Fabrication of Sputtered Ce/La, La/Ce Oxide Bilayers on AA6061 and AA7075 Aluminum Alloys for the Development of Corrosion Protective Coatings
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Coating Deposition
2.2. Characterization of Samples
2.3. Electrochemical Performance
3. Results and Discussion
3.1. Influence of Process Parameters on Ce and La Oxide Sputtered Coatings
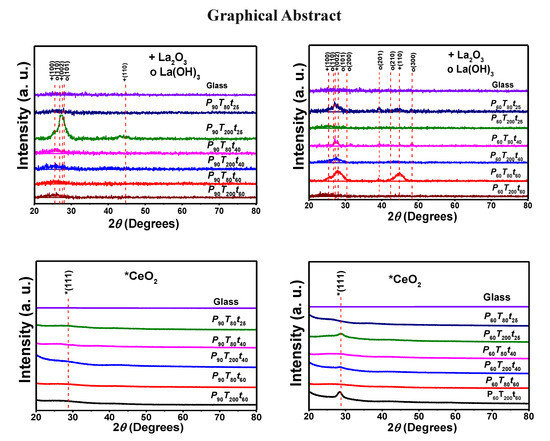
3.1.1. Structural Analysis of Sputtered Ce and La Oxide Coatings
3.1.2. Nature and Chemical Composition of RE Films Deposited on Metallic Substrates
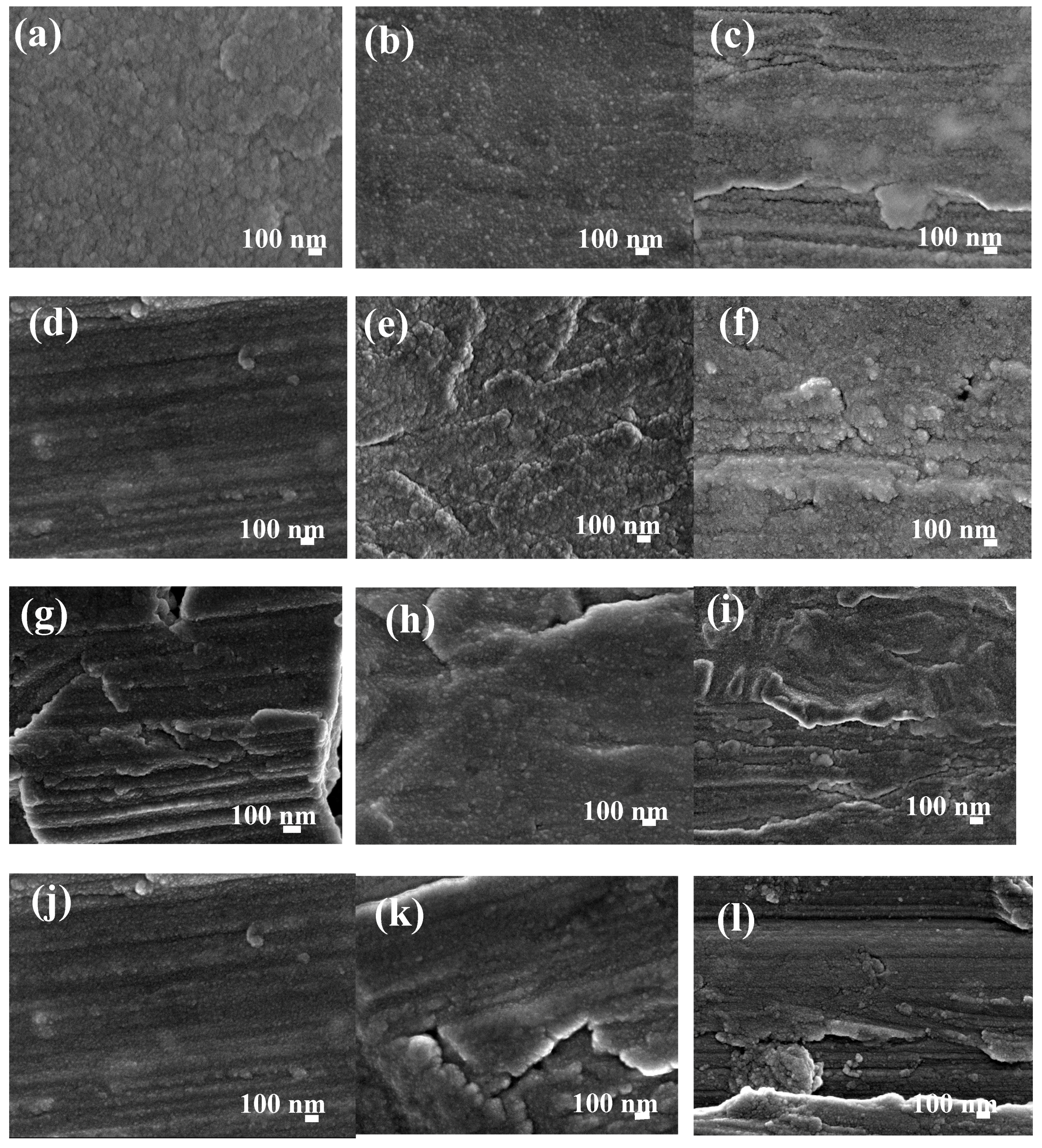
3.1.3. Morphological and Topographical Properties of RE Thin Films on Metallic
3.2. Microstructural Analysis of Ce/La and La/Ce Oxide Bilayered Coatings
3.3. Electrochemical Measurements
3.3.1. Potentiodynamic Curves
3.3.2. EIS Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamdy, A.S.; Butt, D.P. Corrosion mitigation of rare–earth metals containing magnesium EV31A–T6 alloy via chrome–free conversion coating treatment. Electrochim. Acta 2013, 108, 852–859. [Google Scholar] [CrossRef]
- Jana, P.; Jayan, P.S.; Mandal, S.; Biswas, K. Hot corrosion behaviour of rare–earth magnesium hexaaluminate based thermal barrier coatings under molten sulphate–vanadate salts. Mater. Res. Innov. 2014, 18, S4-990–S4-996. [Google Scholar] [CrossRef]
- Zhu, R.; Li, Z.; Li, X.; Sun, Q. Microstructure and properties of the low–power–laser clad coatings on magnesium alloy with different amount of rare earth addition. Appl. Surf. Sci. 2015, 353, 405–413. [Google Scholar] [CrossRef]
- Montemor, M.F. Fostering green inhibitors for corrosion prevention. In Active Protective Coatings, New-Generation Coatings for Metals; Hughes, A.E., Mol, J.M.C., Zheludkevich, M.L., Buchheit, R.G., Eds.; Springer: New York, NY, USA, 2016; pp. 107–137. ISBN 978-94-017-7540-3. [Google Scholar]
- Jamali, S.S.; Moulton, S.E.; Tallman, D.E.; Zhao, Y.; Weber, J.; Wallace, G.G. Self–healing characteristic of praseodymium conversion coating on AZNd Mg alloy studied by scanning electrochemical microscopy. Electrochem. Commun. 2017, 76, 6–9. [Google Scholar] [CrossRef]
- Hughes, A.E.; Mol, J.M.C.; Cole, I.S. The cost and availability of rare earth-based corrosion inhibitors. In Rare Earth-Based Corrosion Inhibitor, 1st ed.; Forsyth, M., Hinton, B., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2014; pp. 291–301. ISBN 9780857093479. [Google Scholar]
- Hughes, A.E.; Harvey, T.G.; Birbilis, N.; Kumar, A.; Buchheit, R.G. Coatings for corrosion prevention based on rare earth. In Rare Earth-Based Corrosion Inhibitor, 1st ed.; Forsyth, M., Hinton, B., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2014; pp. 186–223. ISBN 9780857093479. [Google Scholar]
- Scully, J.R.; Tailleart, N.; Presuel-Moreno, F. Turnable multifunctional corrosion-resistant metallic coatings containing rare earth elements. In Rare Earth-Based Corrosion Inhibitor, 1st ed.; Forsyth, M., Hinton, B., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2014; pp. 267–285. ISBN 9780857093479. [Google Scholar]
