Label-Free Electrochemical Detection of Vanillin through Low-Defect Graphene Electrodes Modified with Au Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Characterisations
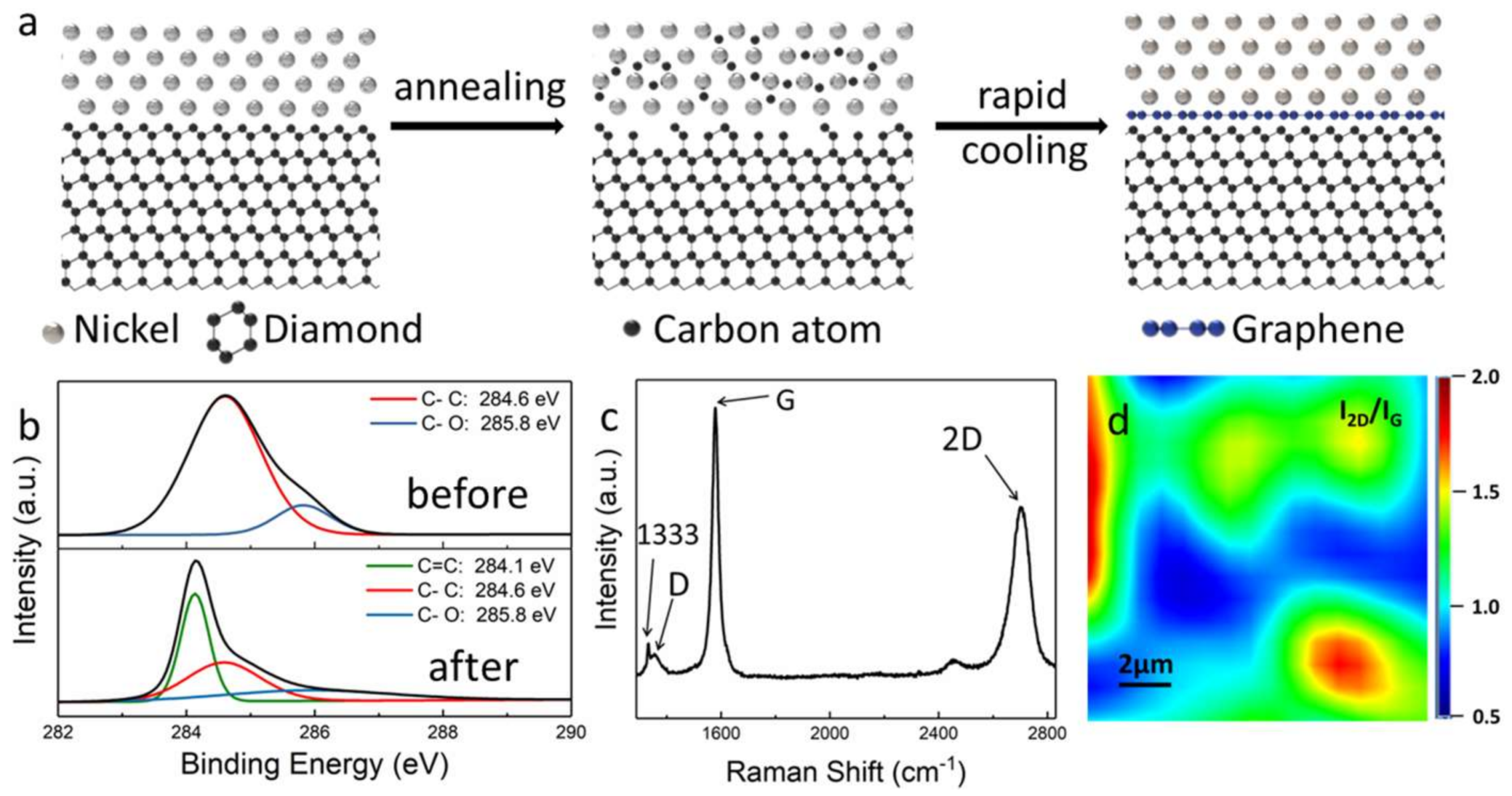
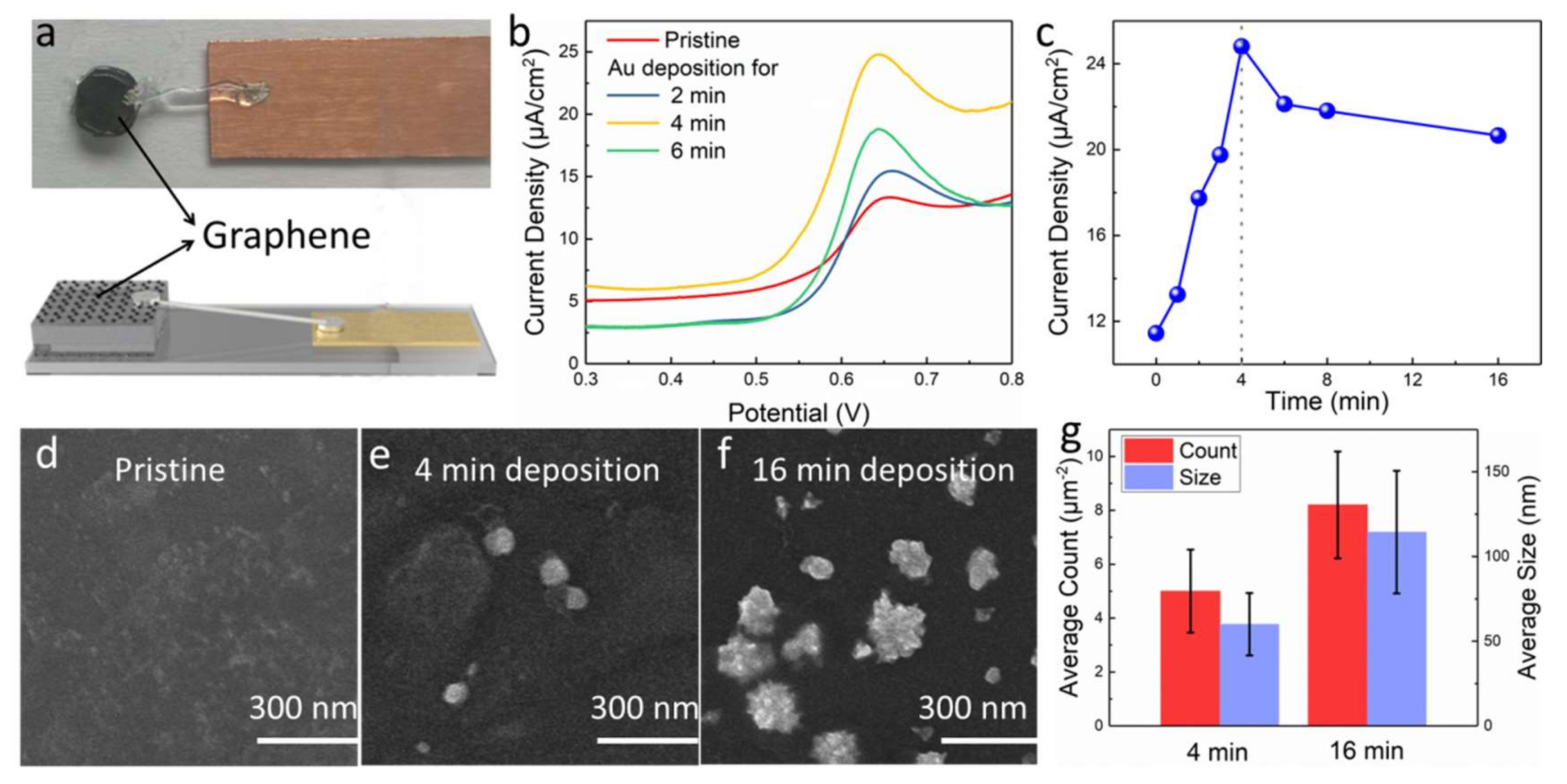
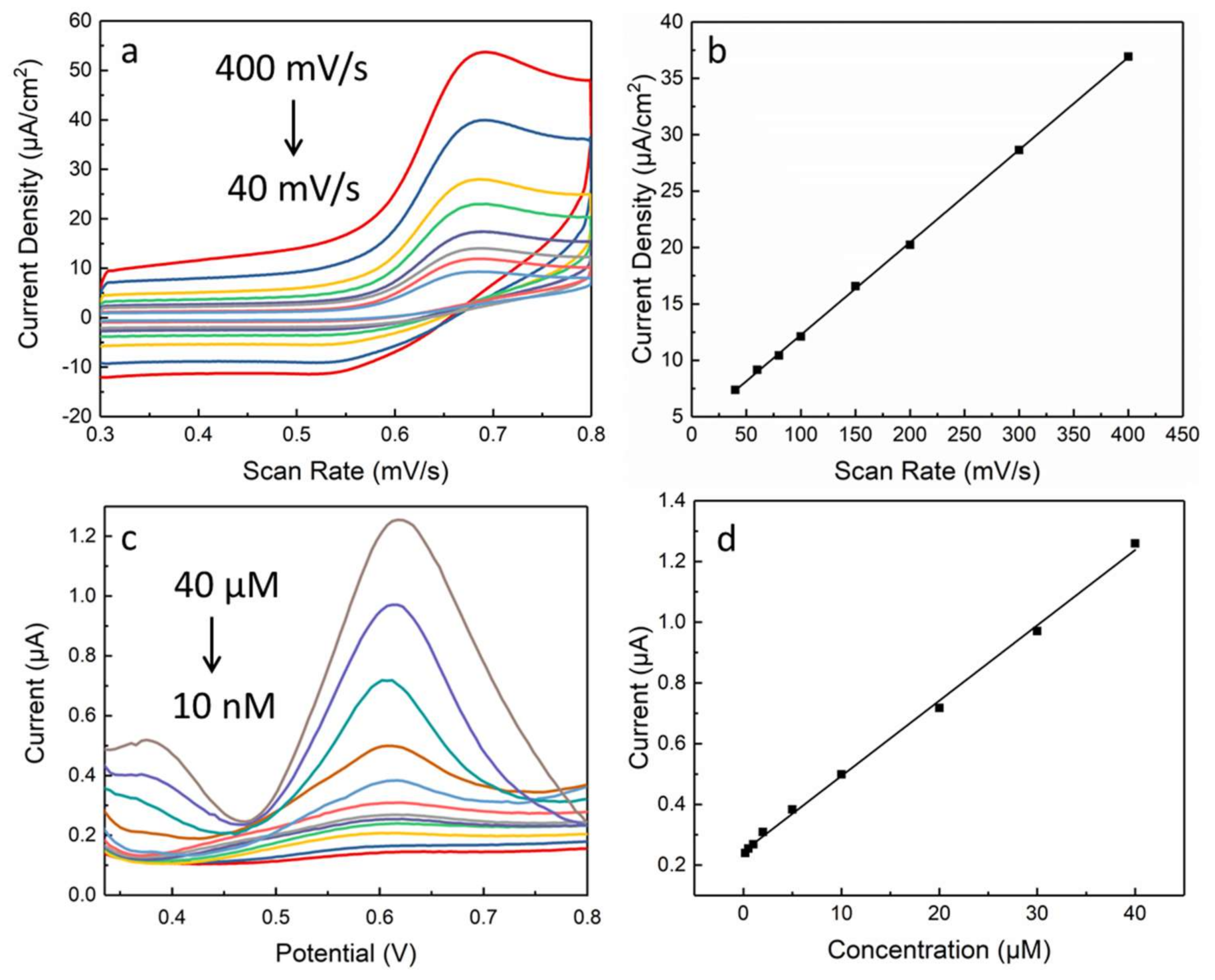
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anklam, E.; Gaglione, S.; Muller, A. Oxidation behaviour of vanillin in dairy products. Food Chem. 1997, 60, 43–51. [Google Scholar] [CrossRef]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Molecules of interest - Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Silva, T.R.; Brondani, D.; Zapp, E.; Vieira, I.C. Electrochemical Sensor Based on Gold Nanoparticles Stabilized in Poly(Allylamine hydrochloride) for Determination of Vanillin. Electroanalysis 2015, 27, 465–472. [Google Scholar] [CrossRef]
- Teissedre, P.L.; Waterhouse, A.L. Inhibition of oxidation of human low-density lipoproteins by phenolic substances in different essential oils varieties. J. Agric. Food Chem. 2000, 48, 3801–3805. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, Y.; Lu, D.; Wang, L.; Ma, D.; Ju, T.; Wu, M. Sensitive determination of vanillin based on an arginine functionalized graphene film. Anal. Methods 2014, 6, 1753–1758. [Google Scholar] [CrossRef]
- Abbasghorbani, M. Electrochemical Determination of Vanillin in Food Samples Using MgO/SWCNTs-ionic Liquid Modified Electrode. Int. J. Electrochem. Sci. 2017, 12, 11656–11665. [Google Scholar] [CrossRef]
