The CiCs(SiI)n Defect in Silicon from a Density Functional Theory Perspective
Abstract
:1. Introduction
2. Methodology
2.1. Details of Calculations
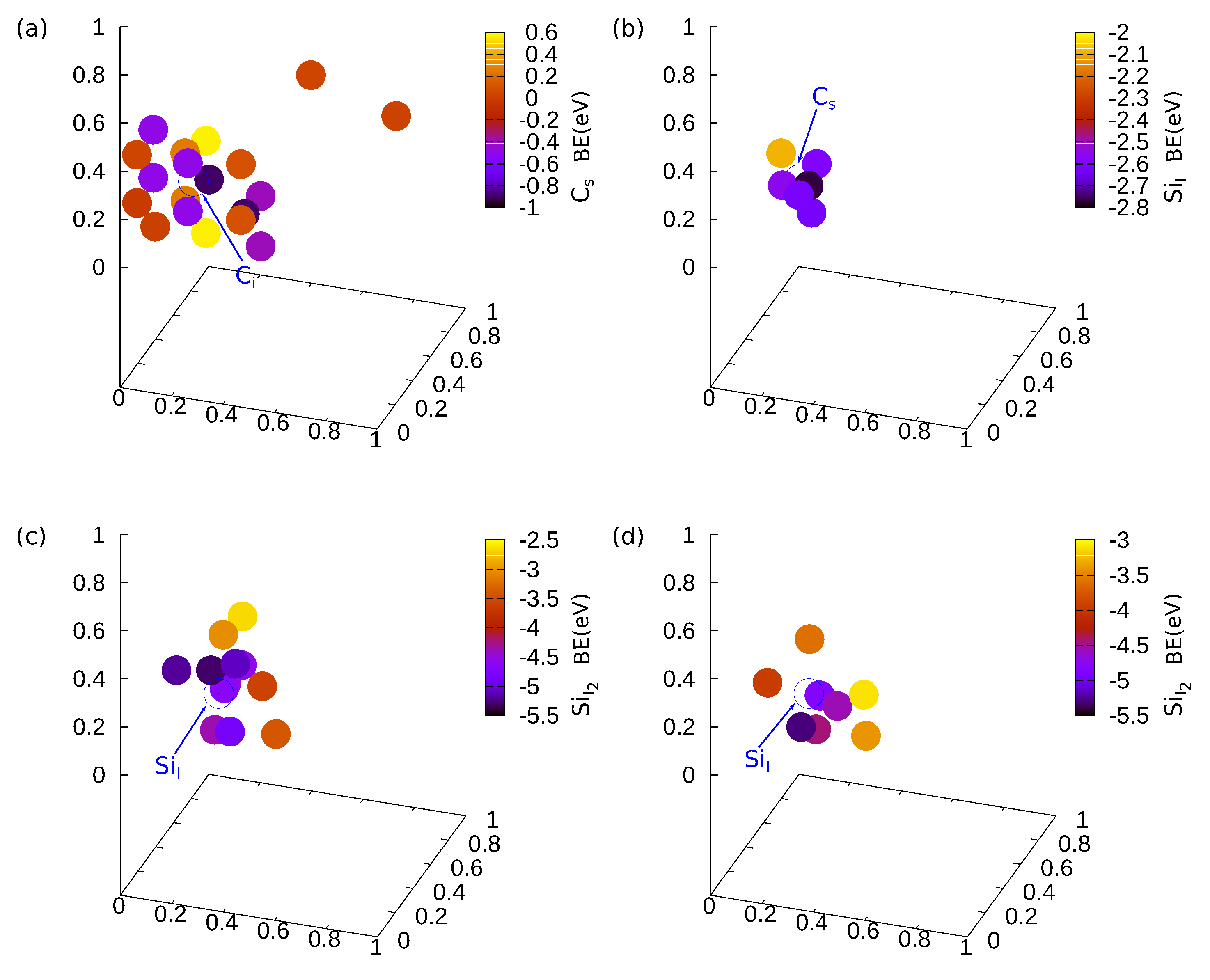
2.2. Definitions of Binding Energies
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Claeys, C.; Simoen, E. Germanium-Based Technologies: From Materials to Devices; Elsevier Science: New York, NY, USA, 2007. [Google Scholar]
- Bracht, H.; Chroneos, A. The vacancy in silicon: A critical evaluation of experimental and theoretical studies. J. Appl. Phys. 2008, 104, 076108. [Google Scholar] [CrossRef]
- Chroneos, A.; Grimes, R.W.; Bracht, H. Impact of germanium on vacancy clustering in germanium-doped silicon. J. Appl. Phys. 2009, 105, 016102. [Google Scholar] [CrossRef]
- Wang, H.; Chroneos, A.; Londos, C.A.; Sgourou, E.N.; Schwingenschlögl, U. A-centers in silicon studied with hybrid density functional theory. Appl. Phys. Lett. 2013, 103, 052101. [Google Scholar] [CrossRef]
- Mostafa, A.; Medraj, M. Binary phase diagrams and thermodynamic properties of silicon and essential doping elements (Al, As, B, Bi, Ga, In, N, P, Sb and Tl). Materials 2017, 10, 676. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Yu, X.G.; Chen, L.; Ma, X.Y.; Yang, D.R. Effect of germanium doping on the formation kinetics of vacancy-dioxygen complexes in high dose neutron irradiated crystalline silicon. J. Appl. Phys. 2017, 122, 095704. [Google Scholar] [CrossRef]
- Chroneos, A. Effect of carbon on dopant–vacancy pair stability in germanium. Semicond. Sci. Technol. 2011, 26, 095017. [Google Scholar] [CrossRef]
- Sun, J.F.; He, Q.X.; Ban, B.Y.; Bai, X.L.; Li, J.W.; Chen, J. Effect of Sn doping on improvement of minority carrier lifetime of Fe contaminated p-type multi-crystalline Si ingot. J. Cryst. Growth 2017, 458, 66. [Google Scholar] [CrossRef]
- Kube, R.; Bracht, H.; Chroneos, A.; Posselt, M.; Schmidt, B. Intrinsic and extrinsic diffusion of indium in germanium. J. Appl. Phys. 2009, 106, 063534. [Google Scholar] [CrossRef]
- Christopoulos, S.-R.G.; Sgourou, E.N.; Vovk, R.V.; Chroneos, A.; Londos, C.A. Impact of isovalent doping on the formation of the CiOi(SiI)n defects in silicon. Solid State Commun. 2017, 263, 19. [Google Scholar] [CrossRef]
- Brozel, M.R.; Newman, R.C.; Totterdell, D.H.J. Interstitial defects involving carbon in irradiated silicon. J. Phys. C Solid State Phys. 1975, 8, 243. [Google Scholar] [CrossRef]
- Davies, G.; Lightowlers, E.C.; Newman, R.C.; Oates, A.S. A model for radiation damage effects in carbon-doped crystalline silicon. Semicond. Sci. Technol. 1987, 2, 524. [Google Scholar] [CrossRef]
