Fabrication of FeAl Intermetallic Foams by Tartaric Acid-Assisted Self-Propagating High-Temperature Synthesis
Abstract
:1. Introduction
2. Materials and Methods
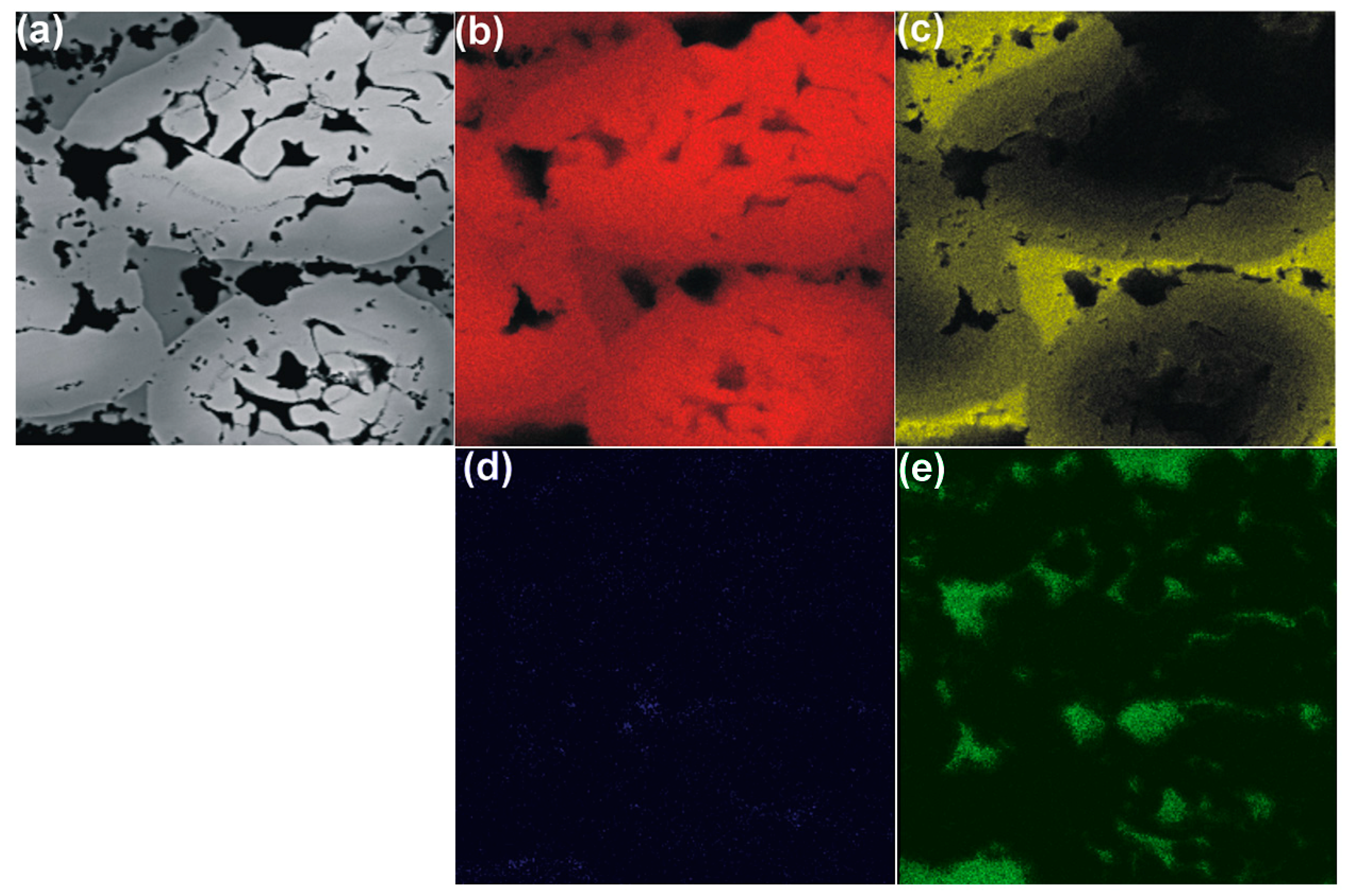
3. Results and Discussion
4. Conclusions
- Sintering of iron and aluminum powders with the addition of tartaric acid allows one to successfully form Fe-Al intermetallic foams.
- Application of tartaric acid as the in situ foaming agent allows one to obtain foams with a more regular distribution of the pores, taking under consideration the surface and core of the specimens.
