Microstructure and Mechanical Properties of Graphene-Reinforced Titanium Matrix/Nano-Hydroxyapatite Nanocomposites
Abstract
:1. Introduction
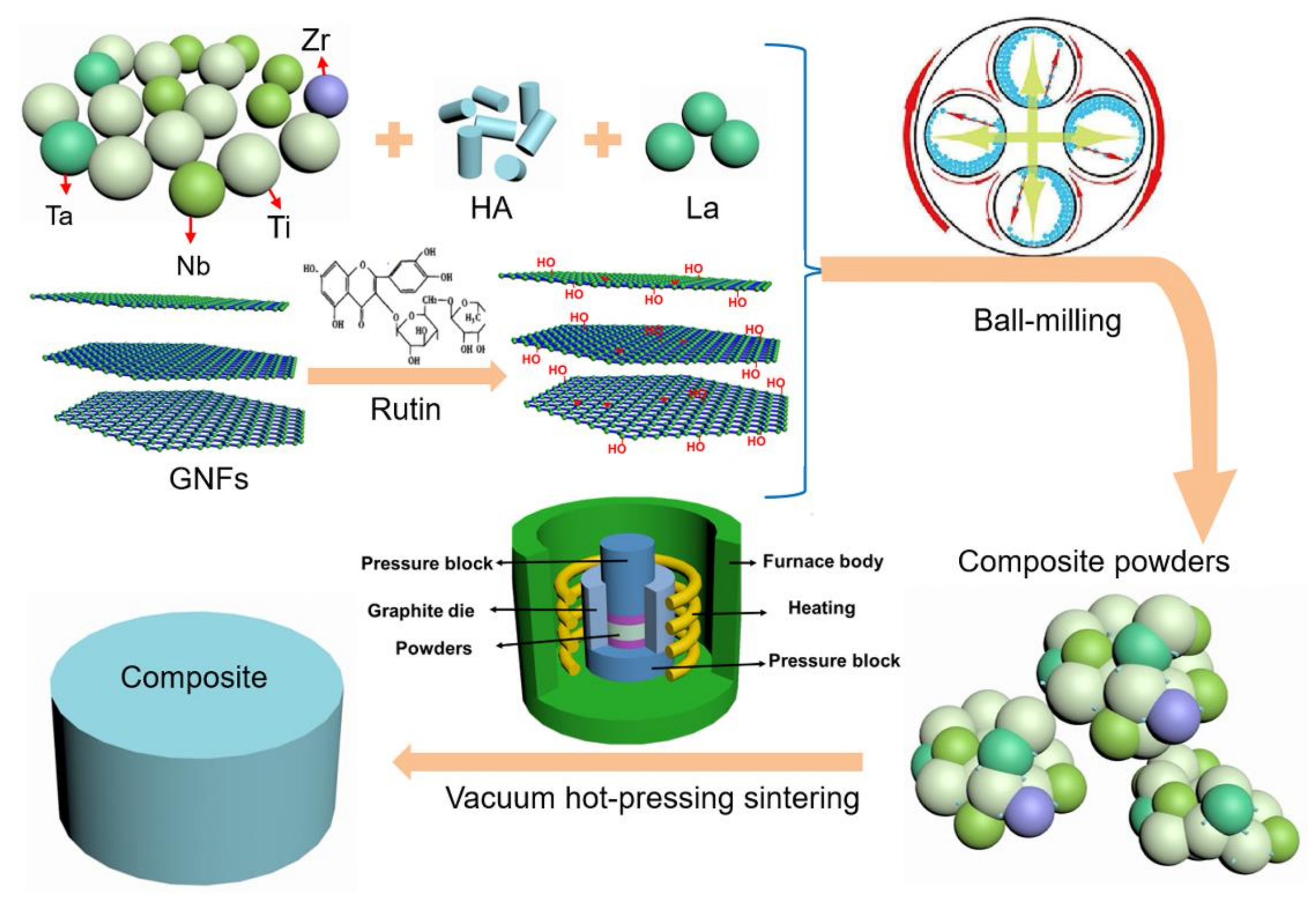
2. Materials and Methods
3. Results
3.1. Microstructure and Phase Composition of Nanocomposite Powders
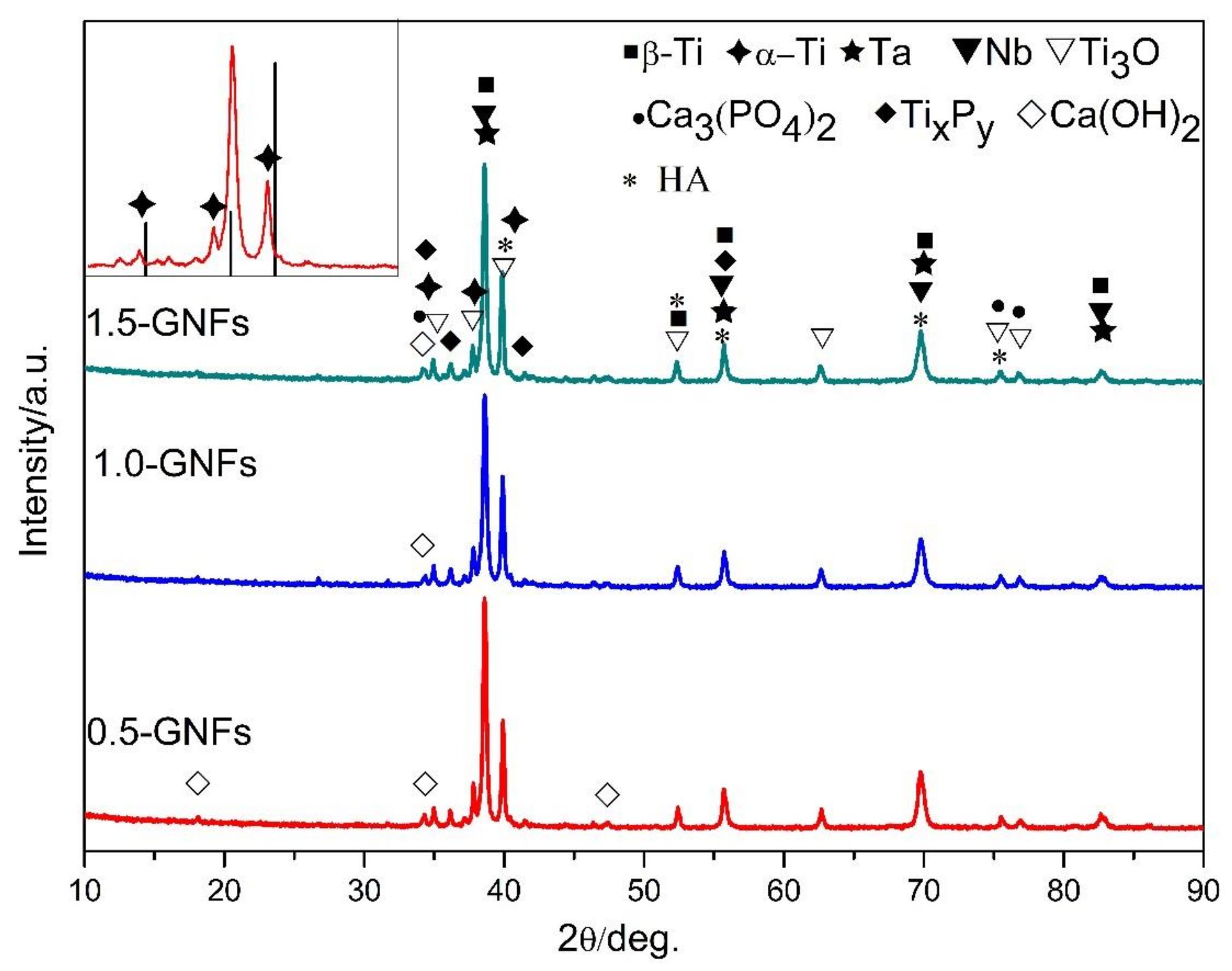
3.1.1. XRD Results Analysis
