz, R.; Thompson, M.; Carreon, V.; Xu, T.; Abetz, C.; Skaff, H.; Dinsmore, A.D.; et al. Hierarchical nanoparticle assemblies formed by decorating breath figures. Nature Mater. 2004, 3, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Meng, Z.; Liu, J.; Schlaich, C.; Yu, Z.; Deng, X. Breath figure lithography for the construction of a hierarchical structure in sponges and their applications to oil/water separation. J. Mater. Chem. A 2017, 5, 16369–16375. [Google Scholar] [CrossRef]
- Widawski, G.; Rawiso, M.; Francois, B. Self-organized honeycomb morphology of star-polymer polystyrene films. Nature 1994, 369, 387–389. [Google Scholar] [CrossRef]
- Connal, L.A.; Qiao, G.G. Honeycomb coated particles: Porous doughnuts, golf balls and hollow porous pockets. Soft Matter 2007, 3, 837–839. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, H.; Zhang, L.; Ran, T.; Zhang, D. Transparent self-cleaning lubricant-infused surfaces made with large-area breath figure patterns. Appl. Surf. Sci. 2015, 355, 1083–1090. [Google Scholar] [CrossRef]
- Zander, N.E.; Orticki, J.A.; Karikari, A.S.; Long, T.E.; Rawlett, A.M. Super-hydrophobic surfaces via micrometer-scale templated pillars. Chem. Mater. 2007, 19, 6145–6149. [Google Scholar] [CrossRef]
- He, Z.; Ma, M.; Xu, X.; Wang, J.; Chen, F.; Deng, H.; Wang, K.; Zhang, Q.; Fu, Q. Fabrication of superhydrophobic coating via a facile and versatile method based on nanoparticle aggregates. Appl. Surf. Sci. 2012, 258, 2544–2550. [Google Scholar] [CrossRef]
- He, Z.; Ma, M.; Lan, X.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Fu, Q. Fabrication of a transparent superamphiphobic coating with improved stability. Soft Matter 2011, 7, 6435–6443. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Bussonniere, A.; Bigdeli, M.B.; Chueh, D.-Y.; Liu, Q.; Chen, P.; Tsai, P.A. Universal wetting transition of an evaporating water droplet on hydrophobic micro- and nano-structures. Soft Matter 2017, 13, 978–984. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Shao, H.; He, Z.; Tang, C.; Yang, J.; Li, Y.; Wang, C.; Li, X.; Shuai, M.; Mei, J. Patternable Poly(chloro-p-xylylene) Film with Tunable Surface Wettability Prepared by Temperature and Humidity Treatment on a Polydimethylsiloxane/Silica Coating. Materials 2018, 11, 486. https://doi.org/10.3390/ma11040486
Yu Y, Shao H, He Z, Tang C, Yang J, Li Y, Wang C, Li X, Shuai M, Mei J. Patternable Poly(chloro-p-xylylene) Film with Tunable Surface Wettability Prepared by Temperature and Humidity Treatment on a Polydimethylsiloxane/Silica Coating. Materials. 2018; 11(4):486. https://doi.org/10.3390/ma11040486
Chicago/Turabian StyleYu, Yonglian, Hong Shao, Zhoukun He, Changyu Tang, Jian Yang, Yongsheng Li, Cong Wang, Xiuyun Li, Maobing Shuai, and Jun Mei. 2018. "Patternable Poly(chloro-p-xylylene) Film with Tunable Surface Wettability Prepared by Temperature and Humidity Treatment on a Polydimethylsiloxane/Silica Coating" Materials 11, no. 4: 486. https://doi.org/10.3390/ma11040486