Hydroxyapatite and Other Calcium Phosphates for the Conservation of Cultural Heritage: A Review
Abstract
1. Introduction
2. Definition of the Treatment Parameters
2.1. Nature of the Phosphate Precursor
2.2. pH of the Phosphate Solution
2.3. Concentration of the Phosphate Solution
2.4. Duration of the Reaction
2.5. Calcium Ion Addition
2.6. Other Ionic Additions
2.7. Organic Additions
2.8. Biomimetic Routes
2.9. Nature of the New Calcium Phosphates
3. Marble
3.1. Protection from Corrosion
Combination of Phosphate and Oxalate Treatments
3.2. Consolidation
3.3. Ability to Arrest and Prevent Bowing of Thin Slabs
3.4. Self-Cleaning Ability
3.5. Anti-Fungal Ability
3.6. Compatibility
3.6.1. Colour Change
3.6.2. Porosity and Pore Size Distribution
3.6.3. Water and Water Vapour Transport Properties
3.7. Durability
3.7.1. Dissolution in Rain
3.7.2. Thermal Weathering
3.7.3. Biodeterioration
4. Limestone
4.1. Consolidation
4.2. Effectiveness as a Coupling Agent for Silicate Consolidants
Combination of TEOS and Hydroxyapatite
4.3. Compatibility
4.3.1. Colour Change
4.3.2. Porosity and Pore Size Distribution
4.3.3. Water and Water Vapour Transport Properties
4.4. Durability
4.4.1. Wetting-Drying Cycles
4.4.2. Freeze-Thaw Cycles
4.4.3. Salt Crystallization Cycles
5. Other Substrates
5.1. Sandstone
5.2. Salt-Bearing Stone
5.3. Sulphated Stone
Ca10(PO4)6(OH)2 + 10(NH4)2SO4 + H2O + 4HPO2−(aq).
5.4. Gypsum Stuccoes
5.5. Concrete
5.6. Archaeological Wall Paintings
5.7. Archaeological Bones
5.8. Paper
6. Field Studies
7. Conclusions
- the DAP solution has low viscosity, so it is able to penetrate deeply into weathered marble (>20 mm) and limestone (>25 mm), while ammonium oxalate is generally affected by low penetration (1–2 mm);
- the phosphate solution causes significant mechanical strengthening after curing for a short time (24–48 h), while curing of ethyl silicate requires more than 6 months;
- the newly formed calcium phosphates do not significantly alter porosity and pore size distribution and leave the treated stone hydrophilic, so that minor changes in water absorption, drying rate and water vapour permeability are experienced. On the contrary, ethyl silicate leaves the treated stone hydrophobic for several months and often causes significant alterations in the pore system;
- as a consequence of the reduced alterations in microstructural and physical properties, stones treated by the phosphate treatment exhibit good durability to heating-cooling cycles, freeze-thaw cycles and salt crystallization cycles; on the contrary, worsened durability was experienced by marble treated with ammonium oxalate and limestone treated with ethyl silicate;
- the phosphate treatment does not cause significant colour change (often below the human eye detection limit and generally below the threshold commonly accepted for conservation treatments); on the contrary, ethyl silicate can cause unacceptable darkening of marble;
- no toxic compound is involved in the phosphate treatment, whereas ethyl silicate is often applied in organic solvents (e.g., white spirit) that can be toxic for human health and the environment;
- because pores are not significantly occluded and the stone remains hydrophilic after treatment, DAP-treated stone can be retreated in the future by either the same treatment or a different one.
- the use of organic additions or templates to optimize marble surface coverage: a few calcite grains, presumably having unfavourable crystallographic orientation, were found to remain uncoated by the calcium phosphate deposits, even when ethanol and isopropanol were added to improve the coating formation. Different organic additions and/or the use of templates should be investigated to favour nucleation on the calcite surface.
- the role of magnesium in the substrate: even small amounts of magnesium have been found to significantly alter formation of calcium phosphates (possibly leading to formation of magnesium phosphates or magnesium ammonium phosphates), hence the influence of magnesium in the substrate should be systematically investigated and methods to prevent its negative influence should be developed. This will be of interest for several different magnesium-containing substrates, ranging from dolomitic marble to ivory.
- the effectiveness and the durability of coatings functionalized with nanoparticles: in addition to combination with nanoTiO2 to achieve self-cleaning ability and with nanosilver to achieve anti-fungal activity, further functionalization of the calcium phosphate coatings by nanoparticle addition is worthy of investigation. In the case of silver nanoparticles and strontium-, barium- and silver-substituted HAP for anti-fungal activity, the effectiveness, the compatibility and the durability in real practical applications should also be investigated.
- the effectiveness and the durability of aluminium phosphates: preliminary results have indicated the high potential of aluminium phosphates for consolidation of marble, thanks to the very good match in lattice parameters between calcite and AlPO4 (the mineral berlinite). Future research should be devoted to further optimize the treatment conditions (in terms of precursors, pH, organic additions, etc.) and to assess the treatment durability.
Acknowledgments
Conflicts of Interest
References
- Sassoni, E.; Naidu, S.; Scherer, G.W. The use of hydroxyapatite as a new inorganic consolidant for damaged carbonate stones. J. Cult. Herit. 2011, 12, 346–355. [Google Scholar] [CrossRef]
- Naidu, S.; Sassoni, E.; Scherer, G.W. New treatment for corrosion-resistant coatings for marble and consolidation of limestone. In Jardins de Pierres—Conservation of Stone in Parks, Gardens and Cemeteries; Stefanaggi, M., Vergès-Belmin, V., Eds.; XL Print: Paris, France, 2011; pp. 289–294. [Google Scholar]