3.1.2. SEM Results Analysis
3.2. Microstructure and Phase Analysis of the Sintered Nanocomposites
3.2.1. XRD Results Analysis
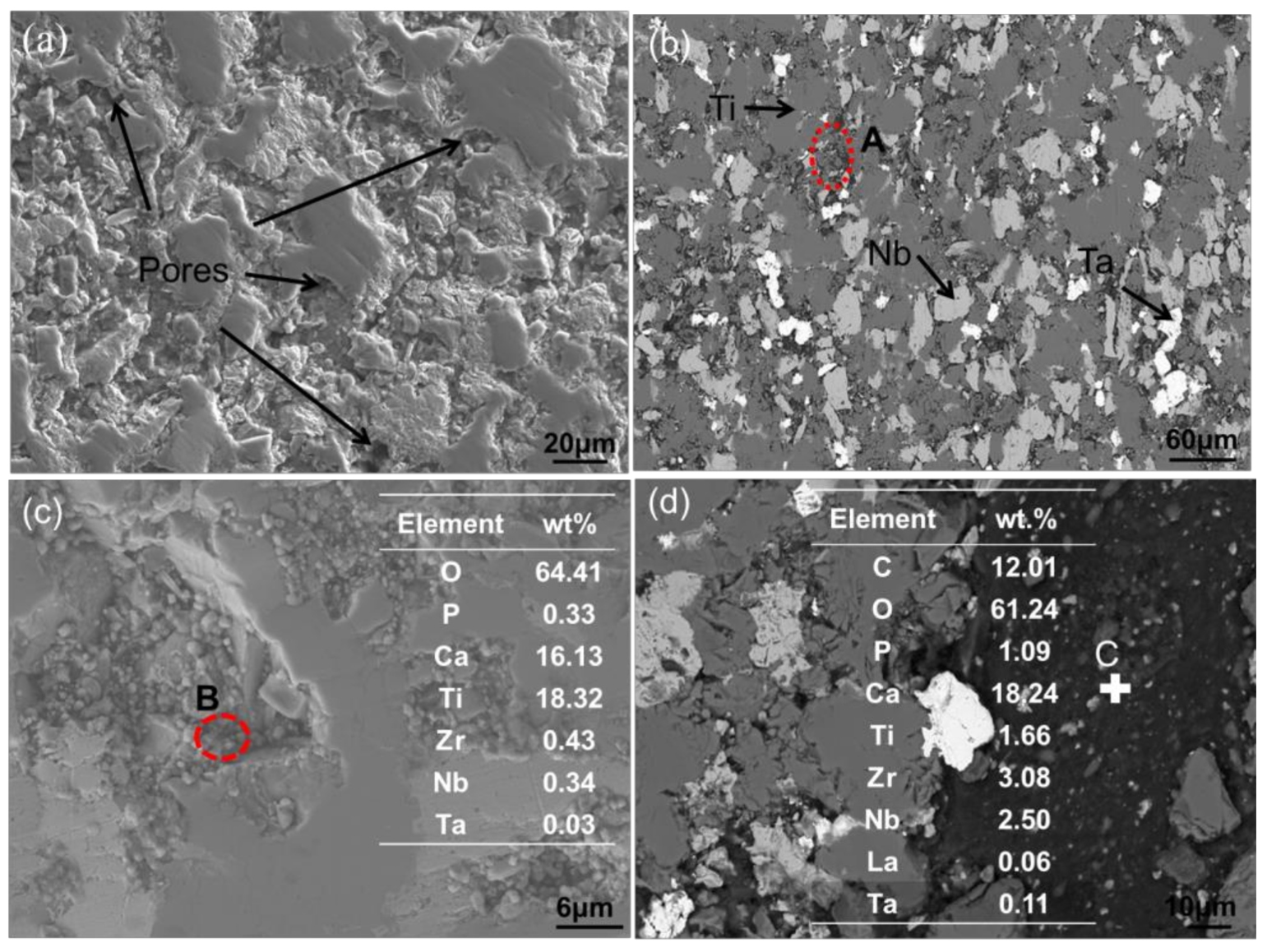
3.2.2. SEM Results Analysis
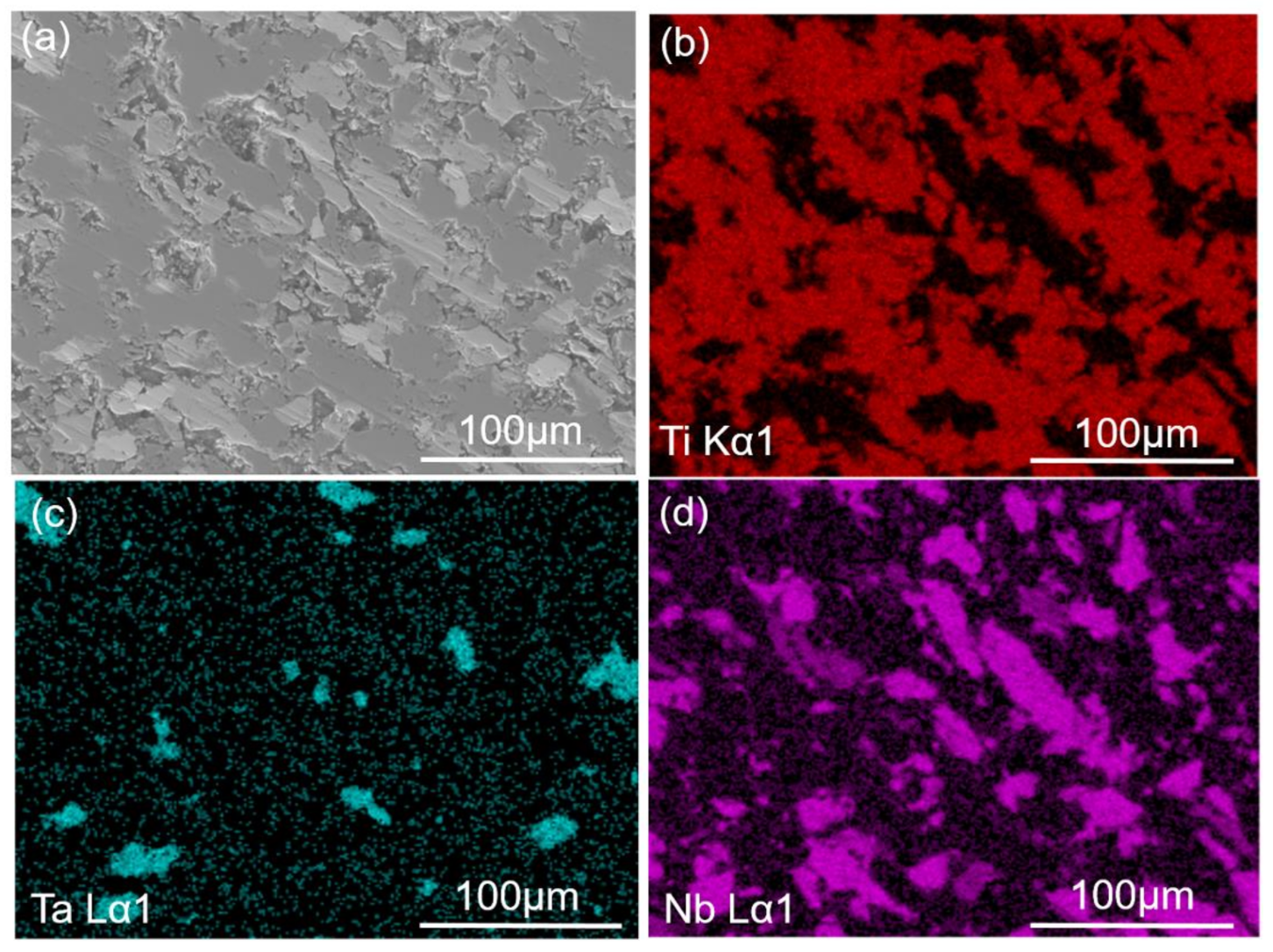
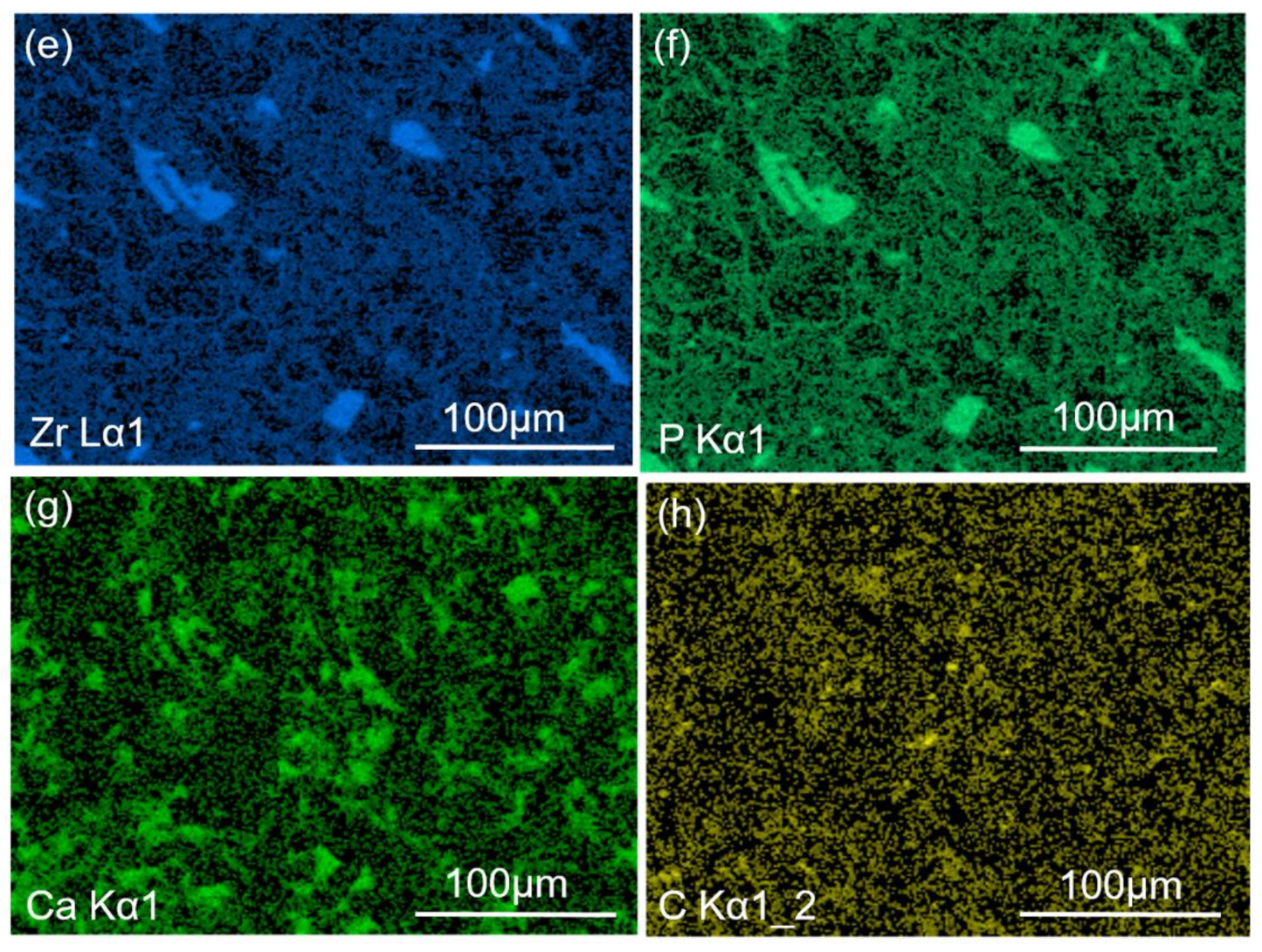
3.2.3. EPMA Results Analysis
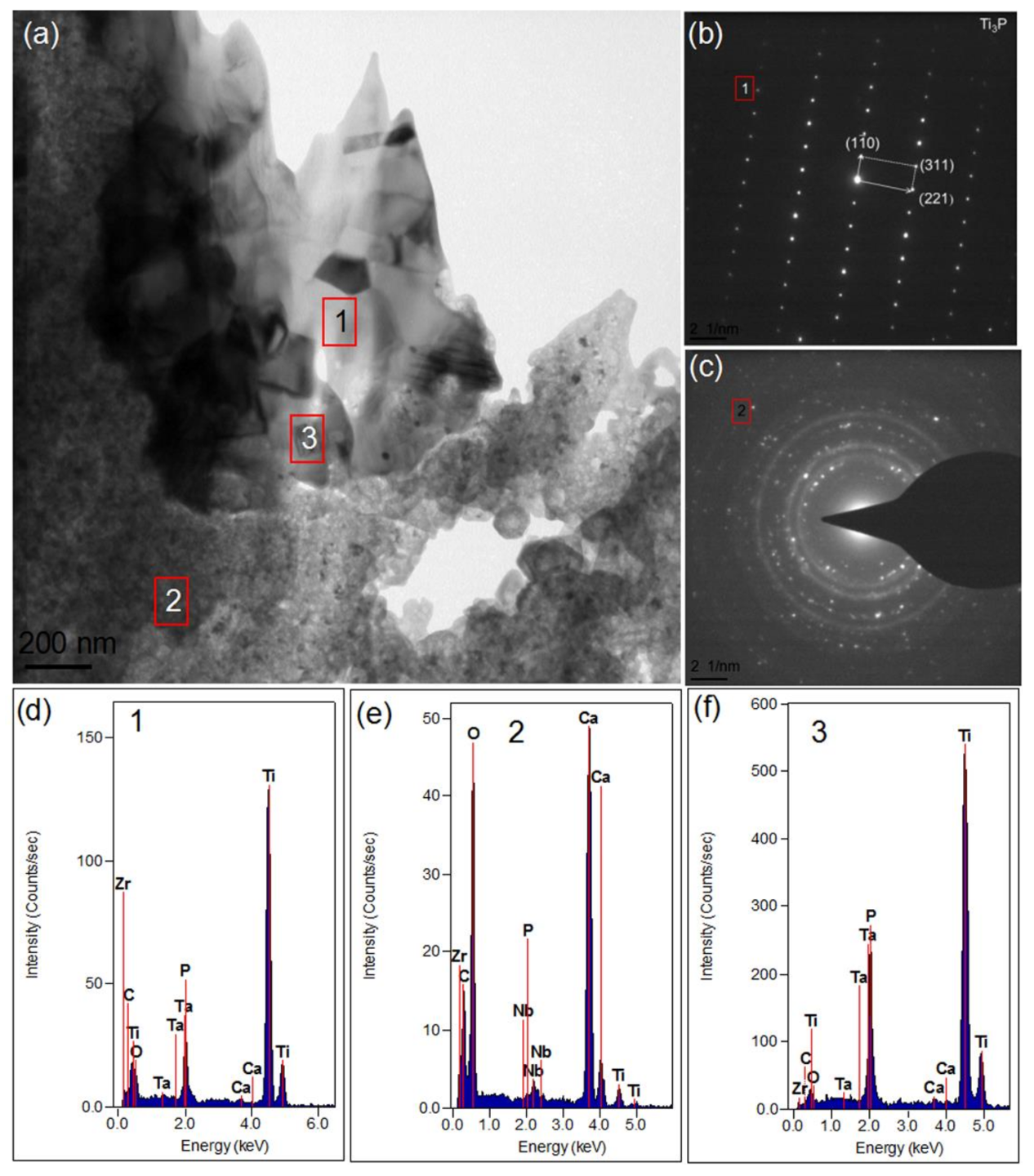
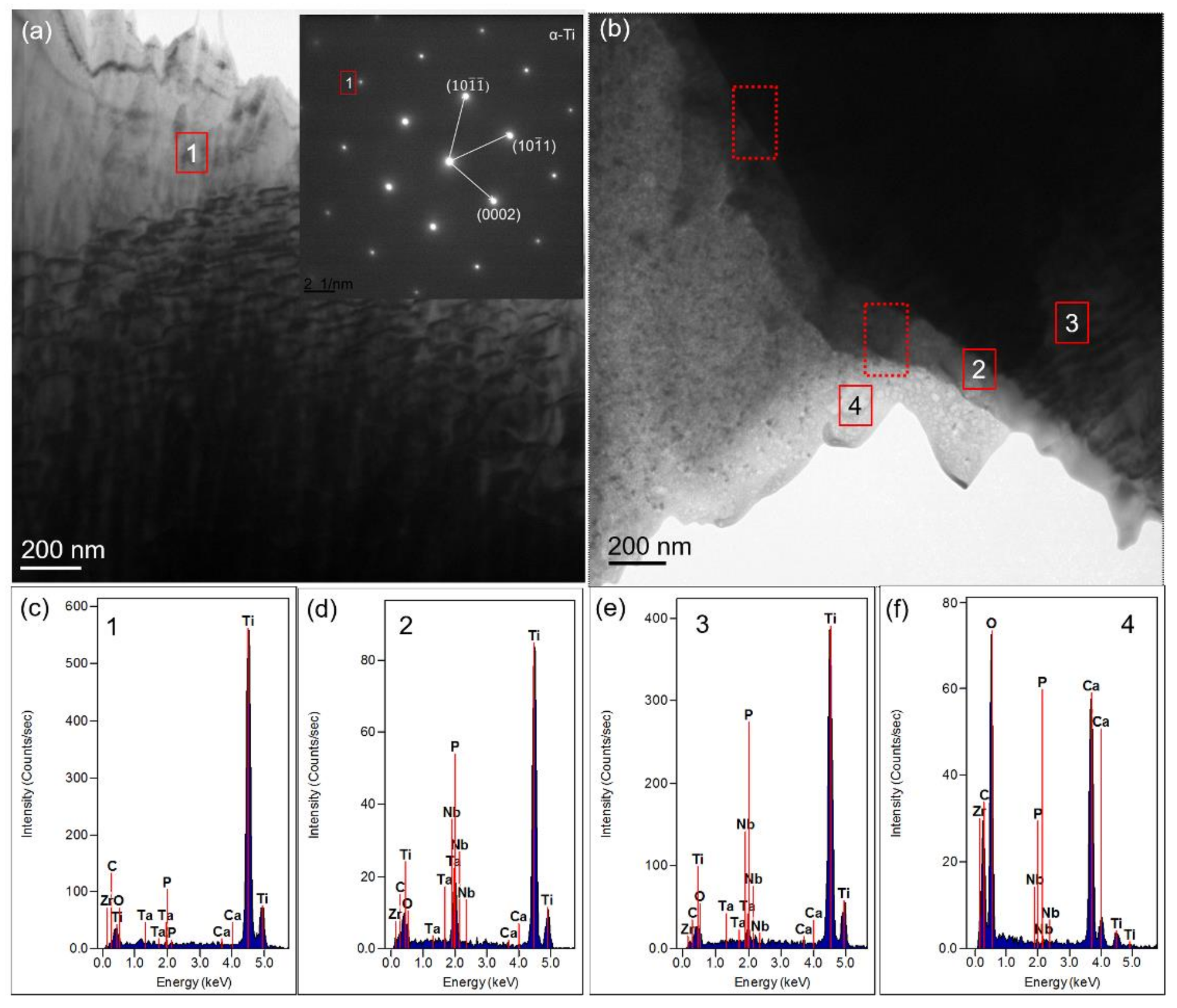
