Microstructure and Corrosion Resistance of PEO Coatings Formed on KBM10 Mg Alloy Pretreated with Nd(NO3)3
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Coatings Preparation
2.2. Coatings Characterization
2.3. Degradation Behavior Examination
3. Results and Discussion
3.1. Morphology and Composition
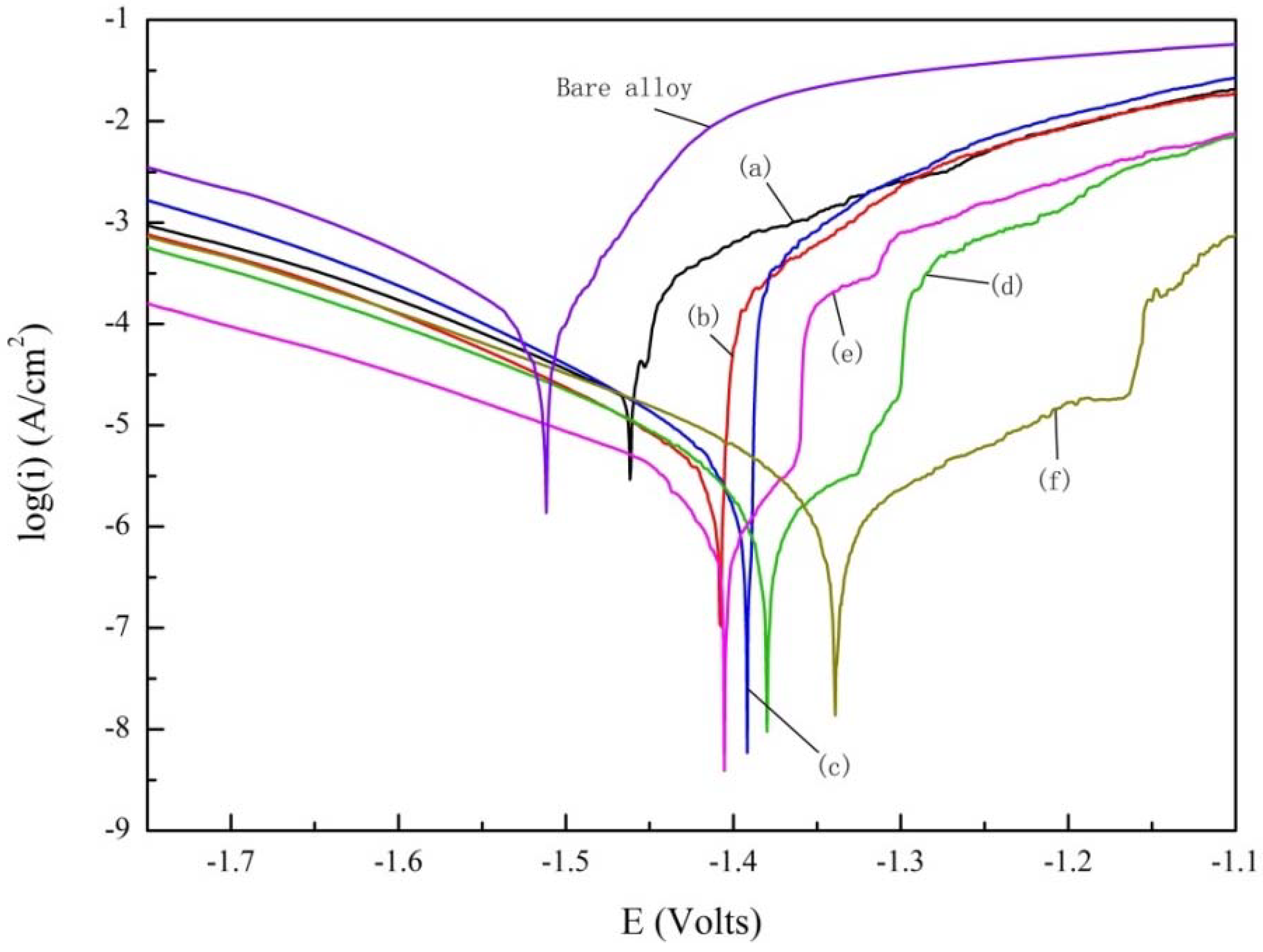
3.2. Polarization Test
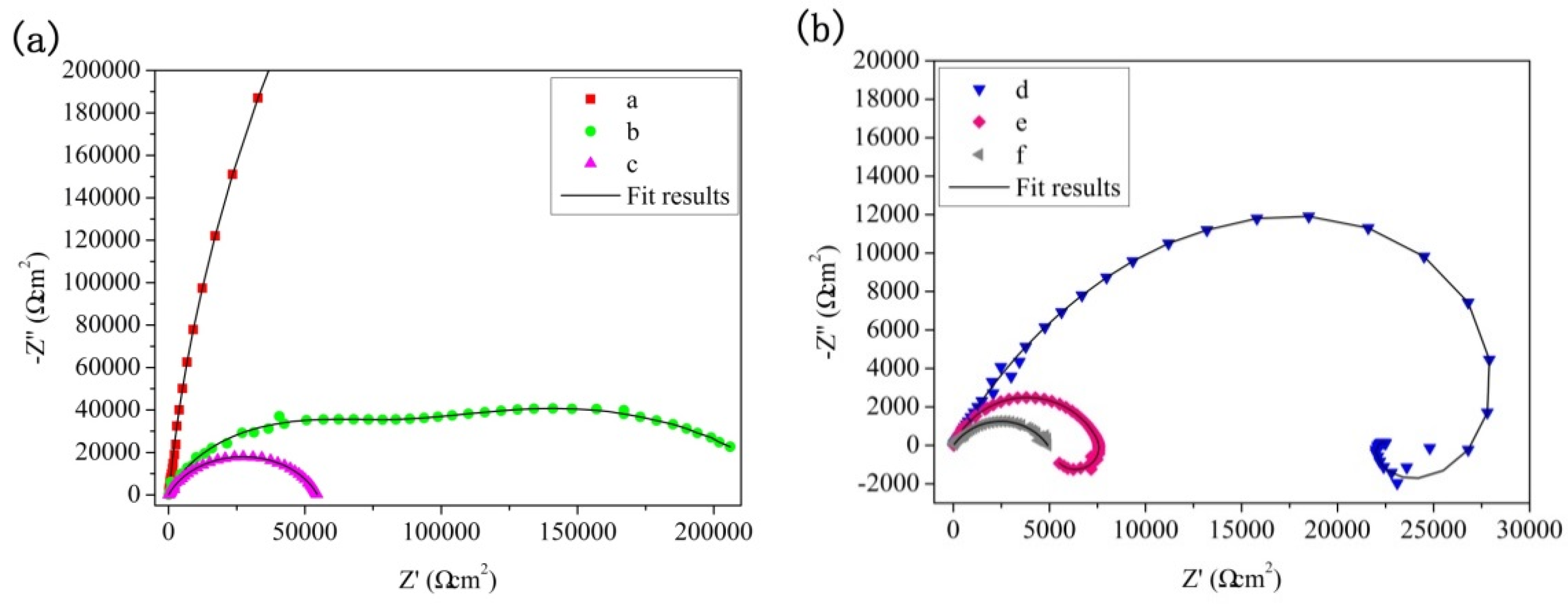
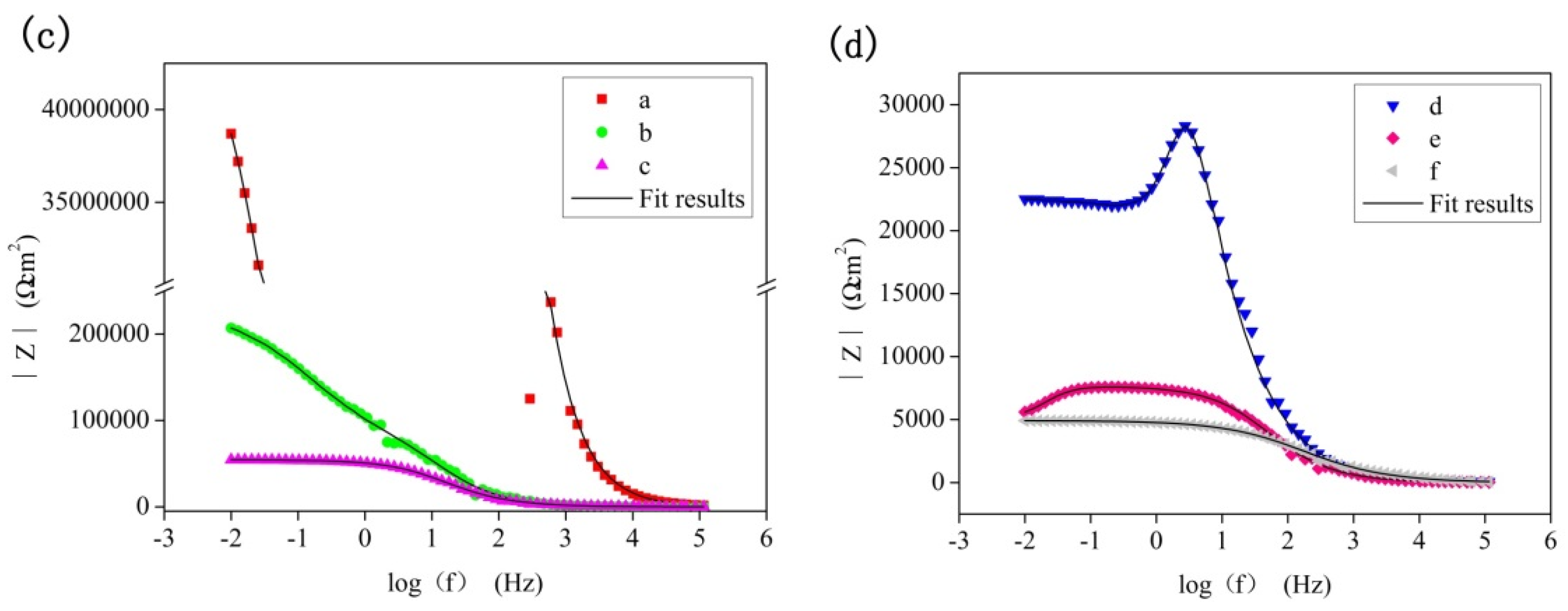
3.3. Long Time Immersion Tests
