Hydrophilic and Hydrophobic Mesoporous Silica Derived from Rice Husk Ash as a Potential Drug Carrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Mesoporous Silica from Rice Husks
2.3. Surface Modification of Mesoporous Silica
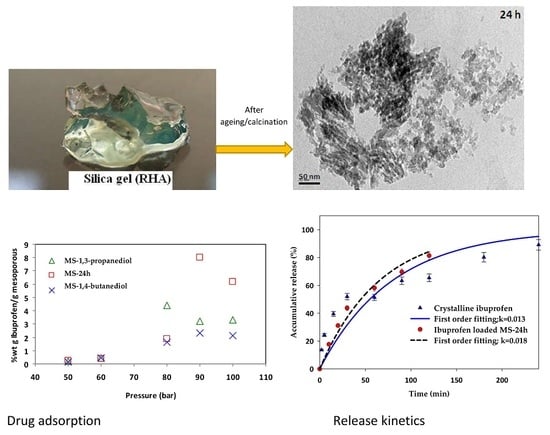
2.4. Drug Adsorption
2.5. In Vitro Release Experiments
3. Results
3.1. Physico-Chemical Properties of As-Synthesized Mesoporous Silica
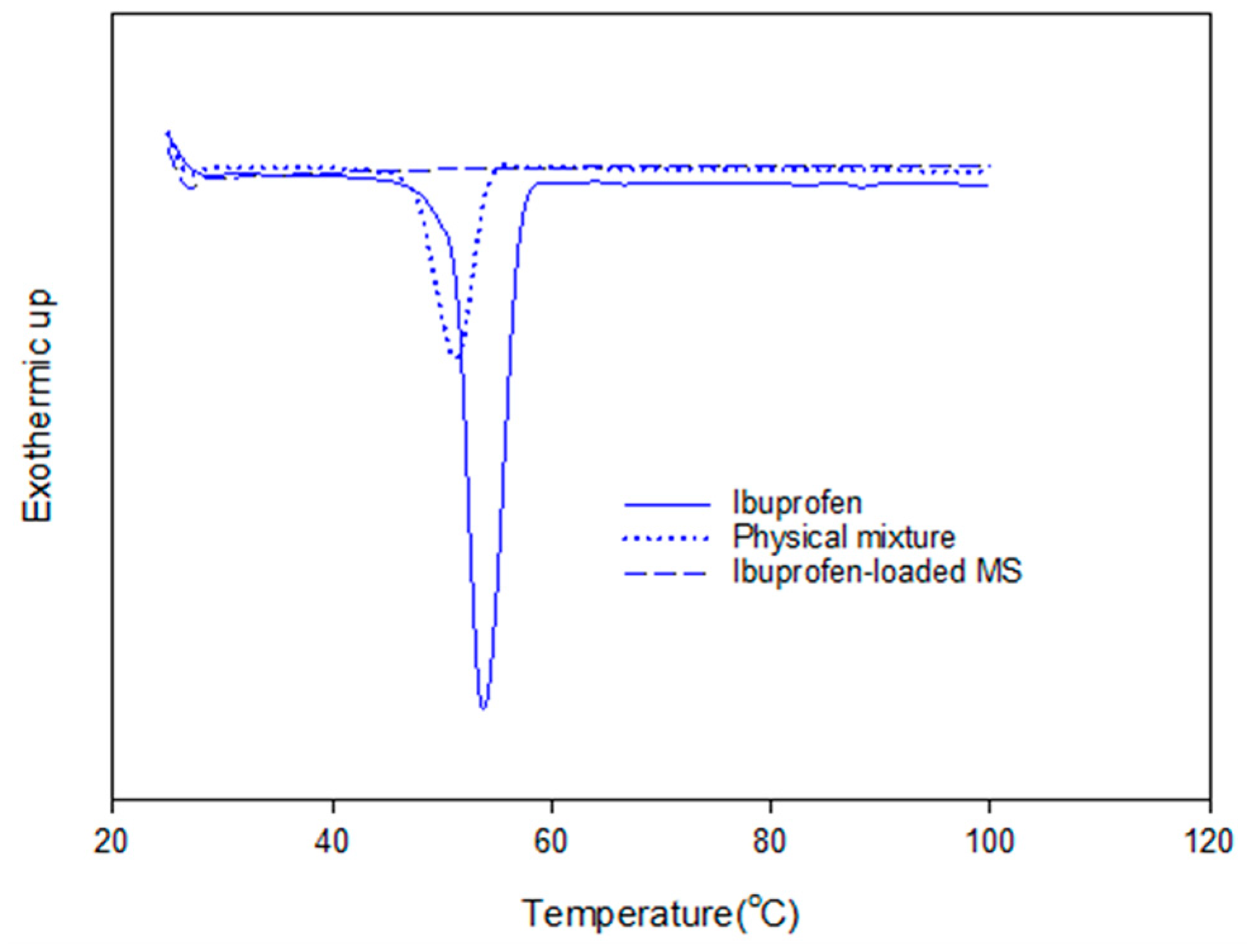
3.2. Drug Loading
3.3. Release Kinetics of Ibuprofen-Loaded Mesoporous Silicas
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, E.C.; Davis-Poynter, N.; Nguyen, C.T.H.; Peters, A.A.; Monteith, G.R.; Strounina, E.; Popat, A.; Ross, B.P. Gag mimetic functionalised solid and mesoporous silica nanoparticles as viral entry inhibitors of herpes simplex type 1 and type 2 viruses. Nanoscale 2016, 8, 16192–16196. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.H.; Webb, R.I.; Lambert, L.K.; Strounina, E.; Lee, E.C.; Parat, M.-O.; McGuckin, M.A.; Popat, A.; Cabot, P.J.; Ross, B.P. Bifunctional succinylated ε-polylysine-coated mesoporous silica nanoparticles for ph-responsive and intracellular drug delivery targeting the colon. ACS Appl. Mater. Interfaces 2017, 9, 9470–9483. [Google Scholar] [CrossRef] [PubMed]
- Juère, E.; Florek, J.; Bouchoucha, M.; Jambhrunkar, S.; Wong, K.Y.; Popat, A.; Kleitz, F. In vitro dissolution, cellular membrane permeability, and anti-inflammatory response of resveratrol-encapsulated mesoporous silica nanoparticles. Mol. Pharm. 2017, 14, 4431–4441. [Google Scholar] [CrossRef] [PubMed]
- Florek, J.; Caillard, R.; Kleitz, F. Evaluation of mesoporous silica nanoparticles for oral drug delivery—Current status and perspective of msns drug carriers. Nanoscale 2017, 9, 15252–15277. [Google Scholar] [CrossRef] [PubMed]
- GIEWS. Crop Prospects and Food Situation; Global Information and Early Warning System on Food and Agriculture, FAO, UN: Rome, Italy, 2017. [Google Scholar]
- Yalçin, N.; Sevinç, V. Studies on silica obtained from rice husk. Ceram. Int. 2001, 27, 219–224. [Google Scholar] [CrossRef]
- Della, V.P.; Kuhn, I.; Hotza, D. Rice husk ash as an alternate source for active silica production. Mater. Lett. 2002, 57, 818–821. [Google Scholar] [CrossRef]
- Chareonpanich, M.; Namto, T.; Kongkachuichay, P.; Limtrakul, J. Synthesis of zsm-5 zeolite from lignite fly ash and rice husk ash. Fuel Process. Technol. 2004, 85, 1623–1634. [Google Scholar] [CrossRef]
- Prasetyoko, D.; Ramli, Z.; Endud, S.; Hamdan, H.; Sulikowski, B. Conversion of rice husk ash to zeolite beta. Waste Manag. 2006, 26, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.P.; Lin, K.S.; Huang, Y.J.; Li, M.C.; Tsaur, L.K. Synthesis of zeolite zsm-48 from rice husk ash. J. Hazard. Mater. 1998, 58, 147–152. [Google Scholar] [CrossRef]