3.2.4. TEM Results Analysis
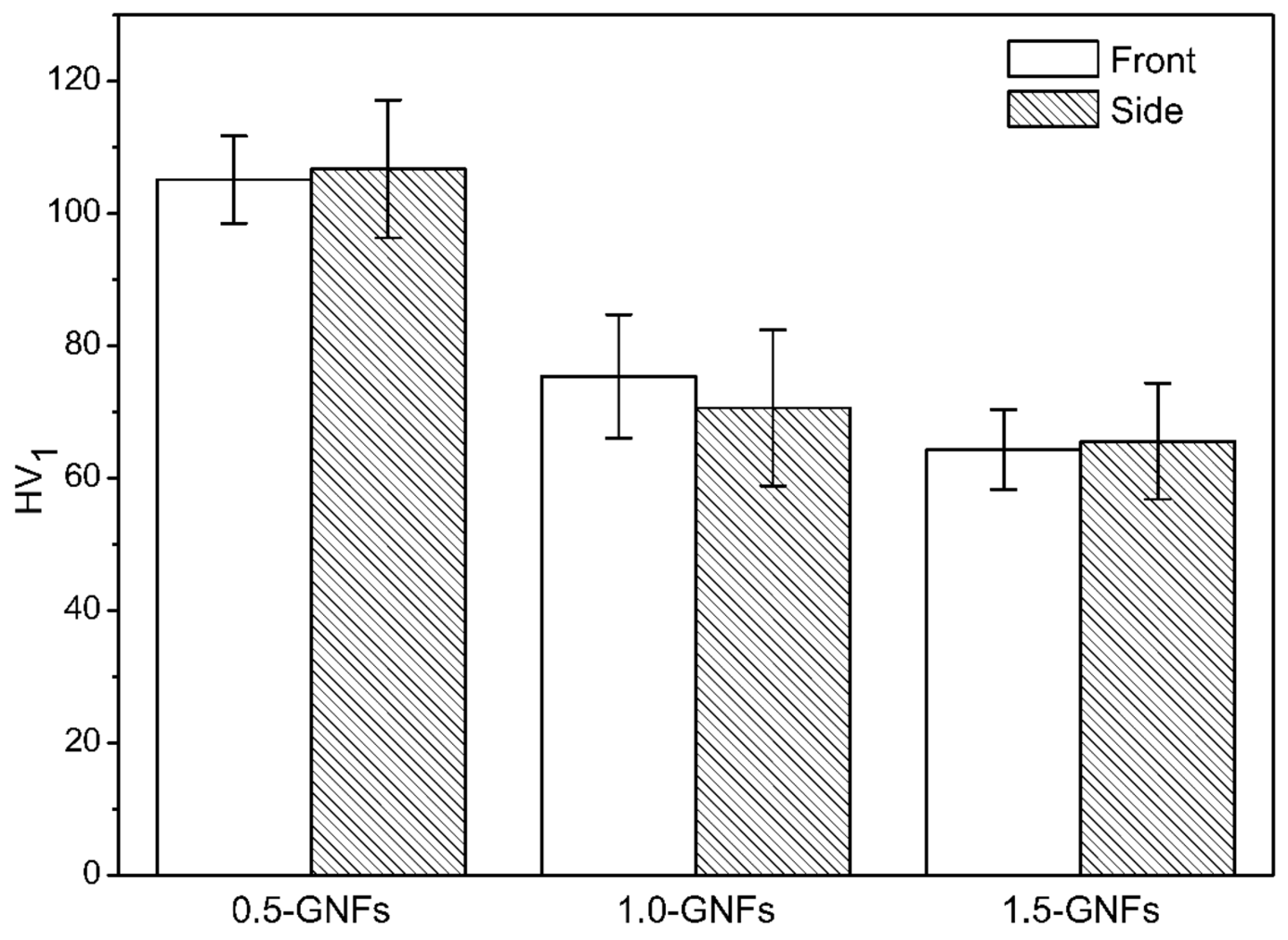
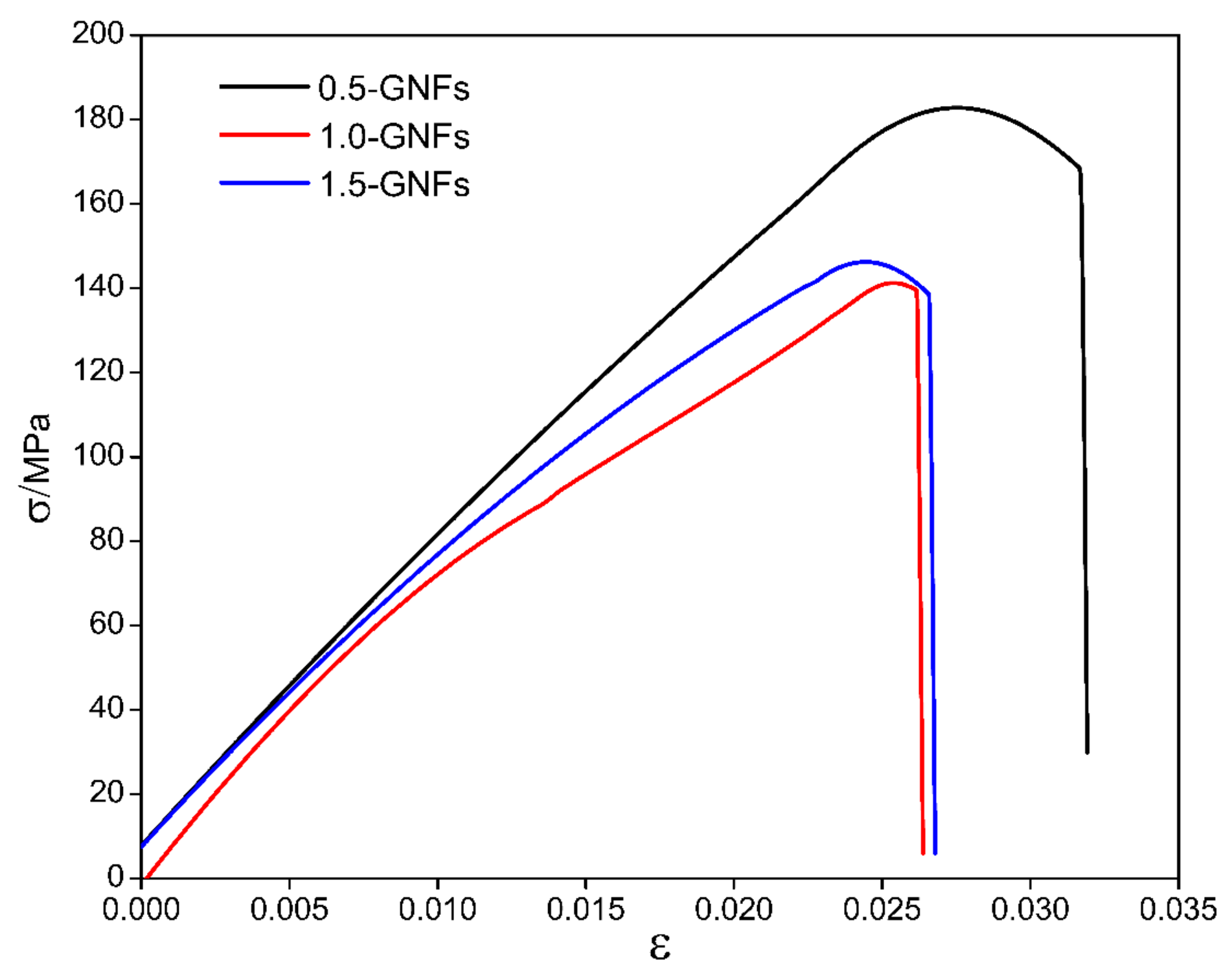
3.3. Mechanical Properties of the Sintered Nanocomposite
3.4. Fracture Surface Analysis of the Sintered Nanocomposites
4. Discussion
5. Conclusions
- Graphene-reinforced titanium matrix/nHA composites were prepared by mechanical alloying and vacuum hot-pressing sintering. Results showed that during the high temperature sintering process, complex chemical reactions occurred, resulting in some new phases of nucleation such as Ca3(PO4)2, TixPy, and Ti3O. The new phases, which easily dropped off under the action of external force due to weak interfacial bonding with the substrate, could hinder the densification of sintering and increase the brittleness of the nanocomposites.
- The shear fractures of the sintered composites were brittle fractures, and there were a lot of detritus and pores on the fracture surface of the composite in which their unique pore structure can give them the characteristic of ultralow density and large surface area for human body with structural and functional integration. There were many tiny particles around the holes, and the reaction and decomposition products of HA were distributed in the alloy matrix. It was believed that the weak interfacial bonding was caused by the difference between reaction products of HA and other materials such as the thermal expansion coefficient.