- Coutinho, J.; Jones, R.; Briddon, P.R.; Öberg, S. Oxygen and dioxygen centers in Si and Ge: Density-functional calculations. Phys. Rev. B 2000, 62, 10824–10840. [Google Scholar] [CrossRef]
- Mattoni, A.; Bernardini, F.; Colombo, L. Self-interstitial trapping by carbon complexes in crystalline silicon. Phys. Rev. B 2002, 66, 195214. [Google Scholar] [CrossRef]
- Londos, C.A.; Potsidi, M.S.; Stakakis, E. Carbon-related complexes in neutron-irradiated silicon. Phys. B Condens. Matter 2003, 340–342, 551–555. [Google Scholar] [CrossRef]
- Potsidi, M.S.; Londos, C.A. The CiCs(SiI) defect in silicon: An infrared spectroscopy study. J. Appl. Phys. 2006, 100, 033523. [Google Scholar] [CrossRef]
- Murin, L.I.; Lindström, J.L.; Davies, G.; Markevich, V.P. Evolution of radiation-induced carbon–oxygen-related defects in silicon upon annealing: LVM studies. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2006, 253, 210–213. [Google Scholar] [CrossRef]
- Londos, C.A.; Andrianakis, A.; Emtsev, V.V.; Ohyama, H. Radiation-induced defects in Czochralski-grown silicon containing carbon and germanium. Semicond. Sci. Technol. 2009, 24, 075002. [Google Scholar] [CrossRef]
- Chroneos, A.; Londos, C.A.; Sgourou, E.N.; Pochet, P. Point defect engineering strategies to suppress A-center formation in silicon. Appl. Phys. Lett. 2011, 99, 241901. [Google Scholar] [CrossRef]
- Chroneos, A. Effect of germanium substrate loss and nitrogen on dopant diffusion in germanium. J. Appl. Phys. 2009, 105, 056101. [Google Scholar] [CrossRef]
- Wang, H.; Chroneos, A.; Londos, C.A.; Sgourou, E.N.; Schwingenschlögl, U. Carbon related defects in irradiated silicon revisited. Sci. Rep. 2014, 4, 4909. [Google Scholar] [CrossRef] [PubMed]
- Murin, L.I.; Svensson, B.G.; Markevich, V.P.; Peaker, A.R. Interactions of Self-Interstitials with Interstitial Carbon-Interstitial Oxygen Center in Irradiated Silicon: An Infrared Absorption Study. Solid State Phenom. 2014, 205–206, 218–223. [Google Scholar] [CrossRef]
- Chroneos, A.; Sgourou, E.N.; Londos, C.A.; Schwingenschlögl, U. Oxygen defect processes in silicon and silicon germanium. Appl. Phys. Rev. 2015, 2, 021306. [Google Scholar] [CrossRef]
- Angeletos, T.; Chroneos, A.; Londos, C.A. Infrared studies of the evolution of the CiOi(SiI) defect in irradiated Si upon isothermal anneals. J. Appl. Phys. 2016, 119, 125704. [Google Scholar] [CrossRef]
- Beaufils, C.; Redjem, W.; Rousseau, E.; Jacques, V.; Kuznetsov, A.Y.; Raynaud, C.; Voisin, C.; Benali, A.; Herzig, T.; Pezzagna, S.; et al. Optical properties of an ensemble of G-centers in silicon. Phys. Rev. B 2018, 97, 035303. [Google Scholar] [CrossRef]