- Matteini, M.; Rescic, S.; Fratini, F.; Botticelli, G. Ammonium phosphates as consolidating agents for carbonatic stone materials used in architecture and cultural heritage: Preliminary research. Int. J. Archit. Herit. 2011, 5, 717–736. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, B.; Liu, Y.; Wei, G.; Zhang, H.; Chen, W.; Xu, Z. Biomimic conservation of weathered calcareous stones by apatite. New J. Chem. 2011, 35, 887–892. [Google Scholar] [CrossRef]
- Yang, F.W.; Liu, Y.; Zhu, Y.C.; Long, S.J.; Zuo, G.F.; Wang, C.Q.; Guo, F.; Zhang, B.J.; Jiang, S.W. Conservation of weathered historic sandstone with biomimetic apatite. Chin. Sci. Bull. 2012, 57, 2171–2176. [Google Scholar] [CrossRef]
- Sassoni, E.; Franzoni, E.; Pigino, B.; Scherer, G.W.; Naidu, S. Consolidation of calcareous and siliceous sandstones by hydroxyapatite: Comparison with a TEOS-based consolidant. J. Cult. Herit. 2013, 14, e103–e108. [Google Scholar] [CrossRef]
- Snethlage, R.; Gruber, C.; Tucic, V.; Wendler, E. Transforming gypsum into calcium phosphate—The better way to preserve lime paint layers on natural stone. In Stone Consolidation in Cultural Heritage; Mimoso, J.M., Delgado Rodrigues, J., Eds.; LNEC: Lisbon, Portugal, 2008; pp. 1–13. [Google Scholar]
- Yang, F.; Liu, Y.; Zuo, G.; Wang, X.; Hua, P.; Ma, Q.; Dong, G.; Yue, Y.; Zhang, B. Hydroxyapatite conversion layer for the preservation of surface gypsification marble relics. Corros. Sci. 2014, 88, 6–9. [Google Scholar] [CrossRef]
- Molina, E.; Rueda-Quero, L.; Benavente, D.; Burgos-Cara, A.; Ruiz-Agudo, E.; Cultrone, G. Gypsum crust as a source of calcium for the consolidation of carbonate stones using a calcium phosphate-based consolidant. Construct. Build. Mater. 2017, 143, 298–311. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E.; Scherer, G.W. Conversion of calcium sulfate dihydrate into calcium phosphates as a route for conservation of gypsum stuccoes and sulfated marble. Construct. Build. Mater. 2018, 170, 290–301. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Scherer, G.W.; Franzoni, E. Preliminary study on the use of ammonium phosphate for the conservation of marble-imitating gypsum-stuccoes. In Proceedings of the 4th Historic Mortars Conference HMC2016, Santorini (GR); Papayianni, I., Stefanidou, M., Pachta, V., Eds.; Aristotle University of Thessaloniki: Thessaloniki, Greece, 2016; pp. 391–398. [Google Scholar]
- Turner, R.J.; Renshaw, J.C.; Hamilton, A. Biogenic Hydroxyapatite: A New Material for the Preservation and Restoration of the Built Environment. ACS Appl. Mater. Interfaces 2017, 9, 31401–31410. [Google Scholar] [CrossRef] [PubMed]
- Balonis-Sant, M.; Ma, X.; Kakoulli, I. Preliminary results on biomimetic methods based on soluble ammonium phosphate precursors for the consolidation of archaeological wall paintings. In Archaeological Chemistry VIII; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1147, pp. 419–447. [Google Scholar] [CrossRef]
- North, A.; Balonis, M.; Kakoulli, I. Biomimetic hydroxyapatite as a new consolidating agent for archaeological bone. Stud. Conserv. 2016, 61, 146–161. [Google Scholar] [CrossRef]
- Yang, F.; He, D.; Liu, Y.; Li, N.; Wang, Z.; Ma, Q.; Dong, G. Conservation of bone relics using hydroxyapatite as protective material. Appl. Phys. A 2016, 122, 479. [Google Scholar] [CrossRef]
- Ion, R.-M.; Doncea, S.M.; Ion, M.-L.; Rǎdiţoiu, V.; Amǎriuţei, V. Surface investigations of old book paper treated with hydroxyapatite nanoparticles. Appl. Surf. Sci. 2013, 285, 27–32. [Google Scholar] [CrossRef]
- Winkler, E.M. Stone in Architecture; Springer: Berlin, Germany, 1997. [Google Scholar]
- Siegesmund, S.; Ullemeyer, K.; Weiss, T.; Tschegg, E.K. Physical weathering of marbles caused by anisotropic thermal expansion. Int. J. Earth Sci. 2000, 89, 170–182. [Google Scholar] [CrossRef]
- Scherer, G.W. Crystallization in pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Baglioni, P.; Carretti, E.; Chelazzi, D. Nanomaterials in art conservation. Nat. Nanotechnol. 2015, 10, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Scherer, G.W.; Wheeler, G.S. Silicate consolidants for stone. Key Eng. Mater. 2009, 391, 1–25. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates: Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef] [PubMed]
- Matteini, M. Inorganic treatments for the consolidation and protection of stone artefacts. Conserv. Sci. Cult. Herit. 2008, 8, 13–27. [Google Scholar]
- Doherty, B.; Pamplona, M.; Selvaggi, R.; Miliani, C.; Matteini, M.; Sgamellotti, A.; Brunetti, B. Efficiency and resistance of the artificial oxalate protection treatment on marble against chemical weathering. Appl. Surf. Sci. 2007, 253, 4477–4484. [Google Scholar] [CrossRef]
- Posner, A.S.; Perloff, A.; Diorio, A.F. Refinement of the hydroxyapatite structure. Acta Crystallogr. 1958, 11, 308–309. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Crystal Structures-Volume 1; Interscience Publishers: New York, NY, USA, 1963. [Google Scholar]
- Patnaik, P. Handbook of Inorganic Chemical Compounds, 1st ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- McComas, W.H.; Rieman, W. The Solubility of Calcium Oxalate Monohydrate in Pure Water and Various Neutral Salt Solutions at 25°. J. Am. Chem. Soc. 1942, 64, 2946–2947. [Google Scholar] [CrossRef]
- Brady, P.V. Physics and chemistry of mineral surfaces; CRC Press: New York, NY, USA, 1996. [Google Scholar]
- Harouiya, N.; Chaïrat, C.; Köhler, S.J.; Gout, R.; Oelkers, E.H. The dissolution kinetics and apparent solubility of natural apatite in closed reactors at temperatures from 5 to 50 °C and pH from 1 to 6. Chem. Geol. 2007, 244, 554–568. [Google Scholar] [CrossRef]
- Mcdowell, H.; Gregory, T.M.; Brown, W.E. Solubility of Ca5(PO4)3OH in the system Ca(OH)2-H3PO4-H2O at 5, 15, 25, and 37 °C. J. Res. Natl. Bur. Stand. A 1977, 81, 273–281. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, M.; Hatta, J.; Shimada, E.; Ikuma, Y.; Yoshimura, M.; Monma, H. AFM analysis of initial stage of reaction between calcite and phosphate. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2004, 111, 226–231. [Google Scholar] [CrossRef]