3.3.1. Morphology and Composition
3.3.2. Corrosion Resistance
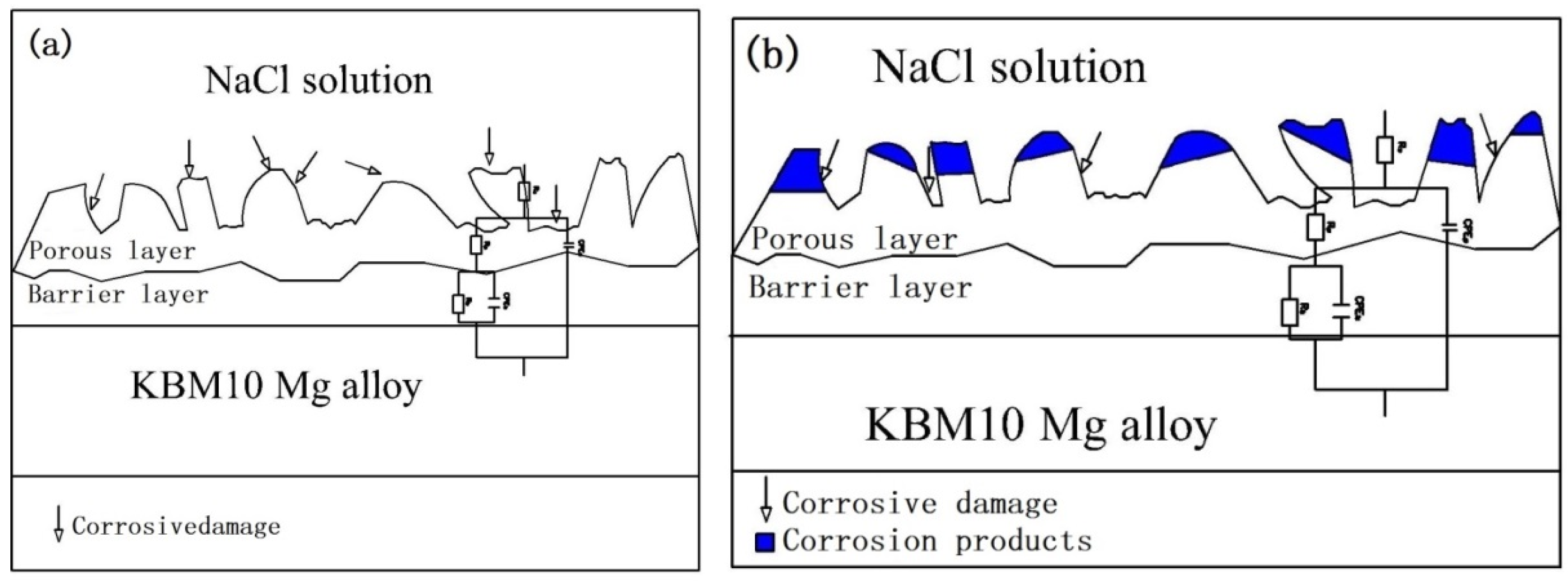
3.3.3. Degradation Mechanism Model
4. Conclusions
- (1)
- The coating thickness exhibits an increase with increasing the Nd(NO3)3 solution concentration.