- Valdez, B.; Kiyota, S.; Stoytcheva, M.; Zlatev, R.; Bastida, J.M. Cerium–based conversion coatings to improve the corrosion resistance of aluminum alloy 6061–T6. Corros. Sci. 2014, 87, 141–149. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, J.; Zhang, L.; Tan, Y.; Li, J. Corrosion behavior of rare earth cerium based conversion coating on aluminum alloy. J. Rare Earths 2010, 28, 371–374. [Google Scholar] [CrossRef]
- Pan, M.; Meng, G.Y.; Xin, H.W.; Chen, C.S.; Peng, D.K.; Lin, Y.S. Pure and doped CeO2 thin films prepared by MOCVD process. Thin Solid Films 1998, 324, 89–93. [Google Scholar] [CrossRef]
- Gnanarajan, S.; Savvides, N. Evolution of texture of CeO2 thin film buffer layers prepared by ion–assisted deposition. Thin Solid Films 1999, 350, 124–129. [Google Scholar] [CrossRef]
- Wang, A.; Belot, J.A.; Marks, T.J.; Markworth, P.R.; Chang, R.P.H.; Chudzik, M.P.; Kannewurf, C.R. Buffers for high temperature superconductor coatings. Low temperature growth of CeO2 films by metal–organic chemical vapor deposition and their implementation as buffers. Physica C 1999, 320, 154–160. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Liu, Q.; Zhang, M.; Qian, Y. Preparation and characterization of cerium (IV) oxide thin films by spray pyrolysis method. Solid State Ion. 2000, 133, 211–215. [Google Scholar] [CrossRef]
- Fahrenholtz, G.W.; O’Keefe, M.J.; Zhou, H.; Grant, J.T. Characterization of cerium–based conversion coatings for corrosion protection of aluminium alloys. Surf. Coat. Technol. 2002, 155, 208–213. [Google Scholar] [CrossRef]
- Arenas, M.A.; de Damborenea, J.J. Growth mechanisms of cerium layers on galvanised steel. Electrochim. Acta 2003, 48, 3693–3698. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Ohashi, M.; Sakamoto, N.; Shida, S. Orientation selective epitaxial growth of CeO2 layers on Si (100) substrates using reactive DC magnetron sputtering with substrate bias. J. Cryst. Growth 2004, 271, 176–183. [Google Scholar] [CrossRef]
- De Souza, J.; da Silva, A.G.P.; Paes, H.R., Jr. Synthesis and characterization of CeO2 thin films deposited by spray pyrolysis. J. Mater. Sci. Mater. Electron. 2007, 18, 951–956. [Google Scholar] [CrossRef]
- Tok, A.I.Y.; Boey, F.Y.C.; Dong, Z.; Sun, X.L. Hydrothermal synthesis of CeO2 nano–particles. J. Mater. Process Technol. 2007, 190, 217–222. [Google Scholar] [CrossRef]
- Murali, K.R. Characteristics of sol–gel dip coated ceria films. J. Mater. Sci. Mater. Electron 2008, 19, 369–371. [Google Scholar] [CrossRef]
- Ta, M.-T.; Briand, D.; Guhel, Y.; Bernard, J.; Pesant, J.C.; Boudart, B. Growth and structural characterization of cerium oxide thin films realized on Si (111) substrates by on–axis rf magnetron sputtering. Thin Solid Films 2008, 517, 450–452. [Google Scholar] [CrossRef]
- Staudt, T.; Lykhach, Y.; Hammer, L.; Schneider, M.A.; Matolín, V.; Libuda, J. A route to continuous ultra–thin cerium oxide films on Cu (111). Surf. Sci. 2009, 603, 3382–3388. [Google Scholar] [CrossRef]
- Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Brachetti-Sibaja, S.B.; Dorantes-Rosales, H.; Hernández-Pérez, M.A.; Lois-Correa, J.A. Preparation of ZnO:CeO2‒x thin films by AP–MOCVD: Structural and optical properties. J. Solid State Chem. 2010, 183, 2205–2217. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Sundari, S.T.; Kuppusami, P.; Mohan, P.C.; Srinivasan, M.P.; Mohandas, E.; Ganesan, V.; Sastikumar, D. A study of microstructural and optical properties of nanocrystalline ceria thin films prepared by pulsed laser deposition. Thin Solid Films 2011, 519, 2520–2526. [Google Scholar] [CrossRef]
- Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Onofre-Bustamante, E.; Alanis-Valdelamar, A.; Escudero-Rincón, M.L.; Brachetti-Sibaja, S.B. Corrosion studies of PPy/Ni organic–inorganic hybrid bilayer coatings on commercial carbon Steel. J. Solid State Electrochem. 2015, 19, 1073–1089. [Google Scholar] [CrossRef]
- Brachetti-Sibaja, S.B.; Domínguez-Crespo, M.A.; Rodil, S.E.; Torres-Huerta, A.M. Optimal conditions for the deposition of novel anticorrosive coatings by RF magnetron sputtering for aluminum alloy AA6082. J. Alloys Compd. 2014, 615, S437–S443. [Google Scholar] [CrossRef]
- Du, Z.; Liu, X.; Zhang, Y. Synthesis of high–quality AZO polycrystalline films via target bias radio frequency magnetron sputtering. Ceram. Int. 2017, 43, 7543–7551. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, H.; Guo, D.; Wu, D.; Tong, Y. Photoluminescence enhancement induced by nanoparticles from La2O3 and CeO2 doped diamond–like carbon films. J. Alloys Compd. 2009, 476, 318–323. [Google Scholar] [CrossRef]
- Bräuer, G.; Szyszka, B.; Vergöhl, M.; Bandorf, R. Magnetron sputtering milestones of 30 years. Vacuum 2010, 84, 1354–1359. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Laguna, O.H.; Centeno, M.A.; Odriozola, J.A. Structural and catalytic properties of lanthanide (La, Eu, Gd) doped ceria. J. Solid State Chem. 2011, 184, 3014–3020. [Google Scholar] [CrossRef]
- Sanchéz, J.E.; Sanchéz, O.M.; Ipaz, L.; Aperador, W.; Caicedo, J.C.; Amaya, C.; Hernández Landaverde, M.A.; Espinoza Beltran, F.; Muñoz-Saldaña, J.; Zambrano, G. Mechanical, tribological, and electrochemical behavior of Cr1−xAlxN coatings deposited by rf reactive magnetron co–sputtering method. Appl. Surf. Sci. 2010, 256, 2380–2387. [Google Scholar] [CrossRef]
- Martínez-Martínez, D.; López-Cartes, C.; Fernández, A.; Sánchez-López, J.C. Corrosion resistance of CrN thin films produced by dc magnetron sputtering. Appl. Surf. Sci. 2013, 270, 150–156. [Google Scholar]
- Liu, Y.; Huang, J.; Claypool, J.B.; Castano, C.E.; O’Keefe, M.J. Structure and corrosion behavior of sputter deposited cerium oxide based coatings with various thickness on Al 2024–T3 alloy substrates. Appl. Surf. Sci. 2015, 355, 805–813. [Google Scholar] [CrossRef]
- Ou, Y.X.; Lin, J.; Tong, S.; Che, H.L.; Sproul, W.D.; Lei, M.K. Wear and corrosion resistance of CrN/TiN superlattice coatings deposited by a combined deep oscillation magnetron sputtering and pulsed dc magnetron sputtering. Appl. Surf. Sci. 2015, 351, 332–343. [Google Scholar] [CrossRef]
- Dhandapani, V.S.; Subbiah, R.; Thangavel, E.; Arumugam, M.; Park, K.; Gasem, Z.M.; Veeraragavan, V.; Kim, D.-E. Tribological properties, corrosion resistance and biocompatibility of magnetron sputtered titanium–amorphous carbon coatings. Appl. Surf. Sci. 2016, 371, 262–274. [Google Scholar] [CrossRef]
- Jullien, M.; Horwat, D.; Manzeh, F.; Escobar-Galindo, R.; Bauer, P.; Pierson, J.; Endrino, J. Influence of the nanoscale structural features on the properties and electronic structure of Al-doped ZnO thin films: An X-ray absorption study. Sol. Energy Mater. Sol. Cells 2011, 95, 2341–2346. [Google Scholar] [CrossRef]