- Naidu, S.; Scherer, G.W. Nucleation, growth and evolution of calcium phosphate films on calcite. J. Colloid Interface Sci. 2014, 435, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Possenti, E.; Colombo, C.; Bersani, D.; Bertasa, M.; Botteon, A.; Conti, C.; Lottici, P.P.; Realini, M. New insight on the interaction of diammonium hydrogen phosphate conservation treatment with carbonatic substrates: A multi-analytical approach. Microchem. J. 2016, 127, 79–86. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineers’ Handbook; McGraw-Hill: London, UK, 1998; ISBN 978-0-07-115982-1. [Google Scholar]
- Graziani, G.; Sassoni, E.; Franzoni, E.; Scherer, G.W. Hydroxyapatite coatings for marble protection: Optimization of calcite covering and acid resistance. Appl. Surf. Sci. 2016, 368, 241–257. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E.; Scherer, G.W. Calcium phosphate coatings for marble conservation: Influence of ethanol and isopropanol addition to the precipitation medium on the coating microstructure and performance. Corros. Sci. 2018, 136, 255–267. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E.; Scherer, G.W. Some Recent Findings on Marble Conservation by Aqueous Solutions of Diammonium Hydrogen Phosphate. MRS Adv. 2017, 2, 2021–2026. [Google Scholar] [CrossRef][Green Version]
- Evans, A.G.; Drory, M.D.; Hu, M.S. The cracking and decohesion of thin films. J. Mater. Res. 1988, 3, 1043–1049. [Google Scholar] [CrossRef]
- Lerner, E.; Azoury, R.; Sarig, S. Rapid precipitation of apatite from ethanol-water solution. J. Cryst. Growth 1989, 97, 725–730. [Google Scholar] [CrossRef]
- Franzoni, E.; Sassoni, E.; Graziani, G. Brushing, poultice or immersion? The role of the application technique on the performance of a novel hydroxyapatite-based consolidating treatment for limestone. J. Cult. Herit. 2015, 16, 173–184. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E. Repair of sugaring marble by ammonium phosphate: Comparison with ethyl silicate and ammonium oxalate and pilot application to historic artifact. Mater. Des. 2015, 88, 1145–1157. [Google Scholar] [CrossRef]
- Sassoni, E.; Andreotti, S.; Scherer, G.W.; Franzoni, E.; Siegesmund, S. Bowing of marble slabs: Can the phenomenon be arrested and prevented by inorganic treatments? Environ. Earth Sci. 2018, in press. [Google Scholar]
- Sassoni, E.; D’Amen, E.; Roveri, N.; Scherer, G.W.; Franzoni, E. Durable self-cleaning coatings for architectural surfaces by incorporation of TiO2 nanoparticles into hydroxyapatite films. Materials 2018, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Balonis, M.; Pasco, H.; Toumazou, M.; Counts, D.; Kakoulli, I. Evaluation of hydroxyapatite effects for the consolidation of a Hellenistic-Roman rock-cut chamber tomb at Athienou-Malloura in Cyprus. Construct. Build. Mater. 2017, 150, 333–344. [Google Scholar] [CrossRef]
- Sassoni, E.; Franzoni, E.; Scherer, G.W.; Naidu, S. Consolidation of a porous limestone by means of a new treatment based on hydroxyapatite. In 12th International Congress on Deterioration and Conservation of Stone; Wheeler, G., Ed.; Columbia University: New York, NY, USA, 2012; pp. 1–11. [Google Scholar]
- Naidu, S.; Liu, C.; Scherer, G.W. Hydroxyapatite-based consolidant and the acceleration of hydrolysis of silicate-based consolidants. J. Cult. Herit. 2015, 16, 94–101. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E. An innovative phosphate-based consolidant for limestone. Part 1: Effectiveness and compatibility in comparison with ethyl silicate. Construct. Build. Mater. 2016, 102, 918–930. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E. An innovative phosphate-based consolidant for limestone. Part 2: Durability in comparison with ethyl silicate. Construct. Build. Mater. 2016, 102, 931–942. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Scherer, G.W.; Franzoni, E. Penetration depth and redistribution of an aqueous ammonium phosphate solution used for porous limestone consolidation by brushing and immersion. Construct. Build. Mater. 2017, 148, 571–578. [Google Scholar] [CrossRef]
- Naidu, S.; Blair, J.; Scherer, G.W. Acid-resistant coatings on marble. J. Am. Ceram. Soc. 2016, 99, 3421–3428. [Google Scholar] [CrossRef]
- Scherer, G.W.; Sassoni, E. Mineral Consolidants. In Proceedings of the International RILEM Conference Materials, Systems and Structures in Civil Engineering 2016—Segment on Historical Masonry; Rörig-Dalgaard, I., Ioannou, I., Eds.; Technical University of Denmark: Lyngby, Denmark, 2016; pp. 1–10. [Google Scholar]
- Yang, F.W.; Liu, Y.; Zuo, G.F.; Zhu, Y.C.; Zhang, B.J.; Hua, P.N. Biomimetic fluorapatite films for conservation of historic calcareous stones. Chin. Sci. Bull. 2012, 57, 1590–1594. [Google Scholar] [CrossRef]
- Pasarín, I.S.; Yang, M.; Bovet, N.; Glyvradal, M.; Nielsen, M.M.; Bohr, J.; Feidenhansâ, R.; Stipp, S.L.S. Molecular ordering of ethanol at the calcite surface. Langmuir 2012, 28, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- Okhrimenko, D.V.; Nissenbaum, J.; Andersson, M.P.; Olsson, M.H.M.; Stipp, S.L.S. Energies of the adsorption of functional groups to calcium carbonate polymorphs: The importance of -OH and -COOH groups. Langmuir 2013, 29, 11062–11073. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, D. The use of CTAB as an addition of DAP for improvement resisting acid rain on limestone. Appl. Surf. Sci. 2017, 422, 1059–1066. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, B.J. Synthesis and characterization of a novel biomaterial for the conservation of historic stone buildings and sculptures. Mater. Sci. Forum 2011, 675, 317–320. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y. Artificial hydroxyapatite film for the conservation of outdoor marble artworks. Mater. Lett. 2014, 124, 201–203. [Google Scholar] [CrossRef]