- Sinha, A.K.; Sharma, U.K.; Sharma, N. A comprehensive review on vanilla flavor: Extraction, isolation and quantification of vanillin and others constituents. Int. J. Food Sci. Nutr. 2008, 59, 299–326. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, L.; Zhao, F.; Zeng, B. A vanillin electrochemical sensor based on molecularly imprinted poly(1-vinyl-3-octylimidazole hexafluoride phosphorus) -multi-walled carbon nanotubes@polydopamine-carboxyl single-walled carbon nanotubes composite. Sens. Actuators B-Chem. 2017, 239, 481–487. [Google Scholar] [CrossRef]
- Barho, F.B.; Gonzalez-Posada, F.; Milla, M.J.; Bomers, M.; Cerutti, L.; Tournie, E.; Taliercio, T. Highly doped semiconductor plasmonic nanoantenna arrays for polarization selective broadband surface-enhanced infrared absorption spectroscopy of vanillin. Nanophotonics 2018, 7, 507–516. [Google Scholar] [CrossRef]
- Butehorn, U.; Pyell, U. Micellar electrokinetic chromatography as a screening method for the analysis of vanilla flavourings and vanilla extracts. J. Chromatogr. A 1996, 736, 321–332. [Google Scholar] [CrossRef]
- Negishi, O.; Ozawa, T. Determination of hydroxycinnamic acids, hydroxybenzoic acids, hydroxybenzaldehydes, hydroxybenzyl alcohols and their glucosides by high-performance liquid chromatography. J. Chromatogr. A 1996, 756, 129–136. [Google Scholar] [CrossRef]
- Alpar, N.; Yardim, Y.; Senturk, Z. Selective and simultaneous determination of total chlorogenic acids, vanillin and caffeine in foods and beverages by adsorptive stripping voltammetry using a cathodically pretreated boron-doped diamond electrode. Sens. Actuators B-Chem. 2018, 257, 398–408. [Google Scholar] [CrossRef]
- Peng, J.; Hou, C.; Hu, X. A Graphene-Based Electrochemical Sensor for Sensitive Detection of Vanillin. Int. J. Electrochem. Sci. 2012, 7, 1724–1733. [Google Scholar]
