Preparation of CaCO3-TiO2 Composite Particles and Their Pigment Properties
Abstract
:1. Introduction
2. Methods
2.1. Raw Materials
2.2. Preparation
2.2.1. Preparation of the CaCO3-TiO2 Composite Particles
2.2.2. Preparation of the Construction Paint for Exterior Wall
2.3. Characterization
2.3.1. Structure and Property Characterization
2.3.2. Property Tests of the CaCO3-TiO2 Composite Particles and the as-Prepared Paint
3. Results and Discussion
3.1. Morphology of CaCO3-TiO2 Composite Particles
3.2. Binding Properties of CaCO3 and TiO2 Particles
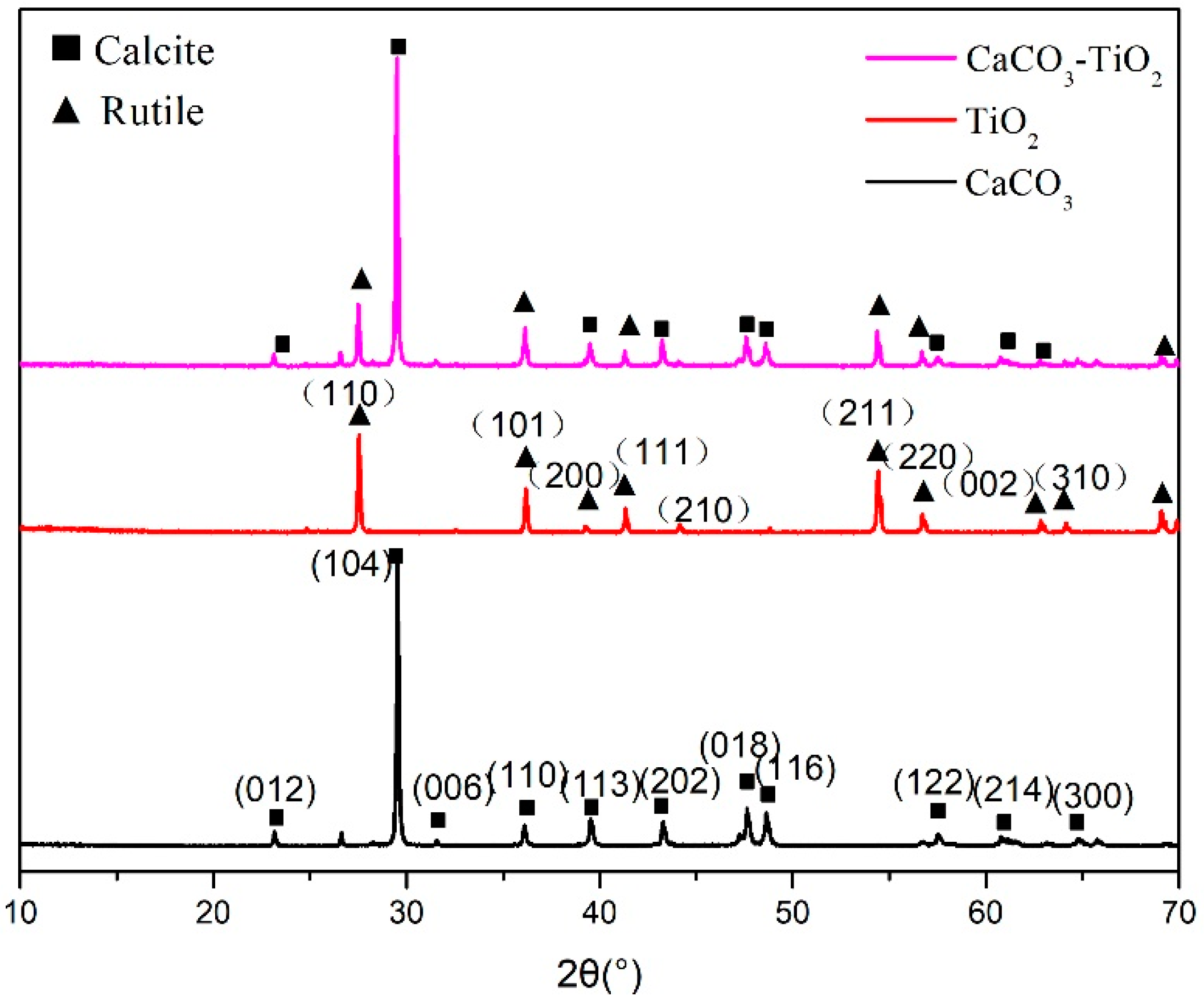
3.2.1. XRD Analysis
3.2.2. Infrared Spectral Analysis
3.2.3. Mechanism Analysis
3.3. Properties of CaCO3-TiO2 Composite Particles
3.3.1. Pigment Properties
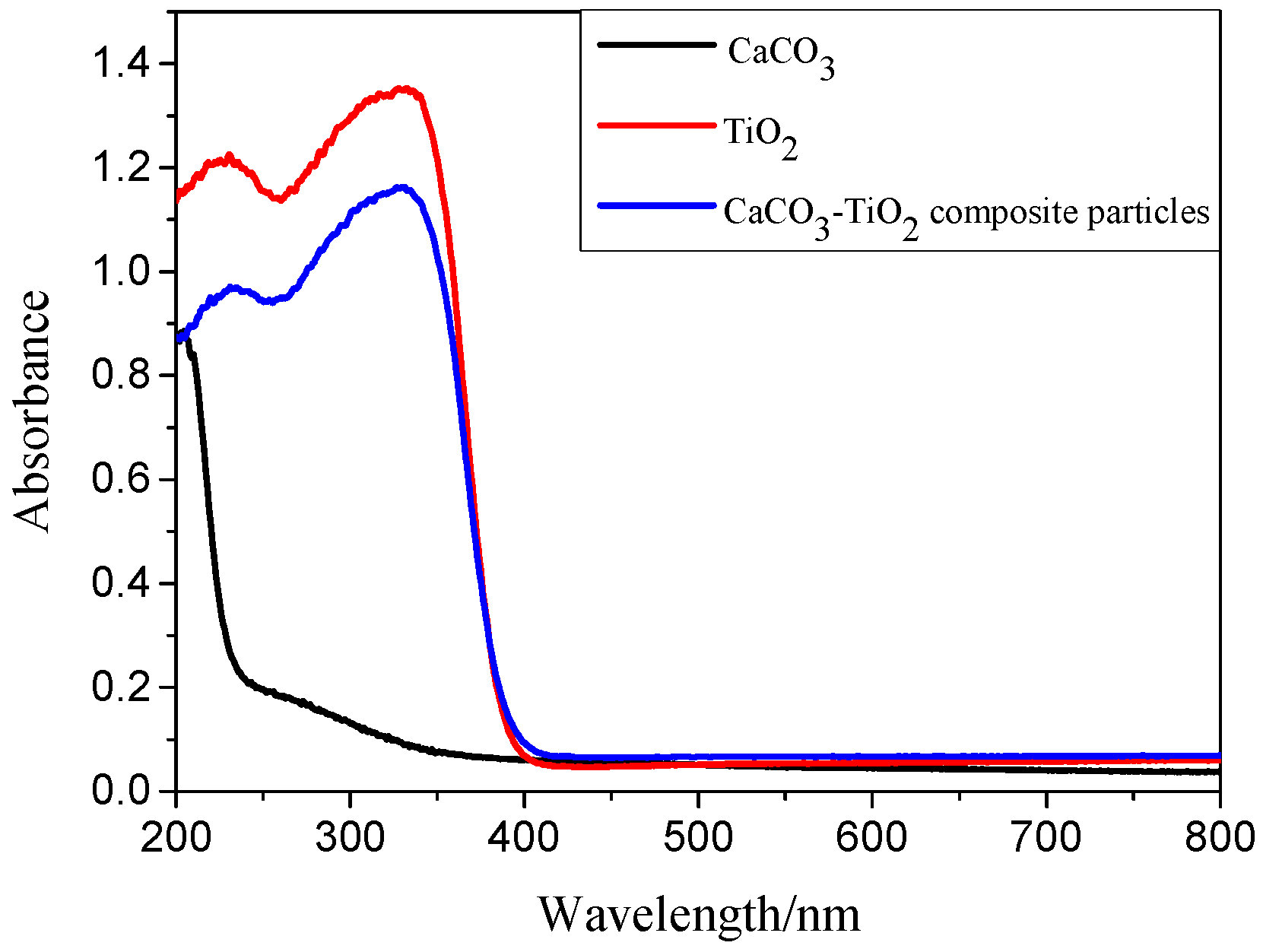
3.3.2. Optical Property of CaCO3-TiO2 Composite Particles
3.3.3. Properties of the Exterior Paint Containing CaCO3-TiO2 Composite Particles
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zheng, S. Processing and Application of Non-Metallic Ore; Chemical Industry Press: Beijing, China, 2003. [Google Scholar]
- Fekete, E.; Pukánszky, B.; Tóth, A.; Bertoti, I. Surface modification and characterization of particulate mineral fillers. J. Colloid Interface Sci. 1990, 135, 200–208. [Google Scholar] [CrossRef]
- Juntuek, P.; Ruksakulpiwat, C.; Chumsamrong, P.; Ruksakulpiwat, Y. Comparison between mechanical and thermal properties of polylactic acid and natural rubber blend using calcium carbonate and vetiver grass fiber as fillers. Adv. Mater. Res. 2012, 410, 59–62. [Google Scholar] [CrossRef]
- Kim, J.J.; Ahn, J.W.; Lee, M.W.; Seo, Y.B. Improving recycled fibres in printing paper by application of an in-situ CaCO3 formation method. Appita J. 2013, 66, 54–58. [Google Scholar]