- (2)
- The PEO coatings were mainly composed of MgO, Al2O3, Mg2SiO4, MgF2, and Nd2O3.
- (3)
- The corrosion resistance of PEO coatings can be improved effectively pretreating the Nd(NO3)3 solution, and the best corrosion resistance was at the Nd(NO3)3 concentration of 0.06 mol/L.
- (4)
- During the immersion tests, the main phase of the coatings, i.e., MgO, was transformed into Mg(OH)2 precipitated into solution.
Author Contributions
Funding
Conflicts of Interest
References
- Jin, S.W.; Li, Y.P.; Nie, S. An integrated bi-level optimization model for air quality management of Beijing’s energy system under uncertainty. J. Hazard. Mater. 2018, 350, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, J.; Wu, H.; Zhang, Z.; Fan, H. A novel approach to estimate the plastic anisotropy of metallic materials using cross-sectional indentation applied to extruded magnesium alloy AZ31B. Materials 2017, 10, 1065. [Google Scholar] [CrossRef] [PubMed]
- Mónica, P.; Bravo, P.M.; Cárdenas, D. Deep cryogenic treatment of HPDC AZ91 magnesium alloys prior to aging and its influence on alloy microstructure and mechanical properties. J. Mater. Process. Technol. 2017, 239, 297–302. [Google Scholar] [CrossRef]
- Baiocco, G.; Rubino, G.; Tagliaferri, V.; Ucciardello, N. Al2O3 coatings on magnesium alloy deposited by the fluidized bed (FB) technique. Materials 2018, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.J.; Song, R.G.; Li, H.X.; Xiang, N. Effect of various additives on performance of plasma electrolytic oxidation coatings formed on AZ31 magnesium alloy in the phosphate electrolytes. J. Wuhan Univ. Techonol. Mater. Sci. Ed. 2018, 33, 703–709. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Shinonaga, Y.; Abe, Y.; Harada, K.; Arita, K. Influence of porous spherical-shaped hydroxyapatite on mechanical strength and bioactive function of conventional glass ionomer cement. Materials 2017, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Koshuro, V.; Fomin, A.; Rodionov, I. Composition, structure and mechanical properties of metal oxide coatings produced on titanium using plasma spraying and modified by micro-arc oxidation. Ceram. Int. 2018, 44, 12593–12599. [Google Scholar] [CrossRef]
- Song, G.L.; Unocic, K.A. The anodic surface film and hydrogen evolution on Mg. Corros. Sci. 2015, 98, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Lu, C.; Wang, C.; Song, R.G. Degradation behavior of n-MAO/EPD bio-ceramic composite coatings on magnesium alloy in simulated body fluid. J. Alloy. Compd. 2015, 625, 258–265. [Google Scholar] [CrossRef]
- Aktug, S.L.; Kutbay, I.; Usta, M. Characterization and formation of bioactive hydroxyapatite coating on commercially pure zirconium by micro arc oxidation. J. Alloy. Compd. 2017, 695, 998–1004. [Google Scholar] [CrossRef]
- Liu, S.; Li, B.; Liang, C.; Wang, H.; Qiao, Z. Formation mechanism and adhesive strength of a hydroxyapatite/TiO2 composite coating on a titanium surface prepared by micro-arc oxidation. Appl. Surf. Sci. 2016, 362, 109–114. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, W.; Du, H.Q.; Zhao, Y.W.; Song, R.G.; Xiang, N. Microstructure and photocatalytic property of TiO2 and Fe3+: TiO2 films produced by micro-arc oxidation. Surf. Coat. Technol. 2017, 315, 196–204. [Google Scholar] [CrossRef]