- The expected performance of the formed foams is promising for future applications of these materials as filters in aggressive environments of hot gases.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, Y.; He, Y.; Liu, C.T. Review of porous intermetallic compounds by reactive synthesis of elemental powders. Intermetallics 2018, 93, 217–226. [Google Scholar] [CrossRef]
- Wang, Z.; Jiao, X.; Feng, P.; Wang, X.; Liu, Z. Intermetallics highly porous open cellular TiAl-based intermetallics fabricated by thermal explosion with space holder process. Intermetallics 2016, 68, 95–100. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, X.; Kang, X.; Feng, P.; Zhang, L.; Wang, J.; Akhtar, F. Hierarchical porous TiAl3 intermetallics synthesized by thermal explosion with a leachable space-holder material. Mater. Lett. 2016, 181, 261–264. [Google Scholar] [CrossRef]
- Łazinska, M.; Durejko, T.; Lipinski, S.; Polkowski, W.; Czujko, T.; Varin, R.A. Porous graded FeAl intermetallic foams fabricated by sintering process using NaCl space holders. Mater. Sci. Eng. A 2015, 636, 407–414. [Google Scholar] [CrossRef]
- Ismail, M.H.; Goodall, R.; Davies, H.A.; Todd, I. Porous NiTi alloy by metal injection moulding/sintering of elemental powders: Effect of sintering temperature. Mater. Lett. 2012, 70, 142–145. [Google Scholar] [CrossRef]
- Pałka, K.; Adamek, G.; Jakubowicz, J. Compression behavior of Ti foams with spherical and polyhedral pores. Adv. Eng. Mater. 2016, 18, 1511–1518. [Google Scholar] [CrossRef]
- Jakubowicz, J.; Adamek, G.; Dewidar, M. Titanium foam made with saccharose as a space holder. J. Porous Mater. 2013, 20, 1137–1141. [Google Scholar] [CrossRef]
- Matsuura, K.; Kitamutra, T.; Kudoh, M. Microstructure and mechanical properties of NiAl intermetallic compound synthesized by reactive sintering under pressure. J. Mater. Process. Technol. 1997, 63, 298–302. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Fung, T.; Zhang, L.P.; Zhang, F.L. Lost carbonate sintering process for manufacturing metal foams. Scr. Mater. 2005, 52, 295–298. [Google Scholar] [CrossRef]
- Karczewski, K.; Stępniowski, W.J.; Jóźwiak, S. Highly-porous FeAl intermetallic foams formed via sintering with Eosin Y as a gas releasing agent. Mater. Lett. 2016, 178, 268–271. [Google Scholar] [CrossRef]
- Karczewski, K.; Stępniowski, W.J.; Chojnacki, M.; Jóźwiak, S. Crystalline oxalic acid aided FeAl intermetallic alloy sintering. Fabrication of intermetallic foam with porosity above 45%. Mater. Lett. 2016, 164, 32–34. [Google Scholar] [CrossRef]
- Karczewski, K.; Stepniowski, W.J.; Salerno, M. Amino acids aided sintering for the formation of highly porous FeAl intermetallic alloys. Materials 2017, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.; Stępniowski, W.; Kaczor, P.; Jóźwiak, S. Fabrication of Fe-Al intermetallic foams via organic compounds assisted sintering. Materials 2015, 8, 2217–2226. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Suzuki, Y.; Goto, T.; Cho, S.H.; Sekino, T. Homogeneously bulk porous calcium hexaaluminate (CaAl12O19): Reactive sintering and microstructure development. Ceram. Int. 2018, 44, 4462–4466. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Jiang, Y. Pore formation process of porous Ti3SiC2 fabricated by reactive sintering. Materials 2017, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, Y.; Feng, P.; Jiao, X.; Zhang, L.; Wang, J. Fe-Al intermetallic foam with porosity above 60% prepared by thermal explosion. J. Alloys Compd. 2018, 732, 443–447. [Google Scholar] [CrossRef]
- Michalcová, A.; Vojtěch, D.; Kubatík, T.F.; Novák, P.; Dvořák, P.; Svobodová, P.; Marek, I. NiAl intermetallic prepared with reactive sintering and subsequent powder-metallurgical plasma-sintering compaction. Mater. Tehnol. 2016, 50, 447–450. [Google Scholar] [CrossRef]
- Novák, P.; Šotka, D.; Novák, M.; Michalcová, A.; Šerák, J.; Vojtěch, D. Production of NiAl–matrix composites by reactive sintering. Powder Metall. 2011, 54, 308–313. [Google Scholar] [CrossRef]
- Wally, Z.J.; van Grunsven, W.; Claeyssens, F.; Goodall, R.; Reilly, G.C. Porous titanium for dental implant applications. Metals 2015, 5, 1902–1920. [Google Scholar] [CrossRef]
- Chen, S.; Bourham, M.; Rabiei, A. Applications of open-cell and closed-cell metal foams for radiation shielding. Proced. Mater. Sci. 2014, 4, 293–298. [Google Scholar] [CrossRef]
- Jin, W.; Liu, J.; Wang, Z.; Wang, Y.; Cao, Z.; Liu, Y.; Zhu, X. Sound absorption characteristics of aluminum foams treated by plasma electrolytic oxidation. Materials 2015, 8, 7511–7518. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, F. Commercial applications of metal foams: Their properties and production. Materials 2016, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Detsi, E.; Tolbert, S.H.; Punzhin, S.; De Hosson, J.T.M. Metallic muscles and beyond: Nanofoams at work. J. Mater. Sci. 2016, 51, 615–634. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karczewski, K.; Stępniowski, W.J.; Salerno, M. Fabrication of FeAl Intermetallic Foams by Tartaric Acid-Assisted Self-Propagating High-Temperature Synthesis. Materials 2018, 11, 621. https://doi.org/10.3390/ma11040621
Karczewski K, Stępniowski WJ, Salerno M. Fabrication of FeAl Intermetallic Foams by Tartaric Acid-Assisted Self-Propagating High-Temperature Synthesis. Materials. 2018; 11(4):621. https://doi.org/10.3390/ma11040621
Chicago/Turabian StyleKarczewski, Krzysztof, Wojciech J. Stępniowski, and Marco Salerno. 2018. "Fabrication of FeAl Intermetallic Foams by Tartaric Acid-Assisted Self-Propagating High-Temperature Synthesis" Materials 11, no. 4: 621. https://doi.org/10.3390/ma11040621