- Mickana, M.; Helmersson, U.; Horwat, D. Effect of substrate temperature on the deposition of Al-doped ZnO thin films using high power impulse magnetron sputtering. Surf. Coat. Technol. 2018, 347, 245–251. [Google Scholar] [CrossRef]
- Kakushima, K.; Tsutsui, K.; Ohmi, S.-I.; Ahmet, P.; Rao, V.R.; Iwai, H. Rare earth oxides in microelectronics. In Rare Earth Oxide Thin Films; Fanciulli, M., Scarel, G., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2007; Volume 106, pp. 345–365. [Google Scholar]
- Patterson, A.L. The Scherrer formula for X–ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Biju, V.; Sugathan, N.; Vrinda, V.; Salini, S. Estimation of lattice strain in nanocrystalline silver from X–ray diffraction line broadening. J. Mater. Sci. 2008, 43, 1175–1179. [Google Scholar] [CrossRef]
- Yogamalar, R.; Srinivasan, R.; Vinu, A.; Ariga, K.; Bose, A.C. X–ray peak broadening analysis in ZnO nanoparticles. Solid State Commun. 2009, 149, 1919–1923. [Google Scholar] [CrossRef]
- Murugan, R.; Vijayaprasath, G.; Ravi, G. The influence of substrate temperature on the optical and micro structural properties of cerium oxide thin films deposited by RF sputtering. Superlattices Microstruct. 2015, 85, 321–330. [Google Scholar] [CrossRef]
- Castano, C.E.; O’Keefe, M.J.; Fahrenholtz, W.G. Cerium–based oxide coatings. Curr. Opin. Solid State Mater. Sci. 2015, 19, 69–76. [Google Scholar] [CrossRef]
- Anandan, C.; Bera, P. XPS studies on the interaction of CeO2 with silicon in magnetron sputtered CeO2 thin films on Si and Si3N4 substrates. Appl. Surf. Sci. 2013, 283, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Rodil, S.E.; Brachetti-Sibaja, S.B.; de la Cruz, W.; Flores-Vela, A. XPS and EIS studies of sputtered Al-Ce films formed on AA6061 aluminium alloy in 3.0 wt % NaCl solution. J. Appl. Electrochem. 2010, 40, 639–651. [Google Scholar] [CrossRef]
- Domínguez-Crespo, M.A.; Rodil, S.E.; Torres-Huerta, A.M.; Ramírez-Meneses, E.; Suárez-Velázquez, G. Structural and electrochemical performance of sputtered Al-Ce films on AA6061 aluminum alloy substrates. Surf. Coat. Technol. 2009, 204, 571–579. [Google Scholar] [CrossRef]
- Sheng, H.W.; Liu, H.Z.; Cheng, Y.Q.; Wen, J.; Lee, P.L.; Luo, W.K.; Shastri, S.D.; Ma, E. Polyamorphism in a metallic glass. Nat. Mater. 2007, 6, 192–197. [Google Scholar] [CrossRef] [PubMed]
- De Asha, A.M.; Critchley, J.T.S.; Nix, R.M. Molecular adsorption characteristics of lanthanum oxide surfaces: The interaction of water with oxide overlayers grown on Cu(111). Surf. Sci. 1998, 405, 201–214. [Google Scholar] [CrossRef]
- Wandelt, K.; Brundle, C.R. The interaction of oxygen with gadolinium: UPS and XPS studies. Surf. Sci. 1985, 157, 162–182. [Google Scholar] [CrossRef]
- Sunding, M.F.; Hadidi, K.; Diplas, S.; Løvvik, O.M.; Norby, T.E.; Gunnæs, A.E. XPS characterization of in situ treated lanthanum oxide and hydroxide using tailored charge referencing and peak fitting procedures. J. Electron Spectrosc Relat. Phenomena 2011, 184, 399–409. [Google Scholar] [CrossRef]