- Tao, H.; He, Y.; Zhao, X. Preparation and Characterization of calcium carbonate-titanium dioxide core-shell (CaCO3@TiO2) nanoparticles and application in the papermaking industry. Powder Technol. 2015, 283, 308–314. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.Y.; Youn, S.H.; Park, M.; Hong, K.S.; Jung, H.S.; Lee, J.K.; Shin, H. Preparation of a nanoporous CaCO3-coated TiO2 electrode and its application to a dye-sensitized solar cell. Langmuir 2007, 23, 11907–11910. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Lin, H.; Deng, Y. Minerals-Titanium Dioxide Micro-Nanometer Scale Particle Composition and Functionalization; Tsinghua University Press: Beijing, China, 2016. [Google Scholar]
- Pei, R. The Production of TiO2 by Sulfuric Acid Process; Chemical Industry Press: Beijing, China, 1982. [Google Scholar]
- Middlemas, S.; Fang, Z.Z.; Fan, P. A new method for production of titanium dioxide pigment. Hydrometallurgy 2013, 131, 107–113. [Google Scholar] [CrossRef]
- Yan, Q.; Lei, Y.; Yuan, J. Preparation of titanium dioxide compound pigments based on kaolin substrates. J. Coat. Technol. Res. 2010, 7, 229–237. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Zhang, Y. Preparation of nanosized anatase TiO2-coated illite composite pigments by Ti(SO4)2 hydrolysis. Powder Technol. 2015, 271, 262–269. [Google Scholar] [CrossRef]
- Zhao, X. Preparation and mechanism of TiO2-coated illite composite pigments. Dyes Pigments 2014, 108, 84–92. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, M.; Ding, H.; Du, G. Preparation and characterization of barite/TiO2 composite particles. Adv. Mater. Sci. Eng. 2015, 2015, 878594. [Google Scholar] [CrossRef]
- Ninness, B.J.; Bousfirld, D.W.; Tripp, C.P. Formation of a thin TiO2 layer on the surfaces of silica and kaolin pigments through atomic layer deposition. Colloids Surf. A 2003, 214, 195–204. [Google Scholar] [CrossRef]