- Li, X.; Shi, J.; Zhu, Y.; Shen, W.; Li, H.; Liang, J.; Gao, J. A template route to the preparation of mesoporous amorphous calcium silicate with high in vitro bone-forming bioactivity. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 83B, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Vempati, R.K.; Borade, R.; Hegde, R.S.; Komarneni, S. Template free zsm-5 from siliceous rice hull ash with varying c contents. Microporous Mesoporous Mater. 2006, 93, 134–140. [Google Scholar] [CrossRef]
- Witoon, T.; Chareonpanich, M.; Limtrakul, J. Synthesis of bimodal porous silica from rice husk ash via sol-gel process using chitosan as template. Mater. Lett. 2008, 62, 1476–1479. [Google Scholar] [CrossRef]
- Sánchez-Flores, N.A.; Pacheco-Malagón, G.; Pérez-Romo, P.; Armendariz, H.; Guzmán-Castillo, M.L. Mesoporous silica from rice hull ash. J. Chem. Technol. Biotechnol. 2007, 82, 614–619. [Google Scholar] [CrossRef]
- Pacheco, G.; Pérez, P.; Guzmán, M.; Sanchez, N.A.; López, C.; Saniger, J. Local order in depolymerized silicate lattices. Inorg. Chem. 2005, 44, 8486–8494. [Google Scholar] [CrossRef] [PubMed]
- Suttiruengwong, S.; Puathawee, P.; Chareonpanich, M. Preparation of mesoporous silica from rice husk ash: Effect of depolymerizing agents on physico-chemical properties. Adv. Mater. Res. 2010, 93–94, 664–667. [Google Scholar] [CrossRef]
- Vitale-Brovarone, C.; Baino, F.; Miola, M.; Mortera, R.; Onida, B.; Verné, E. Glass–ceramic scaffolds containing silica mesophases for bone grafting and drug delivery. J. Mater. Sci. Mater. Med. 2008, 20, 809. [Google Scholar] [CrossRef] [PubMed]
- Renato, M.; Francesco, B.; Gianluca, C.; Sonia, F.; Chiara, V.-B.; Enrica, V.; Barbara, O. Monodisperse mesoporous silica spheres inside a bioactive macroporous glass–ceramic scaffold. Adv. Eng. Mater. 2010, 12, B256–B259. [Google Scholar]
- Vallet-Regi, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A new property of mcm-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Mortera, R.; Onida, B.; Fiorilli, S.; Cauda, V.; Brovarone, C.V.; Baino, F.; Verne, E.; Garrone, E. Synthesis and characterization of mcm-41 spheres inside bioactive glass-ceramic scaffold. Chem. Eng. J. 2008, 137, 54–61. [Google Scholar] [CrossRef]
- Suttiruengwong, S. Silica Aerogels and Hyperbranched Polymers as Drug Delivery Systems. Ph.D. Thesis, Fridrich Alexander Univeristy Erlangen Nuremberg, Erlangen, Germany, 2005. [Google Scholar]
- National Institute of Standards and Technology (NIST). Thermophysical Properties of Fluid Systems. Available online: https://webbook.nist.gov/chemistry/fluid/ (accessed on 23 May 2017).