- Heponiemi, A.; Azalim, S.; Hu, T.; Lassi, U. Cerium oxide based catalysts for wet air oxidation of bisphenol A. Top. Catal. 2015, 58, 1043–1052. [Google Scholar] [CrossRef]
- Zhu, Y.; Jain, N.; Hudait, M.K.; Maurya, D.; Varghese, R.; Priya, S. X–ray photoelectron spectroscopy analysis and band offset determination of CeO2 deposited on epitaxial (100), (110), and (111) Ge. J. Vac. Sci. Technol. B. Nanotechnol. Microelectron. 2014, 32, 011217-1–011217-11. [Google Scholar] [CrossRef]
- Shi, Z.; Shum, P.; Zhou, Z.; Li, L.K.-Y. Effect of oxygen flow ratio on the wetting behavior, microstructure and mechanical properties of CeO2−x coatings prepared by magnetron sputtering. Surf. Coat. Technol. 2017, 320, 333–338. [Google Scholar] [CrossRef]
- Patsalas, P.; Logothetidis, S.; Sygellou, L.; Kennou, S. Structure–dependent electronic properties of nanocrystalline cerium oxide films. Phys. Rev. B 2003, 68, 0351043-1–0351043-13. [Google Scholar] [CrossRef]
- Matolín, V.; Matolínová, I.; Dvořák, F.; Jonhánek, V.; Mysliveček, J.; Prince, K.C.; Skála, T.; Stetsovych, O.; Tsud, N.; Václavů, M.; Šmíd, B. Water interaction with CeO2(111)/Cu(111) model catalyst surface. Catal. Today 2012, 181, 124–132. [Google Scholar] [CrossRef]
- Vook, R.W. Theories of nucleation and growth of thin-films. Thin Film Tech. Special Appl. 1982, 346, 2–8. [Google Scholar]
- Kumar, J.; Kapoor, S.; Gupta, S.K.; Sen, P.K. Theoretical investigation of the effect of asymmetry on optical anisotropy and electronic structure of Stranski-Krastanov quantum dots. Phys. Rev. B 2006, 74, 115326-1–115626-10. [Google Scholar] [CrossRef]
- Webb, H.K.; Truong, V.K.; Hasan, J.; Fluke, C.; Crawford, R.J.; Ivanova, E.P. Roughness Parameters for Standard Description of Surface Nanoarchitecture. Scanning 2012, 34, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Biscarini, F.; Dediu, V.; Greco, O.; Matacotta, F.C.; Migliori, A. Roughness increase and dimensional transitions during the growth of GaBa2Cu3O6+y films on NdGaO3. Nuovo Cimento Della 1997, 19, 1003–1008. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Zhou, Z.; Xu, J. Effects of cerium and lanthanum on the corrosion behavior of Al-3.0 wt % Mg Alloy. J. Mater. Eng. Perform. 2016, 25, 1122–1128. [Google Scholar] [CrossRef]
- Luo, C.; Albu, S.P.; Zhou, X.; Sun, Z.; Zhang, X.; Tang, Z.; Thompson, G.E. Continuous and discontinuous localized corrosion of a 2xxx aluminium–copper–lithium alloy in sodium chloride solution. J. Alloys Compd. 2016, 658, 61–70. [Google Scholar] [CrossRef]
- Bernard, W.J.; Randall, J.J. The reaction between aluminium and water. J. Electrochem. Soc. 1960, 107, 483–487. [Google Scholar] [CrossRef]
- Alwitt, R. The effect of a barrier oxide on evaporation of water from a hydrous aluminium oxide film. J. Electrochem. Soc. 1971, 118, 810–811. [Google Scholar] [CrossRef]
- Martine, A.; Degremont, S.A.; Lyonnaise, E.D. Water Treatment Handbook, 6th ed.; Lavoisier Publishing: Paris, France, 1991; Volume 1, ISBN 13-978-2743009700; ISBN 10-2743009705. [Google Scholar]
- Birbilis, N.; Buchheit, R.G. Electrochemical characteristics of intermetallic phases in aluminium alloys. An experimental survey and discussion. J. Electrochem. Soc. 2005, 152, B140–B151. [Google Scholar] [CrossRef]