- Cao, G.P.; Song, R.G. Microstructure and properties of ceramic coatings prepared by micro-arc oxidation on 7075 aluminum alloy. Mater. Res. Express 2018, 5, 026407. [Google Scholar] [CrossRef] [Green Version]
- Li, O.L.; Tsunakawa, M.; Shimada, Y.; Nakamura, K.; Nishinaka, K.; Ishizaki, T. Corrosion resistance of composite oxide film prepared on Ca-added flame-resistant magnesium alloy AZCa612 by micro-arc oxidation. Corros. Sci. 2017, 125, 99–105. [Google Scholar] [CrossRef]
- Bai, C.Y.; Li, J.W.; Ta, W.B.; Li, B.; Han, Y. In vivo study on the corrosion behavior of magnesium alloy surface treated with micro-arc oxidation and hydrothermal deposition. Orthop. Surg. 2017, 9, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Rajeev, G.P.; Takadama, H.; Bakshi, S.R.; Takadama, H. Fabrication of dense alumina layer on Ti alloy hybrid by cold metal transfer and micro-arc oxidation methods. J. Mater. Res. 2017, 32, 3415–3424. [Google Scholar] [CrossRef]
- Durdu, S.; Korkmaz, K.; Aktuğ, S.L.; Çakır, A. Characterization and bioactivity of hydroxyapatite-based coatings formed on steel by electro-spark deposition and micro-arc oxidation. Surf. Coat. Technol. 2017, 326, 111–120. [Google Scholar] [CrossRef]
- Rocca, E.; Veys-Renaux, D.; Guessoum, K. Electrochemical behavior of zinc in KOH media at high voltage: Micro-arc oxidation of zinc. J. Electroanal. Chem. 2015, 754, 125–132. [Google Scholar] [CrossRef]
- Zhuang, J.J.; Song, R.G.; Xiang, N.; Xiong, Y.; Hu, Q. Effect of current density on microstructure and properties of PEO ceramic coatings on magnesium alloy. Surf. Eng. 2017, 33, 744–752. [Google Scholar] [CrossRef]
- Xiong, Y.; Lu, C.; Wang, C.; Song, R.G. The n-MAO/EPD bio-ceramic composite coating fabricated on ZK60 magnesium alloy using combined micro-arc oxidation with electrophoretic deposition. Appl. Surf. Sci. 2014, 322, 230–235. [Google Scholar] [CrossRef]
- Hong, S.K.; Li, Q.N.; Qu, J.J.; Huang, L.; Zhao, L.B. Effects of Ce (NO3)3 additive on the properties of micro-arc oxidation coatings formed on 7075 aluminum alloy. Chin. Surf. Eng. 2014, 6, 020. [Google Scholar]
- Liu, R.X.; Guo, F.; Li, P.F.; Liu, L.; Wang, S.; Zhao, R.R.; Zhang, Y.L. Effect of RE elements in magnesium alloy on surface morphology and structure of ceramic coating by micro-arc oxidation. Heat Treat. Met. 2008, 11, 020. [Google Scholar]
- Chang, M.; Wu, J.; Chen, D.; Ye, S. The composition of the rare earth based conversion coating formed on AZ91D magnesium alloy. Corros. Sci. Technol. 2018, 17, 1–5. [Google Scholar]
- Di, S.; Guo, Y.; Lv, H.; Yu, J.; Li, Z. Microstructure and properties of rare earth CeO2-doped TiO2 nanostructured composite coatings through micro-arc oxidation. Ceram. Int. 2015, 41, 6178–6186. [Google Scholar] [CrossRef]
- Ardelean, H.; Marcus, P.; Fiaud, C. Enhanced corrosion resistance of magnesium and its alloys through the formation of cerium (and aluminium) oxide surface films. Mater. Corros. 2001, 52, 889–895. [Google Scholar] [CrossRef]