- Sassoni, E.; Franzoni, E. Sugaring marble in the Monumental Cemetery in Bologna (Italy): Characterization of naturally and artificially weathered samples and first results of consolidation by hydroxyapatite. Appl. Phys. A 2014, 117, 1893–1906. [Google Scholar] [CrossRef]
- Osticioli, I.; Botticelli, G.; Matteini, P.; Siano, S.; Pini, R.; Matteini, M. Micro-Raman analysis on the combined use of ammonium oxalate and ammonium phosphate for the consolidation and protection of carbonate stone artifacts. J. Raman Spectrosc. 2017, 48, 966–971. [Google Scholar] [CrossRef]
- Barriuso, B.C.; Botticelli, G.; Cuzman, O.A.; Osticioli, I.; Tiano, P.; Matteini, M. Conservation of calcareous stone monuments: Screening different diammonium phosphate based formulations for countering phototrophic colonization. J. Cult. Herit. 2017, 27, 97–106. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Scherer, G.W.; Franzoni, E. Resistance to simulated rain of hydroxyapatite- and calcium oxalate-based coatings for protection of marble against corrosion. Corros. Sci. 2017, 127, 168–174. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E.; Scherer, G.W. Consolidation of sugaring marble by hydroxyapatite: Some recent developments on producing and treating decayed samples. In Science and Art: A Future for Stone: Proceedings of the 13th International Congress on the Deterioration and Conservation of Stone; Hughes, J., Howind, T., Eds.; University of the West of Scotland: Paisley, UK, 2016; Volume 2, pp. 947–954. [Google Scholar]
- Sassoni, E.; Graziani, G.; Franzoni, E.; Scherer, G.W. New method for controllable accelerated aging of marble: Use for testing of consolidants. J. Am. Ceram. Soc. 2018. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Franzoni, E.; Sassoni, E.; Scherer, G.W.; Naidu, S. Artificial weathering of stone by heating. J. Cult. Herit. 2013, 14, e85–e93. [Google Scholar] [CrossRef]
- Wheeler, G. Alkoxysilanes and the Consolidation of Stone; Research in conservation; The Getty Conservation Institute: Los Angeles, CA, USA, 2005. [Google Scholar]
- Wheeler, G. Sol-Gel Science and Cultural Heritage. In Handbook of Sol-Gel Science and Technology; Klein, L., Ed.; Springer: Berlin, Germany, 2016; pp. 1–18. [Google Scholar]
- Sassoni, E.; Franzoni, E. Influence of porosity on artificial deterioration of marble and limestone by heating. Appl. Phys. A 2014, 115, 809–816. [Google Scholar] [CrossRef]
- Joseph Nathanael, A.; Mangalaraj, D.; Chen, P.C.; Ponpandian, N. Mechanical and photocatalytic properties of hydroxyapatite/titania nanocomposites prepared by combined high gravity and hydrothermal process. Compos. Sci. Technol. 2010, 70, 419–426. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Todorova, N.; Romanos, G.; Vaimakis, T.; Dillert, R.; Bahnemann, D.; Trapalis, C. Composite hydroxyapatite/TiO2 materials for photocatalytic oxidation of NOx. Mater. Sci. Eng. B 2012, 177, 1046–1052. [Google Scholar] [CrossRef]
- Anmin, H.; Tong, L.; Ming, L.; Chengkang, C.; Huiqin, L.; Dali, M. Preparation of nanocrystals hydroxyapatite/TiO2 compound by hydrothermal treatment. Appl. Catal. B Environ. 2006, 63, 41–44. [Google Scholar] [CrossRef]
- Fierăscu, I.; Fierăscu, R.C.; Ion, R.M.; Radovici, C. Synthesized apatitic materials for artefacts protection against biodeterioration. Rom. J. Mater. 2014, 44, 292–297. [Google Scholar]
- Fierăscu, I.; Fierăscu, R.C.; Somoghi, R.; Ion, R.M.; Moanta, A.; Avramescu, S.M.; Damian, C.M.; Ditu, L.M. Tuned apatitic materials: Synthesis, characterization and potential antimicrobial applications. Appl. Surf. Sci. 2018, 438, 127–135. [Google Scholar] [CrossRef]
- Sharma, G. Color fundamentals for digital imaging. In Digital Color Imagining Handbook; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Sassoni, E.; Franzoni, E. Consolidation of Carrara marble by hydroxyapatite and behaviour after thermal ageing. In Built Heritage: Monitoring Conservation Management, Research for Development; Springer: Berlin, Germany, 2015; pp. 379–389. [Google Scholar]
- Sassoni, E.; Graziani, G.; Ridolfi, G.; Bignozzi, M.C.; Franzoni, E. Thermal behavior of Carrara marble after consolidation by ammonium phosphate, ammonium oxalate and ethyl silicate. Mater. Des. 2017, 120, 345–353. [Google Scholar] [CrossRef]
- Sassoni, E.; Naidu, S.; Scherer, G.W. Preliminary results of the use of hydroxyapatite as a consolidant for carbonate stones. In Materials Research Society Symposium Proceedings; Cambridge University Press: Cambridge, UK, 2011; Volume 1319, pp. 189–195. [Google Scholar]
- Maravelaki-Kalaitzaki, P.; Kallithrakas-Kontos, N.; Agioutantis, Z.; Maurigiannakis, S.; Korakaki, D. A comparative study of porous limestones treated with silicon-based strengthening agents. Prog. Org. Coat. 2008, 62, 49–60. [Google Scholar] [CrossRef]
- Ion, R.M.; Turcanu-Carutiu, D.; Fierăscu, R.C.; Fierascu, I. Chalk stone restoration with hydroxyapatite–based nanoparticles. Sci. Bull. Valahia Univ. Mater. Mech. 2014, 9, 16–19. [Google Scholar]
- Ion, R.-M.; Ion, M.-L.; Radu, A.; Şuică-Bunghez, R.-I.; Fierăscu, R.-C.; Fierăscu, I.; Teodorescu, S. Nanomaterials-based mortars for building façades preservation. Rom. J. Mater. 2016, 46, 412–418. [Google Scholar]
- Ion, R.-M.; Turcanu-Caruţiu, D.; Fierăscu, R.C.; Fierăscu, I.; Bunghez, I.-R.; Ion, M.-L.; Teodorescu, S.; Vasilievici, G.; Rădiţoiu, V. Caoxite-hydroxyapatite composition as consolidating material for the chalk stone from Basarabi-Murfatlar churches ensemble. Appl. Surf. Sci. 2015, 358, 612–618. [Google Scholar] [CrossRef]
- Luo, Y.; Xiao, L.; Zhang, X. Characterization of TEOS/PDMS/HA nanocomposites for application as consolidant/hydrophobic products on sandstones. J. Cult. Herit. 2015, 16, 470–478. [Google Scholar] [CrossRef]