- Muñoz, A.G.; Bessone, J.B. Pitting of aluminium in non-aqueous chloride media. Corros. Sci. 1999, 41, 1447–1463. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting corrosion of aluminium. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.P.; Pagetti, J. Characterisation of pitting corrosion in sealed anodic films formed in sulphuric, sulphuric/molybdate and chromic media. Appl. Surf. Sci. 2003, 206, 237–249. [Google Scholar] [CrossRef]
- Mandel, M.; Krüger, L. Determination of pitting sensitivity of the aluminium alloy EN AW-6060-T6 in a carbon-fibre reinforced plastic/aluminium rivet joint by finite element simulation of the galvanic corrosion process. Corros. Sci. 2013, 73, 172–180. [Google Scholar] [CrossRef]
- Gao, M.; Feng, C.R.; Wei, R.P. An analytical electron microscopy study of constitutent particles in commercial 7075-T6 and 2024-T3 alloys. Metall. Mater. Trans. A 1998, 29, 1145–1151. [Google Scholar] [CrossRef]
- Maloney, S.K.; Hono, K.; Polmear, I.J.; Ringer, S.P. The chemistry of precipitates in an aged Al–2.1Zn–1.7Mg at% alloy. Scripta Mater. 1999, 41, 1031–1038. [Google Scholar] [CrossRef]
- Linardi, E.; Collet-Lacoste, J.; Lanzani, L. Characterization of AA6061 alloy oxides obtained in high purity water and in diluted NaCl solution. Procedia Mater. Sci. 2015, 8, 56–64. [Google Scholar] [CrossRef]
- Diaz, B.; Swiatowska, J.; Maurice, V.; Seyeux, A.; Normand, B.; Härkonen, E.; Ritala, M.; Marcus, P. Electrochemical and time-of-flight secondary ion mass spectrometry analysis of ultra-thin metal oxide (Al2O3 and Ta2O5) coatings deposited by atomic layer deposition on stainless steel. Electrochim. Acta 2011, 56, 10516–10523. [Google Scholar] [CrossRef]
- Lazar, A.M.; Yespica, W.P.; Marcelin, S.; Pebere, N.; Samelor, D.; Tendero, C.; Vahlas, C. Corrosion protection of 304 L stainless steel by chemical vapor deposited alumina coatings. Corros. Sci. 2014, 81, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Cao, C.; Yao, Z.; Zhou, D.; Yin, Z. Study of double layer rare earth metal conversion coating on aluminium alloy LY12. Corros. Sci. 2001, 43, 1283–1294. [Google Scholar]
- Yu, X.; Cao, C.; Yao, Z.; Zhou, D.; Yin, Z. Corrosion behavior of rare earth metal (REM) conversion coatings on aluminium alloy LY12. Mat. Sci. Eng. A Struct. 2003, 284, 56–63. [Google Scholar]
- Zivkovic, L.S.; Bajat, J.B.; Popic, J.P.; Jegdic, B.V.; Stevanovic, S.; Miskovic-Stankovic, V.B. Protective properties of cataphoretic epoxy coating on aluminium alloy AA6060 modified with electrodeposited Ce-based coatings: Effect of post-treatment. Prog. Org. Coat. 2015, 79, 43–52. [Google Scholar] [CrossRef]
- Decroly, A.; Petitjean, J.P. Study of the deposition of cerium oxide by conversion on to aluminium alloys. Surf. Coat. Technol. 2005, 194, 1–9. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simoes, A.M.; Carmezim, M.J. Direct chemical assembly of quaternary ammonium groups on a surface of highly dispersed silica. Appl. Surf. Sci. 2006, 253, 784–791. [Google Scholar]
- Yu, P.; Hayes, S.A.; O’Keefe, T.J.; O’Keefe, M.J.; Stoffer, J.O. The Phase Stability of Cerium Species in Aqueous Systems: II. The Formula Systems. Equilibrium Considerations and Pourbaix Diagram Calculations. J. Electrochem. Soc. 2006, 153, C74–C79. [Google Scholar] [CrossRef]