- The microhardness, shear strength, and compressive strength of the composites had a decreasing trend with graphene contents of 0.5–1.5 wt %. This was caused by the agglomeration of graphene. Furthermore, the strengthening mechanisms of composites were mainly second-phase strengthening, fine grain strengthening, dislocation strengthening, and dispersion strengthening.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zavgorodniy, A.V.; Borrero-López, O.; Hoffman, M.; Legeros, R.Z.; Rohanizadehe, R. Characterization of the chemically deposited hydroxyapatite coating on atitanium substrate. J. Mater. Sci. Mater. Med. 2011, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed]
- Surmeneva, M.; Nikityuk, P.; Hans, M.; Surmenev, R. Deposition of ultrathin nano-hydroxyapatite films on laser micro-textured titanium surfaces to prepare a multiscale surface topography for improved surface wettability/energy. Materials 2016, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, S.M.; Silva, A.R.P.; Ciminelli, V.S.; Mansur, H.S. Niobium-doped hydroxyapatite bioceramics: Synthesis, characterization and in vitro cytocompatibility. Materials 2015, 8, 4191–4209. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Abe, Y.; Kobatake, R.; Okazaki, Y.; Oki, Y.; Naito, Y.; Prananingrum, W.; Tsuga, K. Novel development of phosphate treated porous hydroxyapatite. Materials 2017, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Fernández, M.P.; Gehrke, S.A.; Mazón, P.; Calvoguirado, J.L.; De Aza, P.N. Implant stability of biological hydroxyapatites used in dentistry. Materials 2017, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.T.; Biaggio, S.R.; Rocha-Filho, R.C.; Bocchi, N. Influence of hydroxyapatite on the corrosion resistance of the Ti-13Nb-13Zr alloy. J. Mater. Sci. Mater. Med. 2009, 20, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Liu, X.Y.; Hong, H. Characterization of sintered titanium/hydroxyapatite biocomposite using FTIR spectroscopy. J. Mater. Sci. Mater. Med. 2009, 20, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Ru, H.Q.; Chen, D.L.; Zhang, C.P.; Yang, J.L.; Hu, S.L. Interfacial reactions in Ti-Fe particles reinforced hydroxyapatite matrix composites. Mater. Lett. 2014, 128, 245–247. [Google Scholar] [CrossRef]
- Brailovski, V.; Prokoshkin, S.; Gauthier, M.; Inaekyan, K.; Dubinskiy, S.; Petrzhik, M.; Filonov, M. Bulk and porous metastable beta Ti-Nb-Zr(Ta) alloys for biomedical applications. Mater. Sci. Eng. C 2011, 31, 643–657. [Google Scholar] [CrossRef]
- Akmal, M.; Raza, A.; Khan, M.M.; Khan, M.I.; Hussain, M.A. Effect of nano-hydroxyapatite reinforcement in mechanically alloyed NiTi composites for biomedical implant. Mater. Sci. Eng. C 2016, 68, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Xu, L.; Liu, Z.; Woo, K.D. Effects of Sn content on the microstructure, mechanical properties and biocompatibility of Ti-Nb-Sn/hydroxyapatite biocomposites synthesized by powder metallurgy. Mater. Des. 2013, 49, 511–519. [Google Scholar] [CrossRef]
- Miranda, G.; Araújo, A.; Bartolomeu, F.; Buciumeanu, M.; Carvalho, O.; Souza, J.C.M.; Silva, F.S.; Henriques, B. Design of Ti6Al4V-HA composites produced by hot pressing for biomedical applications. Mater. Des. 2016, 108, 488–493. [Google Scholar] [CrossRef]
- Balbinotti, P.; Gemelli, E.; Buerger, G.; Lima, S.A.; Jesus, J.; Camargo, N.H.A.; Henriques, V.A.R.; Soares, G.D.A. Microstructure development on sintered Ti/HA biocomposites produced bypowder metallurgy. Mater. Res. 2011, 14, 384–393. [Google Scholar] [CrossRef]
- Karrholm, J.; Razaznejad, R. Fixation and bone remodeling around alowstiffness stem in revision surgery. Clin. Orthop. Relat. Res. 2008, 466, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedicalapplications. J. Mech. Behav. Biomed. 2008, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Nomura, N.; Sakamoto, K.; Takahashi, K.; Kato, S.; Abe, Y.; Doi, H.; Tsutsumi, Y.; Kobayashi, M.; Kobayashi, E.; Kim, W.J.; et al. Fabrication and mechanical properties of porous Ti/HA composites for bone fixation devices. Mater. Trans. 2010, 51, 1449–1454. [Google Scholar] [CrossRef]
- Kumar, A.; Biswas, K.; Basu, B. On the toughness enhancement in hydroxyapatite-based composites. Acta Mater. 2013, 61, 5198–5215. [Google Scholar] [CrossRef]
- Bovand, D.; Yousefpour, M.; Rasouli, S.; Bagherifard, S.; Bovand, N.; Tamayol, A. Characterization of Ti-HA compositefabricated by mechanical alloying. Mater. Des. 2015, 65, 447–453. [Google Scholar] [CrossRef]
- Zhao, G.; Xia, L.; Zhong, B.; Wen, G.; Song, L.; Wang, X. Effect of milling conditions on the properties of HA/Ti feedstock powders and plasma-sprayed coatings. Surf. Coat. Technol. 2014, 251, 38–47. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Niinomi, M.; Li, H. Inhibited grain growth in hydroxyapatite-graphene nanocomposites during high temperature treatment and their enhanced mechanical properties. Ceram. Int. 2016, 42, 11248–11255. [Google Scholar] [CrossRef]
- Zancanela, D.C.; Simão, A.M.; Francisco, C.G.; Faria, A.N.; Ramos, A.P.; Gonçalves, R.R.; Matsubara, E.Y.; Rosolen, J.M.; Ciancaglini, P. Graphene oxide and titanium: Synergistic effects on the biomineralization ability of osteoblast cultures. J. Mater. Sci. Mater. Med. 2016, 27, 71. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tong, G.; Nian, Q.; Xu, R.; Saei, M.; Chen, F.; Chen, C.; Zhang, M.; Guo, H.; Xu, J. Laser sintered single layer graphene oxide reinforced titanium matrix nanocomposites. Compos. Part B Eng. 2016, 93, 352–359. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Liu, W.W.; Li, W.L.; Wang, Y.G.; Zhao, D.; Liu, X.B. Microscopic mechanicalproperties of titanium composites containing multi-layer graphene nanofillers. Mater. Des. 2016, 109, 256–263. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Jing, P.; Zhai, W. High-temperature tribological performance of TiAl matrix composites reinforced by multilayer graphene. Tribol. Lett. 2015, 58. [Google Scholar] [CrossRef]