- Maravelaki, P.; Verganelaki, A. A Hybrid Consolidant of NanoHydroxyapatite and Silica Inspired from Patinas for Stone Conservation. In Advanced Materials for the Conservation of Stone; Hosseini, M., Karapanagiotis, I., Eds.; Springer: Berlin, Germany, 2018; pp. 79–95. ISBN 978-3-31-972260-3. [Google Scholar]
- Pinto, A.P.F.; Rodrigues, J.D. Impacts of consolidation procedures on colour and absorption kinetics of carbonate stones. Stud. Conserv. 2014, 59, 79–90. [Google Scholar] [CrossRef]
- Franzoni, E.; Graziani, G.; Sassoni, E. TEOS-based treatments for stone consolidation: Acceleration of hydrolysis–condensation reactions by poulticing. J. Sol-Gel Sci. Technol. 2015, 74, 398–405. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Scherer, G.W.; Franzoni, E. The application of hydroxyapatite-based treatments to salt-bearing porous limestones: A study on sodium sulphate-contaminated Lecce Stone. In 4th International Conference on Salt Weathering of Buildings and Stone Sculptures; Laue, S., Ed.; Verlag der Fachhochschule Potsdam: Potsdam, Germany, 2017; pp. 176–186. [Google Scholar]
- Suzuki, Y.; Matsuya, S.; Udoh, K.; Nakagawa, M.; Tsukiyama, Y.; Koyano, K.; Ishikawa, K. Fabrication of hydroxyapatite block from gypsum block based on (NH4)2HPO4 treatment. Dent. Mater. J. 2005, 24, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Suzuki, Y.; Matsuya, S.; Nakagawa, M.; Koyano, K. Effects of pH on the transformation of gypsum to carbonate apatite in the presence of ammonium hydrogen phosphate. Key Eng. Mater. 2006, 309–311, 199–202. [Google Scholar] [CrossRef]
- Ishikawa, K.; Matsuya, S.; Suzuki, Y.; Udoh, K.-I.; Nakagawa, M.; Koyano, K. Fabrication of apatite monolith from calcium sulphate. Key Eng. Mater. 2005, 288–289, 533–536. [Google Scholar] [CrossRef]
- Jroundi, F.; Gonzalez-Muñoz, M.T.; Garcia-Bueno, A.; Rodriguez-Navarro, C. Consolidation of archaeological gypsum plaster by bacterial biomineralization of calcium carbonate. Acta Biomater. 2014, 10, 3844–3854. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Falini, G.; Foresti, E.; Ripamonti, A.; Gazzano, M.; Roveri, N. Magnesium influence on hydroxyapatite crystallization. J. Inorg. Biochem. 1993, 49, 69–78. [Google Scholar] [CrossRef]
- Matteini, M.; Colombo, C.; Botticelli, G.; Casati, M.; Conti, C.; Negrotti, R.; Possenti, E.; Realini, M. Ammonium phosphates to consolidate carbonatic stone materials: An inorganic-mineral treatment greatly promising. In Built Heritage 2013 Monitoring Conservation Management; Boriani, M., Gabaglio, R., Gulotta, D., Eds.; Politecnico di Milano: Milan, Italy, 2013; pp. 1278–1286. [Google Scholar]
- Graziani, G. New Phosphate-Based Treatments for Carbonate Stone Consolidation and Protection. Ph.D. Thesis, University of Bologna, Bologna, Italy, 2016. [Google Scholar]

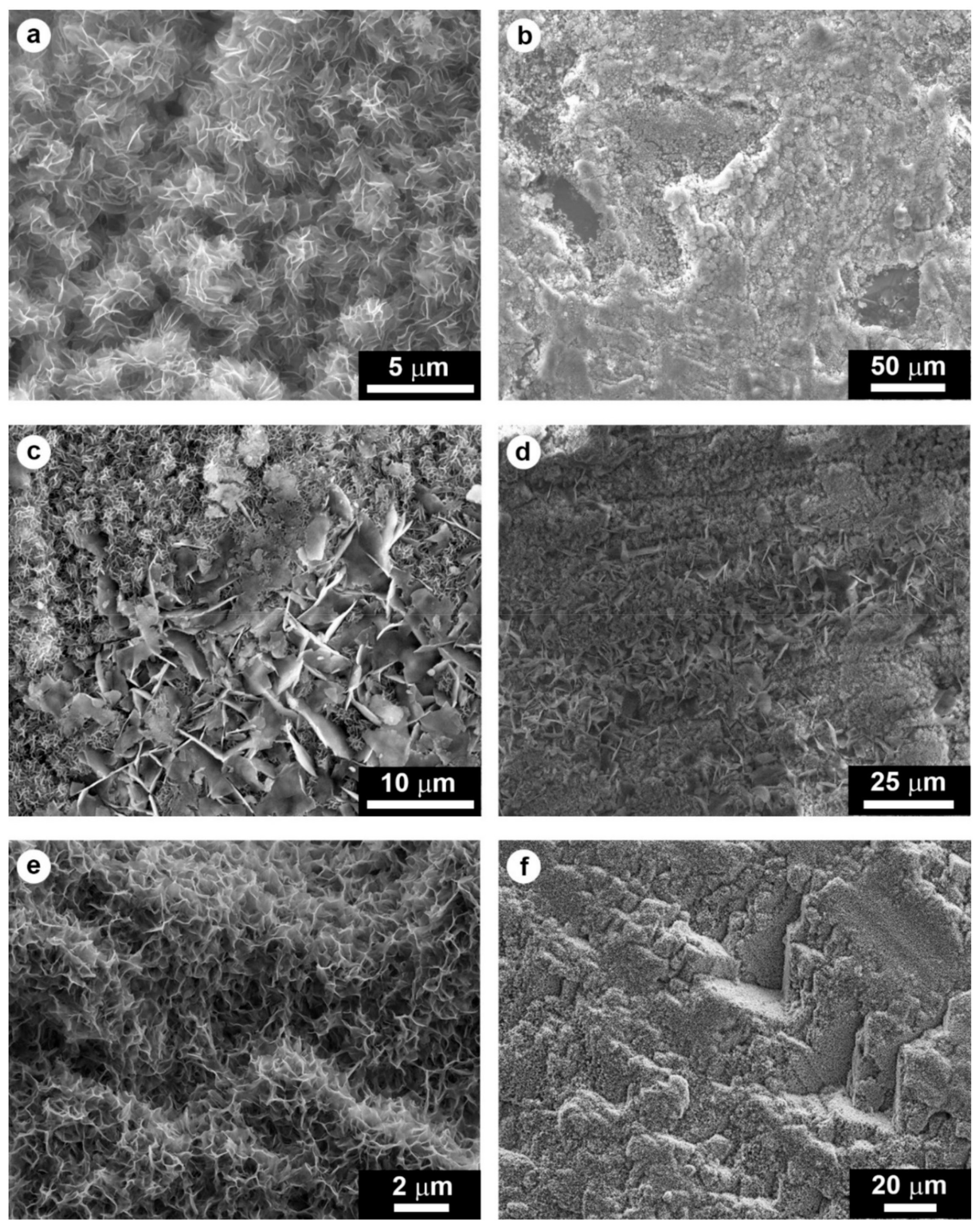
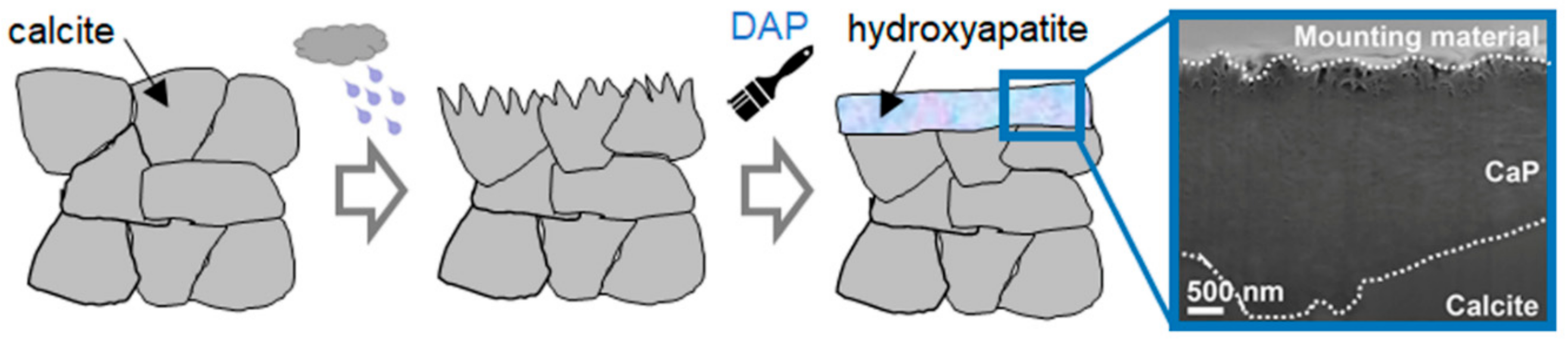









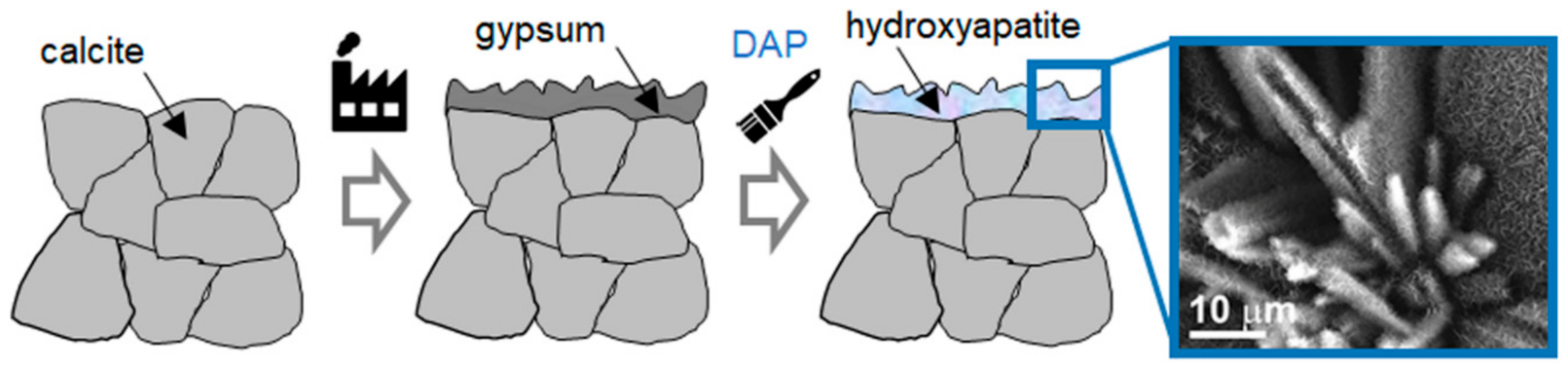
| Mineral | a (Å) | b (Å) | c (Å) | Crystal Structure | Solubility Product Ksp | Dissolution Rate Rdiss (mol/(cm2 s)) |
|---|---|---|---|---|---|---|
| Calcite (2×) | 9.98 | 9.98 | 33.82 | Rhombohedral | 5 × 10−9 | ~10−10 |
| Whewellite | 6.29 | 14.58 | 10.16 | Hexagonal | ~10−9 | n.a. |
| Hydroxyapatite | 9.43 | 9.42 | 6.88 | Monoclinic | ~10−117 | ~10−14 |
| Acronym | Mineral | Formula | Ca/P Ratio | Solubility Product Kps | Solubility at 25 °C (g/L) |
|---|---|---|---|---|---|
| HAP | Hydroxyapatite | Ca10(PO4)6(OH)2 | 1.67 | ~10−117 | ~0.0003 |
| CDHAP | Calcium-deficient HAP | Ca10−x(HPO4)x(PO4)6−x (OH)2−x (0 < x < 1) | 1.5–1.67 | ~10−85 | ~0.0094 |
| ACP | Amorphous calcium phosphate | CaxHy(PO4)z∙nH2O (n = 3–4.5, 15–20% H2O) | 1.2–2.2 | ~10−25–10−33 | n.a. |
| β-TCP | β-Tricalcium phosphate (or calcium phosphate tribasic) | β-Ca3(PO4)2 | 1.5 | ~10−29 | ~0.0005 |
| OCP | Octacalcium phosphate | Ca8H2(PO4)6·5H2O | 1.33 | ~10−97 | ~0.0081 |
| DCPD | Dicalcium phosphate dihydrate (or brushite) | CaHPO4∙2H2O | 1.0 | ~10−7 | ~0.088 |
| DCPA | Dicalcium phosphate anhydrous (or monetite) | CaHPO4 | 1.0 | ~10−7 | ~0.0048 |
| MCPM | Monocalcium phosphate monohydrate | Ca(HPO4)2∙H2O | 0.5 | ~10−1 | ~18 |
| MCPA | Monocalcium phosphate anhydrous (or calcium phosphate monobasic) | Ca(HPO4)2 | 0.5 | ~10−1 | ~17 |
| - | Calcite | CaCO3 | - | 5 × 10−9 | ~0.014 |
| - | Gypsum | CaSO4∙2H2O | - | 9 × 10−6 | ~2.4 |
| - | Whewellite | CaC2O4∙H2O | - | ~10−9 | n.a. |
| Substrate | Treating Solution | pH | Application Method | Characterization Techniques | CaP | Ref |
|---|---|---|---|---|---|---|
| Calcite powder | 10% DAP (24–48 h) | 7.8–8.8 | Immersion | XRD, FT-IR, μ-Raman | HAP, OCP, brushite | [35] |
| Compacted calcite powder | nano-Ca(OH), then 5% TAP (14 days) | n.a. | Spraying | XRD | HAP | [4] |
| Compacted calcite powder | 5% w/v DAP | n.a. | Poultice | μ-Raman | HAP | [61] |
| White marble | Collagen, then 10 mM CaCl2 + 6 mM DAP | n.a. | Dripping | XRD | HAP | [58] |
| White marble | 1 g/L monocalcium phosphate (72 h) | n.a. | Poultice | XRD | HAP | [59] |
| White marble | Collagen, then 5 g/L TAP and 0.6 g/L NH4F (48 h) | n.a. | Poultice | XRD | Fluorapatite | [54] |
| Carrara marble (fresh) | 1 M DAP (24 h) | 8 | Immersion | GID | HAP | [34] |
| Carrara marble (fresh) | 1 M DAP + 1 mM CaCl2 (24 h) | 8 | Immersion | GID | HAP, OCP | [34] |
| Carrara marble (artificially weathered) | 3 M DAP (48 h) | n.a. | Brushing (15 times) | μ-Raman | ACP? MCPA? Residual DAP | [43] |
| Carrara marble (artificially weathered) | 3 M DAP (48 h), then limewater | n.a. | Brushing (15 times) | μ-Raman | OCP, TCP? | [43] |
| Carrara marble (artificially weathered) | 3 M DAP + 1 mM CaCl2 (48 h), then limewater | n.a. | Brushing (15 times) | μ-Raman | OCP, TCP? | [43] |
| Carrara marble (artificially weathered) | 3 M DAP + 3 mM CaCl2 (48 h), then limewater | n.a. | Brushing (15 times) | μ-Raman | OCP | [43] |
| Carrara marble (naturally weathered) | 3 M DAP (48 h), then limewater (24 h) | n.a. | Brushing (15 times) | μ-Raman | HAP | [43] |