- Golabadi, M.; Aliofkhazraei, M.; Toorani, M.; Rouhaghdam, A.S. Evaluation of La containing PEO pretreatment on protective performance of epoxy coating on magnesium. Prog. Org. Coat. 2017, 105, 258–266. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Golabadi, M.; Sabour Rouhaghdam, A. Effect of lanthanum nitrate on the microstructure and electrochemical behavior of PEO coatings on AZ31 Mg alloy. J. Alloys Compd. 2017, 719, 242–255. [Google Scholar] [CrossRef]
- Jüttner, K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on Inhomogeneous surfaces. Electrochim. Acta 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical note: Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corros. Sci. 2001, 57, 747–748. [Google Scholar] [CrossRef]










| Sample | P (W) | Time (min) | Deposition Temperature (°C) | |||||||
| 80 | 200 | |||||||||
| Crystallite Size | Thickness | Ra | Crystallite Size | Thickness | Ra | |||||
| (nm) | (nm) | (nm) | (nm) | (nm) | (nm) | |||||
| AA6061/Ce | 60 | 25 | - | 19.3 | 176.0 | 7.0 | 12.7 | 70.8 | ||
| 40 | - | 30.8 | 88.2 | 1.1 | 22.9 | 95.4 | ||||
| 60 | - | 32.4 | 65.1 | 5.5 | 40.1 | 59.6 | ||||
| 80 | 25 | - | 44.6 | 289.0 | - | 42.2 | 131.6 | |||
| 40 | - | 52.8 | 258.0 | - | 46.1 | 44.2 | ||||
| 60 | - | 149.1 | 127.9 | - | 158.1 | 112.2 | ||||
| Sample | P (W) | Time (min) | Deposition Temperature (°C) | |||||||
| 80 | 200 | |||||||||
| Crystallite Size | Thickness | Ra | Crystallite Size | Thickness | Ra | |||||
| (nm) | (nm) | (nm) | (nm) | (nm) | (nm) | |||||
| La2O3 | La(OH)3 | La2O3 | La(OH)3 | |||||||
| AA6061/La | 60 | 25 | 9.2 | 12.8 | 269.5 | 45.0 | 6.3 | 6.5 | 354.3 | 36.1 |
| 40 | 9.1 | 9.8 | 337.5 | 74.2 | 2.6 | 2.6 | 310.1 | 98.5 | ||
| 60 | 5.3 | 2.8 | 390.2 | 66.0 | 2.0 | 2.0 | 349.1 | 108.0 | ||
| 80 | 25 | 6.2 | 7.0 | 528.5 | 102.0 | 3.5 | 3.3 | 499.0 | 166.0 | |
| 40 | 3.2 | 4.1 | 545.2 | 186.0 | 2.8 | 2.8 | 670.8 | 108.4 | ||
| 60 | 1.5 | 2.1 | 750.6 | 141.0 | 0.9 | 1.0 | 835.5 | 111.0 | ||
| T = 80 °C, t = 60 min | ||||||||||
| Bilayered Coatings | Thickness (nm) | Ra (nm) | Maximum Peak to Valley Height (nm) | |||||||
| AA6061/(Ce (P80T80t60)/La(P60T80t60) | 268.6 | 67.1 | 700.0 | |||||||
| AA7075/(Ce (P80T80t60)/La(P60T80t60) | 182.1 | 71.4 | 400.0 | |||||||
| AA6061/(La(P60T80t60)/(Ce (P80T80t60) | 284.3 | 41.7 | 390.0 | |||||||
| AA7075/La(P60T80t60)/(Ce (P80T80t60) | 193.1 | 27.7 | 204.0 | |||||||
| Sample | Ce4+/Ce3+ Ratio | La Oxide/Hydroxide Ratio |
|---|---|---|
| P60T80t25 | 1.69 | 0.72 |
| P60T80t40 | 2.83 | 0.78 |
| P60T80t60 | 1.54 | 0.62 |
| P80T80t25 | 1.44 | 0.77 |
| P80T80t40 | 2.26 | 0.69 |
| P80T80t60 | 1.53 | 0.76 |
| P60T200t25 | 0.97 | 0.65 |
| P60T200t40 | 2.84 | 0.71 |
| P60T200t60 | 1.24 | 0.78 |
| P80T200t25 | -- | 0.81 |
| P80T200t40 | 2.04 | 0.66 |
| P80T200t60 | 1.60 | 0.63 |
| Sample | βa | βc | icorr | Ecorr | IE |
|---|---|---|---|---|---|
| (mV/dec) | (mV/dec) | (nA cm−2) | (mVSCE) | (%) | |
| AA7075 | |||||
| Substrate | 41.3 | -- | 2297.1 | −806 | -- |
| Ce (P80T80t60) | 60.7 | -- | 1440.6 | −742 | 37.28 |
| La (P60T80t60) | 90.6 | -- | 78.4 | −731 | 96.6 |