- Stiebel, D.; Pichler, P.; Cowern, N.E.B. A reduced approach for modeling the influence of nanoclusters and {113} defects on transient enhanced diffusion. Appl. Phys. Lett. 2001, 79, 2654–2656. [Google Scholar] [CrossRef]
- Huhtinen, M. Simulation of non-ionising energy loss and defect formation in silicon. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 2002, 491, 194–215. [Google Scholar] [CrossRef]
- Docaj, A.; Estreicher, S.K. Three carbon pairs in Si. In Proceedings of the 26th International Conference on Defects in Semiconductors, Nelson, New Zealand, 17–22 July 2012; Volume 407, pp. 2981–2984. [Google Scholar] [CrossRef]
- Payne, M.C.; Teter, M.P.; Allan, D.C.; Arias, T.A.; Joannopoulos, J.D. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Chroneos, A.; Londos, C.A. Interaction of A-centers with isovalent impurities in silicon. J. Appl. Phys. 2010, 107, 093518. [Google Scholar] [CrossRef]
- Chroneos, A.; Londos, C.A.; Bracht, H. A-centers and isovalent impurities in germanium: Density functional theory calculations. Mater. Sci. Eng. B 2011, 176, 453–457. [Google Scholar] [CrossRef]
- Zirkelbach, F.; Stritzker, B.; Nordlund, K.; Lindner, J.K.N.; Schmidt, W.G.; Rauls, E. Combined ab initio and classical potential simulation study on silicon carbide precipitation in silicon. Phys. Rev. B 2011, 84, 064126. [Google Scholar] [CrossRef]
- Sgourou, E.N.; Timerkaeva, D.; Londos, C.A.; Aliprantis, D.; Chroneos, A.; Caliste, D.; Pochet, P. Impact of isovalent doping on the trapping of vacancy and interstitial related defects in Si. J. Appl. Phys. 2013, 113, 239901. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J. Relations between the Concentrations of Imperfections in Crystalline Solids. In Solid State Physics; Seitz, F., Turnbull, D., Eds.; Academic Press: Cambridge, MA, USA, 1956; Volume 3, pp. 307–345. ISBN 0081-1947. [Google Scholar] [CrossRef]

| Title | BE/eV | BE Difference/eV |
|---|---|---|
| CiCs | −0.90 | - |
| CiCs(SiI) | −2.77 | −1.87 |
| CiCs(SiI)2 | −5.30 | −2.53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopoulos, S.-R.G.; Sgourou, E.N.; Vovk, R.V.; Chroneos, A.; Londos, C.A. The CiCs(SiI)n Defect in Silicon from a Density Functional Theory Perspective. Materials 2018, 11, 612. https://doi.org/10.3390/ma11040612
Christopoulos S-RG, Sgourou EN, Vovk RV, Chroneos A, Londos CA. The CiCs(SiI)n Defect in Silicon from a Density Functional Theory Perspective. Materials. 2018; 11(4):612. https://doi.org/10.3390/ma11040612
Chicago/Turabian StyleChristopoulos, Stavros-Richard G., Efstratia N. Sgourou, Ruslan V. Vovk, Alexander Chroneos, and Charalampos A. Londos. 2018. "The CiCs(SiI)n Defect in Silicon from a Density Functional Theory Perspective" Materials 11, no. 4: 612. https://doi.org/10.3390/ma11040612