| Carrara marble (fresh) | 1 M DAP + 1 mM CaCl2 (24 h) | 8 | Immersion | GID | HAP, OCP | [38] |
| Carrara marble (fresh) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % ethanol (24 h) | 8 | Immersion | GID | OCP | [38] |
| Carrara marble (fresh) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % isopropanol (24 h) | 8 | Immersion | GID | OCP | [38] |
| Carrara marble (artificially weathered) | 3 M DAP (48 h), followed by limewater poultice | n.a. | Brushing (8 times) | Raman | HAP (OCP?) | [45] |
| Carrara marble (artificially weathered) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % ethanol (24 h), twice | n.a. | Brushing (5 times) | FT-IR | OCP | [44] |
| Carrara marble (artificially weathered) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % isopropanol (24 h), twice | n.a. | Brushing (8 times) | FT-IR | OCP | [44] |
| Carrara marble (artificially weathered) | 1 M DAP + 1 mM CaCl2 (24 h) | n.a. | Brushing (5 times) | FT-IR | HAP, OCP | [44] |
| Carrara marble (artificially weathered) | 3 M DAP (48 h), followed by limewater poultice | n.a. | Brushing (8 times) | FT-IR | HAP, OCP? | [44] |
| Indiana limestone (artificially weathered) | 1 M DAP (48 h) | 8 | Capillarity | EBSD | HAP, OCP, CDHAP, MCPM | [1] |
| Lecce stone (fresh) | 5% DAP (4–8–17 h) | 8 | Poultice | XRD, FT-IR | HAP | [3] |
| Lecce stone (fresh) | 5% ADP (4–8 h) | 5.6–6 | Poultice | XRD, FT-IR | HAP, brushite | [3] |
| Lecce stone (fresh) | 5% ADP (4–8–17 h) | 7–8 | Poultice | XRD, FT-IR | HAP | [3] |
| Tuffeau de Maastricht (fresh) | 5% DAP (4–8–17 h) | 8 | Poultice | XRD, FT-IR | HAP | [3] |
| Tuffeau de Maastricht (fresh) | 5% ADP | 5.6–6 | Poultice | XRD, FT-IR | brushite | [3] |
| Tuffeau de Maastricht (fresh) | 5% ADP | 7–8 | Poultice | XRD, FT-IR | HAP | [3] |
| Globigerina limestone (artificially weathered) | 3 M DAP (48 h), then limewater | n.a. | Brushing (10 or 20 times), Poultice, Immersion | FT-IR | HAP | [42] |
| Arenisca Ronda (artificially sulphated) | 3 M DAP (1 h) | n.a. | Poultice | XRD | HAP, brushite, TCP? | [9] |
| Marlstone (naturally weathered) | 1 M DAP (3 h) | n.a. | Poultice | XRD, FT-IR | HAP | [46] |
| Substrate | Specimen | Treating Solution | Application Method | Penetration Depth | Consolidating Action | Ref |
|---|---|---|---|---|---|---|
| Compacted calcite powder | n.a. | nano-Ca(OH)2, then 5% TAP (2 weeks) | Spraying | n.a. | - UT: w,STT = 19.4 mg/cm2, σc < 0.05 MPa - TR: w,STT = 0.05 mg/cm2, σc = 4.5 MPa | [4] |
| Compacted calcite powder | Cylinders (D = 39.8 mm, H = 80 mm) | Collagen, then 10 mM CaCl2 + 6 mM DAP | Dripping | n.a. | - UT: Fc = 200 N - TR: Fc = 300 N | [58] |
| Compacted calcite powder | Cylinders (D = 15 mm, H = 4 mm) | 5% w/v DAP | Poultice | HAP formation: 2 mm (μ-Raman) | n.a. | [61] |
| Compacted calcite powder | Cylinders (D = 15 mm, H = 4 mm) | 5% w/v DAP, then 5% w/v AmOx | Poultice | HAP formation: 2.5 mm (μ-Raman) | n.a. | [61] |
| Carrara marble (naturally weathered) | n.a. | 5% w/v DAP, then 5% w/v AmOx | Poultice | HAP formation: 2.5 mm (μ-Raman) | n.a. | [61] |
| Carrara marble (artificially weathered) | Slabs (65 × 65 × 20 mm3) | 3 M DAP (48 h), followed by limewater poultice | Brushing (15 times) | - DAP solution: at least 20 mm - CaP formation: at least 4 mm (abrasion resistance) | - UW,UT: UPV = 2.5 km/s - W,UT: UPV = 0.8 km/s - W,TR: UPV = 4.0 km/s | [43] |
| White marble (naturally weathered) | Slab (120 × 90 × 30 mm3) | 3 M DAP (48 h), followed by limewater poultice | Brushing (15 times) | n.a. | UPV increase from ~88% to ~98% of the maximum UPV value | [43] |
| Carrara marble (artificially weathered) | Slabs (30 × 30 × 20 mm3) | 3 M DAP (48 h), followed by limewater poultice | Brushing (8 times) | n.a. | - UW,UT: UPV = 2.9 km/s - W,UT: UPV = 0.7 km/s - W,TR: UPV = 3.1 km/s | [45] |
| Carrara marble (artificially weathered) | Slabs (30 × 30 × 20 mm3) | 3 M DAP (48 h), followed by limewater poultice (24 h), then nanoTiO2 | Brushing (8 times) | n.a. | - UW,UT: UPV = 2.9 km/s - W,UT: UPV = 0.7 km/s - W,TR: UPV = 3.0 km/s | [45] |
| Carrara marble (artificially weathered) | Slabs (30 × 30 × 20 mm3) | 3 M DAP with nanoTiO2 (48 h), followed by limewater poultice (24 h) | Brushing (8 times) | n.a. | - UW,UT: UPV = 2.9 km/s - W,UT: UPV = 0.7 km/s - W,TR: UPV = 3.1 km/s | [45] |
| Carrara marble (artificially weathered) | Slabs (30 × 30 × 20 mm3) | 0.1 M DAP+0.1 mM CaCl2 + 0.5 wt % ethanol (24 h), twice (the second time without ethanol) | Immersion | n.a. | - UW,UT: UPV = 3.2 km/s - W,UT: UPV = 0.6 km/s - W,TR: UPV = 2.9 km/s | [64] |
| Carrara marble (artificially weathered) | Slabs (30 × 30 × 20 mm3) | 3 M DAP (48 h), followed by limewater poultice | Brushing (15 times) | n.a. | - UW,UT: UPV = 3.2 km/s - W,UT: UPV = 0.6 km/s - W,TR: UPV = 2.2 km/s | [64] |
| Carrara marble (artificially weathered) | Slabs (50 × 50 × 10 mm3) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % ethanol (24 h) | Vacuum saturation | n.a. | - W,UT: Ed = 61% of the initial Ed - W,TR: Ed = 88% of the initial Ed (single application) - W,TR: Ed = 113% of the initial Ed (double application) | [39] |
| Carrara marble (artificially weathered) | Slabs (50 × 50 × 10 mm3) | 1 M DAP + 1 mM CaCl2 (24 h) | Vacuum saturation | n.a. | - UW,UT: Ed = 68–72 GPa * - W,UT: Ed = 38–46 GPa * - W,TR: Ed = 97–98 GPa * | [38] |
| Carrara marble (artificially weathered) | Slabs (50 × 50 × 10 mm3) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % ethanol (24 h) | Vacuum saturation | n.a. | - UW,UT: Ed = 68–72 GPa * - W,UT: Ed = 38–46 GPa * - W,TR: Ed = 61–68 GPa (single application) * - W,TR: Ed = 61–76 GPa * (double application) * | [38] |
| Carrara marble (artificially weathered) | Slabs (50 × 50 × 10 mm3) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % isopropanol (24 h) | Vacuum saturation | n.a. | - UW,UT: Ed = 68–72 GPa * - W,UT: Ed = 38–46 GPa * - W,TR: Ed = 58–60 GPa (single application) * - W,TR: Ed = 67–80 GPa * (double application) * | [38] |