- Pan, J.S.; Li, X.L.; Zhang, W.M. The current status and prospects of heat treatment and surface engineering in China. Heat Treat. Met. 2005, 1, 1–8. [Google Scholar]
- Cai, J.; Cao, F.; Chang, L.; Zheng, J.; Zhang, J.; Cao, C. The preparation and corrosion behaviors of MAO coating on AZ91D with rare earth conversion precursor film. Appl. Surf. Sci. 2011, 257, 3804–3811. [Google Scholar] [CrossRef]
- Lu, J.P.; Cao, G.P.; Quan, G.F.; Wang, C.; Zhuang, J.J.; Song, R.G. Effects of voltage on microstructure and corrosion resistance of micro-arc oxidation ceramic coatings formed on KBM10 magnesium alloy. J. Mater. Eng. Perform. 2018, 27, 147–154. [Google Scholar] [CrossRef]
- Zhuang, J.J.; Guo, Y.Q.; Xiang, N.; Xiong, Y.; Hu, Q.; Song, R.G. A study on microstructure and corrosion resistance of ZrO2-containing PEO coatings formed on AZ31 Mg alloy in phosphate-based electrolyte. Appl. Surf. Sci. 2015, 357, 1463–1471. [Google Scholar] [CrossRef]
- Shen, D.J.; Ma, H.J.; Guo, C.H.; Cai, J.R.; Li, G.L.; He, D.L.; Yang, Q.X. Effect of cerium and lanthanum additives on plasma electrolytic oxidation of AZ31 magnesium alloy. J. Rare Earths 2013, 31, 1208–1213. [Google Scholar] [CrossRef]
- Li, J.Z.; Tian, Y.W.; Gui, Z.X.; Huang, Z.Q. Effects of rare earths on the microarc oxidation of a magnesium alloy. Rare Met. 2008, 27, 50–54. [Google Scholar] [CrossRef]
- Pezzato, L.; Brunelli, K.; Babbolin, R.; Dolcet, P.; Dabalà, M. Sealing of PEO coated AZ91 magnesium alloy using La-based solutions. Int. J. Corros. 2017, 2017, 5305218. [Google Scholar] [CrossRef]
- Xiang, N.; Song, R.G.; Li, H.; Wang, C.; Mao, Q.Z.; Xiong, Y. Study on microstructure and electrochemical corrosion behavior of PEO coatings formed on aluminum alloy. J. Mater. Eng. Perform. 2015, 24, 5022–5031. [Google Scholar] [CrossRef]
- He, X.; Kong, D.J.; Song, R.G. Microstructures and properties of laser cladding Al-TiC-CeO2 composite coatings. Materials 2018, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.Y.; Gao, S.D.; Li, P.P.; Zeng, R.C.; Zhang, F.; Li, S.Q.; Han, E.H. Corrosion resistance of a self-healing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2017, 11, 84–95. [Google Scholar] [CrossRef]
- Pezzato, L.; Brunelli, K.; Dabalà, M. Corrosion properties of plasma electrolytic oxidation coated AA7075 treated using an electrolyte containing lanthanum-salts. Surf. Interface Anal. 2016, 48, 729–738. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, Q.; Hu, X.X.; Song, R.G. Microstructure and corrosion resistance of Ti3O5-HA bio-ceramic coating fabricated on AZ80 magnesium alloy. Surf. Coat. Technol. 2017, 325, 239–247. [Google Scholar] [CrossRef]
- Mohedano, M.; Blawert, C.; Zheludkevich, M.L. Cerium-based sealing of PEO coated AM50 magnesium alloy. Surf. Coat. Technol. 2015, 269, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.L.; Li, H.T.; Jiang, B.L.; Liu, C.C.; Gu, X.; Chen, D.C. A comparative study of corrosion behavior of hard anodized and micro-arc oxidation coatings on 7050 aluminum alloy. Metals 2018, 8, 165. [Google Scholar] [CrossRef]
- Dong, K.; Song, Y.; Shan, D.; Han, E.H. Corrosion behavior of a self-sealing pore micro-arc oxidation film on AM60 magnesium alloy. Corros. Sci. 2015, 100, 275–283. [Google Scholar] [CrossRef]