- Lu, Z.; Ren, M.; Yin, H.; Wang, A.; Ge, C.; Zhang, Y.; Yu, L.; Jiang, T. Preparation of nanosized anatase TiO2-coated kaolin composites and their pigmentary properties. Powder Technol. 2009, 196, 122–125. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, X.; Fan, Y.; Zhou, X. Low temperature synthesis and characterization of rutile TiO2-coated mica-titania pigments. Dyes Pigments 2012, 95, 534–539. [Google Scholar] [CrossRef]
- Ao, Q.; Wu, X.; Fan, Y. The effect of iron ions on the anatase-rutile phase transformation of titania (TiO2) in mica-titania pigments. Dyes Pigments 2012, 95, 96–101. [Google Scholar]
- Tanabe, K.; Anabe, K.; Minitsuhashi, K.; Yoshida, T. Titanium Dioxide–Calcium Carbonate Composite Particles. EP US6991677B2, 31 January 2006. [Google Scholar]
- Liu, G.; Hu, A.; Zeng, H. Preparation of nano CaCO3/TiO2 composite particles. Mater. Rev. 2004, 18, 80–82. [Google Scholar]
- Sun, S.; Ding, H.; Hou, X. Study on preparation of TiO2-coated CaCO3 composite pigments by hydrophobic agglomeration method. Non-Met. Mines 2017, 40, 26–29. [Google Scholar]
- Wang, B.; Ding, H.; Wang, Y. Preparation of barite/TiO2 composite particle and interaction mechanism between TiO2 and barite particles. Rare Met. Mater. Eng. 2011, 40, 193–197. [Google Scholar]
- Chen, W.; Liang, Y.; Hou, X.; Zhang, J.; Ding, H.; Sun, S.; Cao, H. Mechanical grinding preparation and characterization of TiO2-coated wollastonite composite pigments. Materials 2018, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ding, H.; Zhou, H. Preparation of TiO2-coated barite composite pigments by the hydrophobic aggregation method and their structure and properties. Sci. Rep. 2017, 7, 10083. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, C.; Guo, L.; Cui, L.; Zhou, B. Mass production and application of TiO2@CaCO3 composites in interior emulsion coatings. Colloids Surf. A 2016, 498, 98–105. [Google Scholar] [CrossRef]
- GB/T 5211.15-2014, General Methods of Test for Pigments and Extenders; Standards Press of China: Beijing, China, 2014.