- Yang, W.Z.; Huang, W.M.; Wang, Z.F.; Shang, F.J.; Huang, W.; Zhang, B.Y. Thermal and mechanical properties of graphene-titanium composites synthesized by microwave sintering. Acta Metall. Sin. 2016, 29, 707–713. [Google Scholar] [CrossRef]
- Zou, C.; Kang, H.; Wang, W.; Chen, Z.; Li, R.; Gao, X.; Li, T.; Wang, T. Effect of La addition on the particle characteristics, mechanical and electrical properties of in situ Cu-TiB2 composites. J. Alloys Compd. 2016, 687, 312–319. [Google Scholar] [CrossRef]
- Xiao, L.; Lu, W.J.; Qin, J.N.; Chen, Y.F.; Zhang, D.; Wang, M.M.; Zhu, F.; Ji, B. Steady state creep of insitu TiB plus La2O3 reinforced high temperature titanium matrix composite. Mater. Sci. Eng. A 2009, 499, 500–506. [Google Scholar] [CrossRef]
- Hadi, M.; Shafyei, A.; Meratian, M. A comparative study of microstructure and high temperature mechanical properties of a β-stabilized TiAl alloy modified by lanthanum and erbium. Mater. Sci. Eng. A 2015, 624, 1–8. [Google Scholar] [CrossRef]
- Shao, Z.; Sun, Y.; Liu, W.; Zhang, X.; Jiang, X. Effects of multi-phase reinforcements on microstructures, mechanical and tribological properties of Cu/Ti3SiC2/C/BN/GNPs nanocomposites sintered by vacuum hot-pressing and hot isostatic pressing. Metals 2016, 6, 324. [Google Scholar] [CrossRef]
- Nouri, A.; Hodgson, P.D.; Wen, C.E. Effect of process control agent on the porousstructure and mechanical properties of abiomedical Ti-Sn-Nb alloy producedby powder metallurgy. Acta Biomater. 2010, 6, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- He, Y.H.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Effect of HA (hydroxyapatite) content on the microstructure, mechanical and corrosion properties of (Ti, 13Nb, 13Zr)-xHA biocomposites synthesized by sparkle plasma sintering. Vacuum 2016, 131, 176–180. [Google Scholar] [CrossRef]
- Arifin, A.; Sulong, A.B.; Muhamad, N.; Syarif, J.; Ramli, M.I. Material processing of hydroxyapatite and titanium alloy (HA/Ti) composite as implant materials using powder metallurgy: Areview. Mater. Des. 2014, 55, 165–175. [Google Scholar] [CrossRef]
- Zhu, S.L.; Wang, X.M.; Xie, G.Q.; Qin, F.X.; Yoshimura, M.; Inoue, A. Formation of Ti-based bulk glassy alloy/hydroxyapatite composite. Scr. Mater. 2008, 58, 287–290. [Google Scholar] [CrossRef]
- Zhang, L.; He, Z.Y.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Rapidly sintering of interconnected porous Ti-HA biocomposite with high strength and enhanced bioactivity. Mater. Sci. Eng. C 2016, 67, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Omran, A.M.; Woo, K.D.; Kang, D.S.; Abdel-Gaber, G.T.; Fouad, H.; Abdo, H.S.; Khalil, K.A. Fabrication and evaluation of porous Ti-HA bio-nanomaterial by leaching process. Arab. J. Chem. 2015, 8, 372–379. [Google Scholar] [CrossRef]
- Zhang, L.; He, Z.Y.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Enhanced in vitro bioactivity of porous NiTi-HA composites with interconnected pore characteristics prepared by spark plasma sintering. Mater. Des. 2016, 101, 170–180. [Google Scholar] [CrossRef]
- Nath, S.; Tripathi, R.; Basu, B. Understanding Phase Stability, Microstructure development and biocompatibility in calcium phosphate-titania composites, synthesized from hydroxyapatite and titanium powder mix. Mater. Sci. Eng. C 2009, 29, 97–107. [Google Scholar] [CrossRef]





| Region | O | P | Ca | Ti | Zr | Nb | La | Ta |
|---|---|---|---|---|---|---|---|---|
| 1 | 46.98 | 13.70 | 29.21 | 6.76 | 2.70 | 0.52 | --- | 0.13 |
| 2 | 69.18 | 8.38 | 14.71 | 3.39 | 2.42 | 1.73 | 0.01 | 0.18 |
| Sample | Microhardness/HV | Compressive Strength/MPa | Shear Strength/MPa | |
|---|---|---|---|---|
| Front | Side | |||
| 0.5-GNFs | 105.10 | 106.71 | 178.08 | 37.45 |
| 1.0-GNFs | 75.39 | 70.63 | 140.76 | 30.60 |
| 1.5-GNFs | 64.32 | 65.57 | 145.04 | 28.56 |
| Region | O | P | Ca | Ti | Zr | Nb | La | Ta |
|---|---|---|---|---|---|---|---|---|
| A | 61.91 | 1.97 | 5.83 | 0.76 | 1.58 | 27.83 | 0.06 | 0.05 |
| B | 41.34 | 1.46 | 0.61 | 55.19 | 0.40 | 0.94 | --- | 0.06 |
| C | 81.94 | 0.16 | 1.52 | 2.12 | 0.76 | 0.28 | 13.21 | --- |
| D | 41.81 | 1.69 | 4.37 | 10.75 | 0.02 | 2.81 | --- | 38.54 |
| E | 21.91 | 1.40 | 2.18 | 9.98 | 0 | 1.88 | --- | 62.85 |
| Element | Treatment Condition | 2 | Crystal Face | Grain Size/Å |
|---|---|---|---|---|
| α-Ti | milled for 5 h | 40.147/38.440/35.135 | (101)/(002)/(100) | 371/333/311 |
| sintered at 1000 °C | 39.818/37.777/34.885 | 441/470/399 | ||
| Ta/Nb | milled for 5 h | 38.440/69.664/55.625 | (110)/(211)/(200) | 333/272/275 |
| sintered at 1000 °C | 38.613/69.795/55.724 | 305/204/249 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Jiang, X.; Shao, Z.; Zhu, D.; Zhu, M. Microstructure and Mechanical Properties of Graphene-Reinforced Titanium Matrix/Nano-Hydroxyapatite Nanocomposites. Materials 2018, 11, 608. https://doi.org/10.3390/ma11040608
Li F, Jiang X, Shao Z, Zhu D, Zhu M. Microstructure and Mechanical Properties of Graphene-Reinforced Titanium Matrix/Nano-Hydroxyapatite Nanocomposites. Materials. 2018; 11(4):608. https://doi.org/10.3390/ma11040608
Chicago/Turabian StyleLi, Feng, Xiaosong Jiang, Zhenyi Shao, Degui Zhu, and Minhao Zhu. 2018. "Microstructure and Mechanical Properties of Graphene-Reinforced Titanium Matrix/Nano-Hydroxyapatite Nanocomposites" Materials 11, no. 4: 608. https://doi.org/10.3390/ma11040608
APA StyleLi, F., Jiang, X., Shao, Z., Zhu, D., & Zhu, M. (2018). Microstructure and Mechanical Properties of Graphene-Reinforced Titanium Matrix/Nano-Hydroxyapatite Nanocomposites. Materials, 11(4), 608. https://doi.org/10.3390/ma11040608