- Zheng, D.; Hu, C.; Gan, T.; Dang, X.; Hu, S. Preparation and application of a novel vanillin sensor based on biosynthesis of Au-Ag alloy nanoparticles. Sens. Actuators B-Chem. 2010, 148, 247–252. [Google Scholar] [CrossRef]
- Chen, C.Y.; Dai, D.; Chen, G.X.; Yu, J.H.; Nishimura, K.; Lin, C.T.; Jiang, N.; Zhan, Z.L. Rapid growth of single-layer graphene on the insulating substrates by thermal CVD. Appl. Surf. Sci. 2015, 346, 41–45. [Google Scholar] [CrossRef]
- Li, X.; Tao, L.; Chen, Z.; Fang, H.; Li, X.; Wang, X.; Xu, J.B.; Zhu, H. Graphene and related two-dimensional materials: Structure-property relationships for electronics and optoelectronics. Appl. Phys. Rev. 2017, 4, 021306. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Wang, K.; Cao, A.; Wei, J.; Li, C.; Jia, Y.; Li, Z.; Li, X.; Wu, D. Graphene-On-Silicon Schottky Junction Solar Cells. Adv. Mater. 2010, 22, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, D.; Wu, Y.; Yuan, Q.; Guo, L.; Dai, D.; Xu, Y.; Zhao, P.; Jiang, N.; Lin, C.-T. High quality graphene films with a clean surface prepared by an UV/ozone assisted transfer process. J. Mater. Chem. C 2017, 5, 1880–1884. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Z.; Chen, G.; Dai, D.; Sun, H.; Dai, W.; Jiang, N.; Jiang, Y.H.; Lin, C.T. A study of the growth-time effect on graphene layer number based on a Cu-Ni bilayer catalyst system. RSC Adv. 2016, 6, 23956–23960. [Google Scholar] [CrossRef]