- HG/T 3851-2006, Covering Power Determination of Dyestuff; National Development and Reform Commission: Beijing, China, 2006.
- GB/T 23981-2009, Determination of Contrast Ratio of White and Light Coloured Paints; Standards Press of China: Beijing, China, 2009.
- Bezrodna, T.; Gavrilko, T.; Puchkovska, G.; Shimanovska, V.; Baran, J.; Marchewka, M. Spectroscopic study of TiO2 (rutile)–benzophenone heterogeneous systems. J. Mol. Struct. 2002, 614, 315–324. [Google Scholar] [CrossRef]
- Wei, H.; McMaster, W.A.; Tan, J.Z.Y.; Cao, L.; Chen, D.; Caruso, R.A. Mesoporous TiO2/g-C3N4 Microspheres with enhanced visible-light photocatalytic activity. J. Phys. Chem. C 2017, 121, 22114–22122. [Google Scholar] [CrossRef]
- Trezza, M.A.; Lavat, A.E. Analysis of the system 3CaO·Al2O3–CaSO4·2H2O–CaCO3–H2O by FT-IR spectroscopy. Cem. Concr. Res. 2001, 31, 869–872. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, L. Controllable synthesis of calcium carbonate polymorphs at different temperatures. Powder Technol. 2009, 189, 64–69. [Google Scholar] [CrossRef]
- Bezrodna, T.; Puchkovska, G.; Shymanovska, V.; Baran, J.; Ratajczak, H. IR-analysis of H-bonded H2O on the pure TiO2 surface. J. Mol. Struct. 2004, 700, 175–181. [Google Scholar] [CrossRef]
- Wang, D.; Hu, Y. Solution Chemistry of Flotation; Hunan Science & Technology Press: Changsha, China, 1988. [Google Scholar]
- Iv, T.D.P.; Cygan, R.T.; Mitchell, R. Molecular models of a hydrated calcite mineral surface. Geochim. Cosmochim. Acta 2007, 71, 5876–5887. [Google Scholar]
- Perrin, D.D. Stability Constants of Metal-Ion-Complexes; Pergamon Press: Oxford, UK, 1979. [Google Scholar]
- Inam, M.A.; Ouattara, S.; Frances, C. Effects of concentration of dispersions on particle sizing during production of fine particles in wet grinding process. Powder Technol. 2011, 208, 329–336. [Google Scholar] [CrossRef] [Green Version]
- GB/T 9756-2009, Synthetic Resin Emulsion Coatings for Interior Wall; Standards Press of China: Beijing, China, 2009.






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Ding, H.; Hou, X. Preparation of CaCO3-TiO2 Composite Particles and Their Pigment Properties. Materials 2018, 11, 1131. https://doi.org/10.3390/ma11071131
Sun S, Ding H, Hou X. Preparation of CaCO3-TiO2 Composite Particles and Their Pigment Properties. Materials. 2018; 11(7):1131. https://doi.org/10.3390/ma11071131
Chicago/Turabian StyleSun, Sijia, Hao Ding, and Xifeng Hou. 2018. "Preparation of CaCO3-TiO2 Composite Particles and Their Pigment Properties" Materials 11, no. 7: 1131. https://doi.org/10.3390/ma11071131