- Liou, T.H. Preparation and characterization of nano-structured silica from rice husk. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2004, 364, 313–323. [Google Scholar] [CrossRef]
- Zong, S.; Wei, W.; Jiang, Z.; Yan, Z.; Zhu, J.; Xie, J. Characterization and comparison of uniform hydrophilic/hydrophobic transparent silica aerogel beads: Skeleton strength and surface modification. RSC Adv. 2015, 5, 55579–55587. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids; Academic Press: New York, NY, USA, 1999. [Google Scholar]
- Smirnova, I.; Suttiruengwong, S.; Seiler, M.; Arlt, W. Dissolution rate enhancement by adsorption of poorly soluble drugs on hydrophilic silica aerogels. Pharm. Dev. Technol. 2004, 9, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.; Suttiruengwong, S.; Arlt, W. Feasibility study of hydrophilic and hydrophobic silica aerogels as drug delivery systems. J. Non-Cryst. Solids 2004, 350, 54–60. [Google Scholar] [CrossRef]









| Samples | ISi–OH/ISi–O–Si Ratio | Compositions (%) | Surface Area BET (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 a | K2O a | CaO a | MnO a | Fe2O3 a | Weight Loss (%) b | |||||
| RHA | – | 86.74 | 7.53 | 2.19 | 0.23 | 0.27 | 3.02 | 46.26 | 0.315 | 27.41 |
| MS-24h | 3.628 | 94.5 | 0.2 | 0.3 | 0.1 | 0.1 | 4.84 | 149.4 | 0.549 | 14.69 |
| MS-48h | 2.286 | 95.1 | 0.2 | 0.3 | 0.1 | – | 4.28 | 205.7 | 0.384 | 7.46 |
| MS-120h | 2.070 | 95.0 | 0.1 | 0.2 | 0.1 | – | 4.49 | 500.7 | 0.655 | 5.23 |
| MS-360h | 1.700 | 95.5 | 0.2 | 0.2 | 0.1 | – | 4.06 | 453.8 | 0.518 | 4.56 |
| MS-672h | 1.241 | 95.3 | 0.2 | 0.2 | 0.1 | – | 4.19 | 451.9 | 0.613 | 6.42 |
| TMMS-m-MS | – | – | – | – | – | – | – | 144.3 | 0.544 | 14.83 |
| FS c | – | ≥99.8 c | – | – | – | – | – | 200 ± 25 c | 0.338 | 8.96 |
| FS-1 | – | 98.9 b | – | – | – | – | 1.05 b | 208 | 0.820 | 24.56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suttiruengwong, S.; Pivsa-Art, S.; Chareonpanich, M. Hydrophilic and Hydrophobic Mesoporous Silica Derived from Rice Husk Ash as a Potential Drug Carrier. Materials 2018, 11, 1142. https://doi.org/10.3390/ma11071142
Suttiruengwong S, Pivsa-Art S, Chareonpanich M. Hydrophilic and Hydrophobic Mesoporous Silica Derived from Rice Husk Ash as a Potential Drug Carrier. Materials. 2018; 11(7):1142. https://doi.org/10.3390/ma11071142
Chicago/Turabian StyleSuttiruengwong, Supakij, Sommai Pivsa-Art, and Metta Chareonpanich. 2018. "Hydrophilic and Hydrophobic Mesoporous Silica Derived from Rice Husk Ash as a Potential Drug Carrier" Materials 11, no. 7: 1142. https://doi.org/10.3390/ma11071142
APA StyleSuttiruengwong, S., Pivsa-Art, S., & Chareonpanich, M. (2018). Hydrophilic and Hydrophobic Mesoporous Silica Derived from Rice Husk Ash as a Potential Drug Carrier. Materials, 11(7), 1142. https://doi.org/10.3390/ma11071142