- Loan, P.T.K.; Wu, D.; Ye, C.; Li, X.; Tra, V.T.; Wei, Q.; Fu, L.; Yu, A.; Li, L.J.; Lin, C.T. Hall effect biosensors with ultraclean graphene film for improved sensitivity of label-free DNA detection. Biosens. Bioelectron. 2018, 99, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Zhao, F.; Zeng, B. Sensitive voltammetric determination of vanillin with an AuPd nanoparticles-graphene composite modified electrode. Food Chem. 2014, 151, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.S.H.; Fu, L.; Mahon, P.; Lai, G.; Yu, A. Fabrication of beta-Cyclodextrin-Functionalized Reduced Graphene Oxide and Its Application for Electrocatalytic Detection of Carbendazim. Electrocatalysis 2016, 7, 411–419. [Google Scholar] [CrossRef]
- Zhao, H.; Ji, X.; Wang, B.; Wang, N.; Li, X.; Ni, R.; Ren, J. An ultra-sensitive acetylcholinesterase biosensor based on reduced graphene oxide-Au nanoparticles-beta-cyclodextrin/Prussian blue-chitosan nanocomposites for organophosphorus pesticides detection. Biosens. Bioelectron. 2015, 65, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, J.; Yang, G.; Wang, C.; Zhu, J.J. Direct electrochemistry and electrocatalysis of hemoglobin based on poly(diallyldimethylammonium chloride) functionalized graphene sheets/room temperature ionic liquid composite film. Electrochem. Commun. 2010, 12, 402–405. [Google Scholar] [CrossRef]
- Sun, H.; Li, X.; Li, Y.; Chen, G.; Liu, Z.; Alam, F.E.; Dai, D.; Li, L.; Tao, L.; Xu, J.B.; et al. High-Quality Monolithic Graphene Films via Laterally Stitched Growth and Structural Repair of Isolated Flakes for Transparent Electronics. Chem. Mater. 2017, 29, 7808–7815. [Google Scholar] [CrossRef]
- He, D.; Li, S.; Zhang, P.; Luo, H. CVD graphene incorporating polymerized L-cysteine as an electrochemical sensing platform for simultaneous determination of dopamine and ascorbic acid. Anal. Methods 2017, 9, 6689–6697. [Google Scholar] [CrossRef]