| Carrara marble (artificially weathered) | Cylinders (D = 15 mm, H = 50 mm) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % ethanol (24 h), twice | Brushing (5 times) | DAP solution: at least 7.5 mm | - UW,UT: Ed = 53–76 GPa ** - W,UT: Ed = 14–24 GPa ** - W,TR: Ed = 64–93 GPa ** | [44] |
| Carrara marble (artificially weathered) | Slabs (400 × 100 × 20 mm3) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % ethanol (24 h), twice | Brushing (8 times) | DAP solution: at least 20 mm | - UW,UT: Ed = 58–60 GPa ** - W,UT: Ed = 16–19 GPa ** - W,TR: Ed = 22–32 GPa ** | [44] |
| Carrara marble (artificially weathered) | Cylinders (D = 15 mm, H = 50 mm) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % isopropanol (24 h), twice | Brushing (5 times) | DAP solution: at least 7.5 mm | - UW,UT: Ed = 53–76 GPa ** - W,UT: Ed = 14–24 GPa ** - W,TR: Ed = 70–91 GPa ** | [44] |
| Carrara marble (artificially weathered) | Slabs (400 × 100 × 20 mm3) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % isopropanol (24 h), twice | Brushing (8 times) | DAP solution: at least 20 mm | - UW,UT: Ed = 58–60 GPa ** - W,UT: Ed = 16–19 GPa ** - W,TR: Ed = 27–37 GPa ** | [44] |
| Carrara marble (artificially weathered) | Cylinders (D = 15 mm, H = 50 mm) | 1 M DAP + 1 mM CaCl2 (24 h) | Brushing (5 times) | DAP solution: at least 7.5 mm | - UW,UT: Ed = 53–76 GPa ** - W,UT: Ed = 14–24 GPa ** - W,TR: Ed = 110–112 GPa ** | [44] |
| Carrara marble (artificially weathered) | Slabs (400 × 100 × 20 mm3) | 1 M DAP + 1 mM CaCl2 (24 h) | Brushing (7 times) | DAP solution: at least 20 mm | - UW,UT: Ed = 58–60 GPa ** - W,UT: Ed = 16–19 GPa ** - W,TR: Ed = 52–59 GPa ** | [44] |
| Carrara marble (artificially weathered) | Cylinders (D = 15 mm, H = 50 mm) | 3 M DAP (24 h), followed by limewater poultice | Brushing (5 times) | DAP solution: at least 7.5 mm | - UW,UT: Ed = 53–76 GPa ** - W,UT: Ed = 14–24 GPa ** - W,TR: Ed = 79–93 GPa ** | [44] |
| Carrara marble (artificially weathered) | Slabs (400 × 100 × 20 mm3) | 3 M DAP (24 h), followed by limewater poultice | Brushing (4 times) | DAP solution: at least 20 mm | - UW,UT: Ed = 58–60 GPa ** - W,UT: Ed = 16–19 GPa ** - W,TR: Ed = 62–69 GPa ** | [44] |
| Substrate | Treating Solution | Application Method | ΔL* | Δa* | Δb* | ΔE* | Ref |
|---|---|---|---|---|---|---|---|
| Carrara marble (fresh) | 0.1 M DAP + 0.1 mM CaCl2 in 0.5 wt % ethanol (24 h), twice (the second time without ethanol) | Immersion | n.a. | n.a. | n.a. | 0.4 | [63] |
| Carrara marble (fresh) | 3 M DAP (48 h), followed by limewater poultice | Brushing (8 times) | n.a. | n.a. | n.a. | 0.6 | [63] |
| White marble (fresh) | 1 g/L monocalcium phosphate | Poultice | +1.7 | −1.2 | −0.8 | 2.2 | [59] |
| White marble (fresh) | 0.1 g/L Type I collagen + 5 g/L TAP + 0.6 g/L NH4F | Poultice | +1.5 | −1.4 | −0.7 | 2.1 | [54] |
| Carrara marble (artificially weathered) | 0.1 M DAP+0.1 mM CaCl2 in 0.5 wt % ethanol (24 h), twice (the second time without ethanol) | Immersion | n.a. | n.a. | n.a. | 1.1 | [64] |
| Carrara marble (artificially weathered) | 3 M DAP (48 h), followed by limewater poultice | Brushing (15 times) | n.a. | n.a. | n.a. | 1.5 | [64] |
| Carrara marble (artificially weathered) | 3 M DAP (48 h), followed by limewater poultice | Brushing (15 times) | −1.9 | −0.1 | −0.4 | 1.9 | [43] |
| White marble (naturally weathered) | 3 M DAP (48 h), followed by limewater poultice | Brushing (15 times) | +2.1 | −0.1 | −1.7 | 2.7 | [43] |
| Carrara marble (artificially weathered) | 1 M DAP + 1 mM CaCl2 (24 h) | Vacuum saturation | −2.0 | −0.2 | −0.6 | 2.1 | [38] |
| Carrara marble (artificially weathered) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % ethanol (24 h) | Vacuum saturation | −1.4 | +0.0 | −0.1 | 1.4 | [38] |
| Carrara marble (artificially weathered) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % ethanol (24 h), twice | Vacuum saturation | −1.5 | +0.0 | −0.6 | 1.6 | [38] |
| Carrara marble (artificially weathered) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % isopropanol (24 h) | Vacuum saturation | −1.4 | −0.4 | −0.7 | 1.6 | [38] |
| Carrara marble (artificially weathered) | 0.1 M DAP + 0.1 mM CaCl2 in 10 vol % isopropanol (24 h), twice | Vacuum saturation | −2.0 | −0.3 | −1.0 | 2.2 | [38] |
| Carrara marble (artificially weathered) | 3 M DAP (48 h), followed by limewater poultice | Brushing (8 times) | +1.8 | −0.3 | −2.2 | 2.2 | [45] |
| Carrara marble (artificially weathered) | 3 M DAP (48 h), followed by limewater poultice (24 h), then nanoTiO2 | Brushing (8 times) | +1.2 | 0.2 | −1.5 | 1.2 | [45] |
| Carrara marble (artificially weathered) | 3 M DAP with nanoTiO2 (48 h), followed by limewater poultice (24 h) | Brushing (8 times) | −0.2 | −0.3 | −1.7 | 0.6 | [45] |
| White marble (artificially sulphated) | 0.02 M DAP at pH 9.2 (5 days) | Poultice | n.a. | n.a. | n.a. | 1.5 | [8] |
| Substrate | Specimen | Treating Solution | Application Method | Penetration Depth | Consolidating Action | Ref |
|---|---|---|---|---|---|---|
| Indiana limestone (artificially weathered) | Cylinders (D = 20 mm, H = 50 mm) | 1 M DAP (48 h) | Immersion | CaP formation: 20 mm (EBSD, MIP) | - UW,UT: Ed = 35 GPa, Δσt = 4.8 MPa - W,UT: Ed = 19 GPa, Δσt = 3.3 MPa - W,TR: Ed = 36 GPa, Δσt = 4.1 MPa | [1] |
| Indiana limestone (artificially weathered) | Cylinders (D = 20 mm, H = 50 mm) | 1 M DAP (48 h) | Brushing | n.a. | - UW,UT: Ed = 35 GPa, Δσt = 4.8 MPa - W,UT: Ed = 23 GPa, Δσt = 3.5 MPa - W,TR: Ed = 40 GPa, Δσt = 4.1 MPa | [79] |
| Indiana limestone (artificially weathered) | Cubes (50 mm side) | 1 M DAP + 1 mM CaCl2 (48 h) | Capillarity | n.a. | - UW,UT: Ed = 35 GPa - W,UT: Ed = 22 GPa W,TR: Ed = 46 GPa | [48] |