| Ce/La(P80T80t60)/(P60T80t60) | 92.1 | -- | 949.8 | −746 | 58.6 |
| La/Ce (P60T80t60)/(P80T80t60) | 92.8 | 360.1 | 566.6 | −751 | 75.3 |
| AA6061 | |||||
| Substrate | 93.9 | -- | 963.1 | −863 | -- |
| Ce (P80T80t60) | 105.0 | -- | 524.5 | −694 | 45.7 |
| La (P60T80t60) | 39.3 | 408.5 | 17.7 | −932- | 98.2 |
| Ce/La (P80T80t60)/(P60T80t60) | 93.9 | -- | 195.4 | −928 | 79.7 |
| La/Ce (P60T80t60)/(P80T80t60) | 35.1| | -- | 99.1 | −719 | 89.7 |
| Sample | Rs (Ω cm2) | Rfilm (Ω cm2) | Rox (Ω cm2) | Rct (Ω cm2) | CPEfilm Ω−1 cm−2sn | nfilm | CPEox Ω−1 cm−2sn | nox | CPEdl Ω−1 cm−2sn | ndl | χ2 | Equivalent Circuit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA7075 | ||||||||||||
| Bare aluminum | 16.1 | -- | 38.3 | 7.3 × 103 | -- | -- | 87 × 10−6 | 0.87 | 1.9 × 10−6 | 0.86 | 1.01 × 10−3 | (a) |
| La (P60T80t60) | 16.7 | 1.3 × 106 | 514.4 | 1.8 × 106 | 29.8 × 10−9 | 0.96 | 57 × 10−6 | 0.75 | 115.1 × 10−9 | 0.72 | 1.36 × 10−3 | |
| Ce (P80T80t60) | 14.8 | 8.9 × 103 | 115.1 | 12.9 × 103 | 3.0 × 10−6 | 0.85 | 96 × 10−6 | 0.84 | 0.34 × 10−8 | 0.67 | 0.56 × 10−3 | (b) |
| Ce/La(P80T80t60)/(P60T80t60) | 15.9 | 9.8 × 103 | 756.3 | 16.3 × 103 | 0.13 × 10−6 | 0.66 | 0.15 × 10−6 | 0.84 | 2.1 × 10−6 | 0.67 | 1.64 × 10−3 | |
| La/Ce(P60T80t60)/(P80T80t60) | 14.0 | 7.7 × 104 | 598 | 6.6 × 104 | 61.3 × 10−6 | 0.88 | 68.4 × 10−6 | 0.77 | 61.0 × 10−7 | 0.89 | 0.67 × 10−3 | |
| AA6061 | ||||||||||||
| Bare aluminum | 15.1 | -- | 630.0 | 2.0×103 | -- | -- | 9.6 × 10−5 | 0.88 | 4.3 × 10−6 | 0.85 | 7.9 × 10−3 | (a) |
| La (P60T80t60) | 15.2 | 20.8 × 103 | 132.5 | 2.3×106 | 1.3 × 10−6 | 0.86 | 12.2 × 10−9 | 0.86 | 6.4 × 10−6 | 0.86 | 3.37 × 10−3 | |
| Ce (P80T80t60) | 14.3 | 5.7 × 103 | 282.2 | 2.2×105 | 2.6 × 10−6 | 0.87 | 170.1 × 10−9 | 0.70 | 13.2 × 10−7 | 0.87 | 2.7 × 10−3 | (b) |
| Ce/La (P80T80t60)/(P60T80t60) | 15.3 | 8.0 × 103 | 383.5 | 5.2×105 | 6.6 × 10−6 | 0.89 | 2.7 × 10−6 | 0.78 | 21.0 × 10−6 | 0.79 | 1.39 × 10−3 | |
| La/Ce (P60T80t60)/(P80T80t60) | 15.7 | 13.2 × 103 | 1120.1 | 3.5×105 | 40.8 × 10−6 | 0.89 | 2.1 × 10−6 | 0.78 | 5.8 × 10−6 | 0.83 | 1.07 × 10−3 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brachetti-Sibaja, S.B.; Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Rodil-Posada, S.E.; López-Oyama, A.B.; García-Zaleta, D.S.; Onofre-Bustamante, E. Fabrication of Sputtered Ce/La, La/Ce Oxide Bilayers on AA6061 and AA7075 Aluminum Alloys for the Development of Corrosion Protective Coatings. Materials 2018, 11, 1114. https://doi.org/10.3390/ma11071114
Brachetti-Sibaja SB, Domínguez-Crespo MA, Torres-Huerta AM, Rodil-Posada SE, López-Oyama AB, García-Zaleta DS, Onofre-Bustamante E. Fabrication of Sputtered Ce/La, La/Ce Oxide Bilayers on AA6061 and AA7075 Aluminum Alloys for the Development of Corrosion Protective Coatings. Materials. 2018; 11(7):1114. https://doi.org/10.3390/ma11071114
Chicago/Turabian StyleBrachetti-Sibaja, Silvia B., Miguel A. Domínguez-Crespo, Aidé M. Torres-Huerta, Sandra E. Rodil-Posada, Ana B. López-Oyama, David S. García-Zaleta, and Edgar Onofre-Bustamante. 2018. "Fabrication of Sputtered Ce/La, La/Ce Oxide Bilayers on AA6061 and AA7075 Aluminum Alloys for the Development of Corrosion Protective Coatings" Materials 11, no. 7: 1114. https://doi.org/10.3390/ma11071114