- Lin, C.C.; Huang, S.Y. Synthesis of Graphene by CVD and its Nitrogen-plasma Treatment for Electrodes in Electrochemical Capacitor: Significance of Cooling and Plasma Conditions. Electrochemistry 2016, 84, 506–510. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J.; Tuan, N.T.; Jeong, H.; Lee, S.H.; Kim, N.H.; Lee, J.H. Enhanced electrocatalytic performance of an ultrafine AuPt nanoalloy framework embedded in graphene towards epinephrine sensing. Biosens. Bioelectron. 2017, 89, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Guo, S.; Zhu, C.; Zhai, Y.; Wang, E. Self-Assembly of Cationic Polyelectrolyte-Functionalized Graphene Nanosheets and Gold Nanoparticles: A Two-Dimensional Heterostructure for Hydrogen Peroxide Sensing. Langmuir 2010, 26, 11277–11282. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Gracio, J. Surface Modification of Graphene Nanosheets with Gold Nanoparticles: The Role of Oxygen Moieties at Graphene Surface on Gold Nucleation and Growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Hong, W.; Bai, H.; Xu, Y.; Yao, Z.; Gu, Z.; Shi, G. Preparation of Gold Nanoparticle/Graphene Composites with Controlled Weight Contents and Their Application in Biosensors. J. Phys. Chem. C 2010, 114, 1822–1826. [Google Scholar] [CrossRef]
- Hu, Y.; Jin, J.; Wu, P.; Zhang, H.; Cai, C. Graphene-gold nanostructure composites fabricated by electrodeposition and their electrocatalytic activity toward the oxygen reduction and glucose oxidation. Electrochim. Acta 2010, 56, 491–500. [Google Scholar] [CrossRef]
- Wu, J.; Xu, H.; Zhang, J. Raman Spectroscopy of Graphene. Acta Chim. Sin. 2014, 72, 301–318. [Google Scholar] [CrossRef]