- San, H.S.; Hu, J.; Zhang, Y.F.; Han, J.P.; Tang, S.W. Corrosion behavior of cathodic electrodeposition coatings on micro-arc oxidized titanium alloy in simulated body fluid. J. Electrochem. Soc. 2017, 164, D785–D794. [Google Scholar] [CrossRef]












| Element | Mg | Al | Zn | Mn | Li | Zr | Sb | Mo |
|---|---|---|---|---|---|---|---|---|
| wt % | Bal. | 4–7 | 0.5–2.5 | 1–3 | 0.2–0.8 | 2–1.0 | <1 | <1 |
| Element | 350–0 | 350–2 | 350–4 | 350–6 | 350–8 | 350–10 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt % | at% | wt % | at% | wt % | at% | wt % | at% | wt % | at% | wt % | at% | |
| O | 46.51 | 57.09 | 46.9 | 57.38 | 47.29 | 57.79 | 44.69 | 57.02 | 44.69 | 55.48 | 43.63 | 54.99 |
| F | 3.64 | 3.77 | 4.41 | 4.54 | 4.34 | 4.46 | 4.77 | 5.13 | 6.07 | 6.34 | 6.12 | 6.5 |
| Mg | 39.12 | 31.61 | 37.96 | 30.57 | 37.37 | 30.05 | 35.38 | 29.71 | 36.44 | 29.77 | 36.01 | 29.86 |
| Al | 1.33 | 0.97 | 1.26 | 0.91 | 1.15 | 0.83 | 1.3 | 0.98 | 1.42 | 1.05 | 1.09 | 0.81 |
| Si | 9.4 | 6.57 | 9.46 | 6.6 | 9.86 | 6.86 | 8.88 | 6.45 | 10.18 | 7.2 | 10.38 | 7.45 |
| Nd | - | - | - | - | - | - | 4.98 | 0.71 | 1.19 | 0.16 | 2.77 | 0.39 |
| Sample | Ecorr/(V vs. SCE) | Icorr/(μA/cm2) | βa/(mV) | βc/(mV) | Rp/(Ωcm2) |
|---|---|---|---|---|---|
| Bare alloy | −1.512 | 1.80 × 10−4 | 81.60 | 180.64 | 1.36 × 105 |
| (350–0) | −1.463 | 5.21 × 10−5 | 97.76 | 283.55 | 6.06 × 105 |
| (350–2) | −1.408 | 5.98 × 10−6 | 58.88 | 148.63 | 3.06 × 106 |
| (350–4) | −1.392 | 4.19 × 10−6 | 53.45 | 114.53 | 3.78 × 106 |
| (350–6) | −1.380 | 8.02 × 10−7 | 55.67 | 93.241 | 1.89 × 107 |
| (350–8) | −1.405 | 1.39 × 10−6 | 57.18 | 134.28 | 1.26 × 107 |
| (350–10) | −1.339 | 2.28 × 10−6 | 175.59 | 137.09 | 1.46 × 107 |
| Immersion time | Rs (Ωcm2) | Qp (Ω−1 sn cm−2) | n1 | Rp (Ωcm2) | Qb (Ω−1 sn cm−2) | n2 | Rb (Ωcm2) | L (H) | RL (Ωcm2) |
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 184.3 | 1.31 × 10−9 | 0.97 | 1.16 × 106 | 8.43 × 10−8 | 0.52 | 1.88 × 107 | - | - |
| 48 h | 4.96 | 4.19 × 10−7 | 0.80 | 8.32 × 104 | 2.56 × 10−6 | 1.00 | 3.48 × 104 | - | - |
| 96 h | 5.61 | 2.84 × 10−7 | 0.82 | 1.36 × 103 | 6.12 × 10−7 | 0.70 | 5.42 × 104 | - | - |
| 192 h | 9.52 | 1.62 × 10−6 | 0.70 | 1.06 × 103 | 3.41 × 10−7 | 0.78 | 3.65 × 104 | 4.35 × 103 | 5.79 × 104 |
| 384 h | 9.44 | 1.40 × 10−6 | 0.79 | 4.31 × 103 | 1.98 × 10−6 | 1.00 | 3.06 × 103 | 1.87 × 105 | 1.44 × 104 |
| 768 h | 7.10 | 1.27 × 10−7 | 0.88 | 8.55 × 102 | 7.64 × 10−6 | 0.58 | 3.97 × 103 | 2.66 × 109 | 2.44 × 109 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; He, X.; Li, H.; Song, R. Microstructure and Corrosion Resistance of PEO Coatings Formed on KBM10 Mg Alloy Pretreated with Nd(NO3)3. Materials 2018, 11, 1062. https://doi.org/10.3390/ma11071062
Lu J, He X, Li H, Song R. Microstructure and Corrosion Resistance of PEO Coatings Formed on KBM10 Mg Alloy Pretreated with Nd(NO3)3. Materials. 2018; 11(7):1062. https://doi.org/10.3390/ma11071062
Chicago/Turabian StyleLu, Junpeng, Xing He, Hongxia Li, and Renguo Song. 2018. "Microstructure and Corrosion Resistance of PEO Coatings Formed on KBM10 Mg Alloy Pretreated with Nd(NO3)3" Materials 11, no. 7: 1062. https://doi.org/10.3390/ma11071062