| Globigerina limestone (artificially weathered) | Cylinders (D = 20 mm, H = 50 mm) | 3 M DAP, followed by limewater poultice | Brushing (10 times) | - DAP solution: 8–9 mm - HAP formation: at least 7.5 mm (abrasion resistance), at least 10 mm (FT-IR) | - UW,UT: Ed = 16 GPa, Δσt = 3.0 MPa - W,UT: Ed = 11 GPa, Δσt = 2.7 MPa - W,TR: Ed = 16 GPa, Δσt = 3.4 MPa | [42] |
| Globigerina limestone (artificially weathered) | Cylinders (D = 20 mm, H = 50 mm) | 3 M DAP, followed by limewater poultice | Brushing (20 times) | - DAP solution: 8–9 mm - HAP formation: at least 7.5 mm (abrasion resistance), at least 10 mm (FT-IR) | - UW,UT: Ed = 16 GPa, Δσt = 3.0 MPa - W,UT: Ed = 11 GPa, Δσt = 2.7 MPa - W,TR: Ed = 16 GPa, Δσt = 3.2 MPa | [42] |
| Globigerina limestone (artificially weathered) | Cylinders (D = 20 mm, H = 50 mm) | 3 M DAP, followed by limewater poultice | Poultice | - DAP solution: 25 mm - HAP formation: at least 7.5 mm (abrasion resistance), at least 10 mm (FT-IR) | - UW,UT: Ed = 16 GPa, Δσt = 3.0 MPa - W,UT: Ed = 11 GPa, Δσt = 2.7 MPa - W,TR: Ed = 16 GPa, Δσt = 3.4 MPa | [42] |
| Globigerina limestone (artificially weathered) | Cylinders (D = 20 mm, H = 50 mm) | 3 M DAP, followed by limewater poultice | Immersion | - DAP solution: 25 mm - HAP formation: at least 7.5 mm (abrasion resistance), at least 10 mm (FT-IR) | - UW,UT: Ed = 16 GPa, Δσt = 3.0 MPa - W,UT: Ed = 11 GPa, Δσt = 2.7 MPa - W,TR: Ed = 17 GPa, Δσt = 3.5 MPa | [42] |
| Lecce stone (fresh) | Slabs (50 × 50 × 20 mm3) | 5% DAP solution for 4–8–17 h | Poultice | HAP formation: at least 10 mm (MDR) | - UT: MDR = 33 N - TR: MDR = 38–39 N | [3] |
| Lecce stone (fresh) | Slabs (50 × 50 × 20 mm3) | 5% ADP solution for 4–8 h | Poultice | HAP formation: at least 10 mm (MDR) | - UT: MDR = 33 N - TR: MDR = 37–39 N | [3] |
| Lecce stone (fresh) | Slabs (50 × 50 × 20 mm3) | 5% ADP solution at pH 8 for 4–8–17 h | Poultice | HAP formation: at least 10 mm (MDR) | - UT: MDR = 33 N - TR: MDR = 38–41 N | [3] |
| Tuffeau de Maastricht (fresh) | Slabs (50 × 50 × 20 mm3) | 5% DAP solution for 4–8–17 h | Poultice | HAP formation: at least 10 mm (MDR) | - UT: MDR = 3.1 N - TR: MDR = 3.0–3.8 N | [3] |
| Tuffeau de Maastricht (fresh) | Slabs (50 × 50 × 20 mm3) | 5% ADP solution for 4–8 h | Poultice | brushite formation: at least 10 mm (MDR) | - UT: MDR = 3.1 N - TR: MDR = 4.1–4.3 N | [3] |
| Tuffeau de Maastricht (fresh) | Slabs (50 × 50 × 20 mm3) | 5% ADP solution at pH 8 for 4–8–17 h | Poultice | HAP formation: at least 10 mm (MDR) | - UT: MDR = 3.1 N - TR: MDR = 4.1–4.8 N | [3] |
| Compacted limestone powder | Cylinders (D = 32 mm, H = n.a.) | 1 M DAP (3 months) | Brushing (until refusal) | n.a. | - UT: HD = 60 HD, σc = 77 MPa, w,STT = 1.2 mg/cm2 - TR: HD = 83 HD, σc = 177 MPa, w,STT = 0.01 mg/cm2 | [57] |
| Compacted limestone powder | Cylinders (D = 32 mm, H = n.a.) | 1 M DAP + 0.1 M CTAB (3 months) | Brushing (until refusal) | n.a. | - UT: HD = 60 HD, σc = 77 MPa, w,STT = 1.2 mg/cm2 - TR: HD = 84 HD, σc = 180 MPa, w,STT = 0.01 mg/cm2 | [57] |
| Arenisca Ronda (artificially sulphated) | Slabs (25 × 20 × 10 mm3) | 3 M DAP for 60 min | Poultice | CaP formation: 3.5 mm | - W,UT: MDR = 1.27 N/mm - W,TR: MDR = 1.51 N/mm | [9] |
| Substrate | Treating Solution | Application Method | ΔL* | Δa* | Δb* | ΔE* | Ref |
|---|---|---|---|---|---|---|---|
| Indiana limestone (artificially weathered) | 1 M DAP | Immersion | n.a. | n.a. | n.a. | 7.6 * | [1] |
| Lecce stone (fresh) | 5% DAP solution for 4–8–17 h | Poultice | −2.5 to −0.5 | 0 to +0.4 | −2.8 to +0.6 | 2.1 to 2.9 | [3] |
| Lecce stone (fresh) | 5% ADP solution for 4–8 h | Poultice | −0.7 to 0 | +0.9 to +1.1 | −1.7 to −1.3 | 1.6 to 2.2 | [3] |
| Lecce stone (fresh) | 5% ADP solution at pH 8 for 4–8–17 h | Poultice | −1.2 to +3.7 | 0.1 to 0.6 | −4.3 to −0.1 | 1.2 to 5.7 | [3] |
| Tuffeau de Maastricht (fresh) | 5% DAP solution for 4–8–17 h | Poultice | +0.3 to +0.5 | −0.1 to 0 | −1.7 to −0.9 | 0.9 to 1.7 | [3] |
| Tuffeau de Maastricht (fresh) | 5% ADP solution for 4–8 h | Poultice | +0.6 to +2.4 | −0.1 to 0 | −3.2 to +0.3 | 0.7 to 4.0 | [3] |
| Tuffeau de Maastricht (fresh) | 5% ADP solution at pH 8 for 4–8–17 h | Poultice | +0.1 to +3.1 | +0.2 | −4.0 to −1.0 | 1.1 to 5.1 | [3] |
| Globigerina limestone (artificially weathered) | 3 M DAP, followed by limewater poultice | Brushing (10 times) | −0.7 | +1.9 | +1.9 | 2.8 | [42] |
| Globigerina limestone (artificially weathered) | 3 M DAP, followed by limewater poultice | Brushing (20 times) | −3.3 | +1.1 | 0.0 | 3.5 | [42] |
| Globigerina limestone (artificially weathered) | 3 M DAP, followed by limewater poultice | Poultice | −0.2 | 0.0 | −3.4 | 3.4 | [42] |
| Globigerina limestone (artificially weathered) | 3 M DAP, followed by limewater poultice | Immersion | −0.5 | 0.0 | −2.2 | 2.2 | [42] |
| Globigerina limestone (artificially weathered) | 3 M DAP, followed by limewater poultice | Brushing (10 times) | −4.0 | +1.5 | +1.3 | 4.4 | [49] |
| Compacted limestone powder | 1 M DAP (3 months) | Brushing (until refusal) | n.a. | n.a. | n.a. | 2.3 | [57] |
| Compacted limestone powder | 1 M DAP + 0.1 M CTAB (3 months) | Brushing (until refusal) | n.a. | n.a. | n.a. | 3.3 | [57] |
| Arenisca Ronda (artificially sulphated) | 3 M DAP for 60 min | Poultice | n.a. | n.a. | n.a. | <3 | [9] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sassoni, E. Hydroxyapatite and Other Calcium Phosphates for the Conservation of Cultural Heritage: A Review. Materials 2018, 11, 557. https://doi.org/10.3390/ma11040557
Sassoni E. Hydroxyapatite and Other Calcium Phosphates for the Conservation of Cultural Heritage: A Review. Materials. 2018; 11(4):557. https://doi.org/10.3390/ma11040557
Chicago/Turabian StyleSassoni, Enrico. 2018. "Hydroxyapatite and Other Calcium Phosphates for the Conservation of Cultural Heritage: A Review" Materials 11, no. 4: 557. https://doi.org/10.3390/ma11040557
APA StyleSassoni, E. (2018). Hydroxyapatite and Other Calcium Phosphates for the Conservation of Cultural Heritage: A Review. Materials, 11(4), 557. https://doi.org/10.3390/ma11040557