- Ueda, K.; Aichi, S.; Asano, H. Direct formation of graphene layers on diamond by high-temperature annealing with a Cu catalyst. Diam. Relat. Mater. 2016, 63, 148–152. [Google Scholar] [CrossRef]
- Pandey, P.C.; Pandey, A.K. Size-dependence enhancement in electrocatalytic activity of NiHCF-gold nanocomposite: potential application in electrochemical sensing. Analyst 2012, 137, 3306–3313. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Yuan, Q.; Ye, C.; Guo, P.; Du, S.; Lai, G.; Yu, A.; Jiang, N.; Fu, L.; Lin, C.-T.; et al. Label-Free Electrochemical Detection of Vanillin through Low-Defect Graphene Electrodes Modified with Au Nanoparticles. Materials 2018, 11, 489. https://doi.org/10.3390/ma11040489
Gao J, Yuan Q, Ye C, Guo P, Du S, Lai G, Yu A, Jiang N, Fu L, Lin C-T, et al. Label-Free Electrochemical Detection of Vanillin through Low-Defect Graphene Electrodes Modified with Au Nanoparticles. Materials. 2018; 11(4):489. https://doi.org/10.3390/ma11040489
Chicago/Turabian StyleGao, Jingyao, Qilong Yuan, Chen Ye, Pei Guo, Shiyu Du, Guosong Lai, Aimin Yu, Nan Jiang, Li Fu, Cheng-Te Lin, and et al. 2018. "Label-Free Electrochemical Detection of Vanillin through Low-Defect Graphene Electrodes Modified with Au Nanoparticles" Materials 11, no. 4: 489. https://doi.org/10.3390/ma11040489
APA StyleGao, J., Yuan, Q., Ye, C., Guo, P., Du, S., Lai, G., Yu, A., Jiang, N., Fu, L., Lin, C.-T., & Chee, K. W. A. (2018). Label-Free Electrochemical Detection of Vanillin through Low-Defect Graphene Electrodes Modified with Au Nanoparticles. Materials, 11(4), 489. https://doi.org/10.3390/ma11040489






