Modified Porous SiO2-Supported Cu3(BTC)2 Membrane with High Performance of Gas Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment of the SiO2 Disk
2.3. Surface Modification of the SiO2 Disk with N-[3-(Trimethoxysilyl)propyl]ethylenediamine
2.4. Synthetic Cu3(BTC)2 Membrane with the Modified SiO2 Disk
2.5. Characterization of the Cu3(BTC)2 Membranes
2.6. Low-Pressure N2 Sorption Measurements
2.7. The Gas Separation Test
3. Results
3.1. The FTIR of the Modified SiO2 Disk
3.2. The XRD of the Cu3(BTC)2 Membranes
3.3. The TGA of the Cu3(BTC)2 Membranes
3.4. The Low-Pressure N2 Sorption Measurements and the Pore Size of the Cu3(BTC)2 Membranes
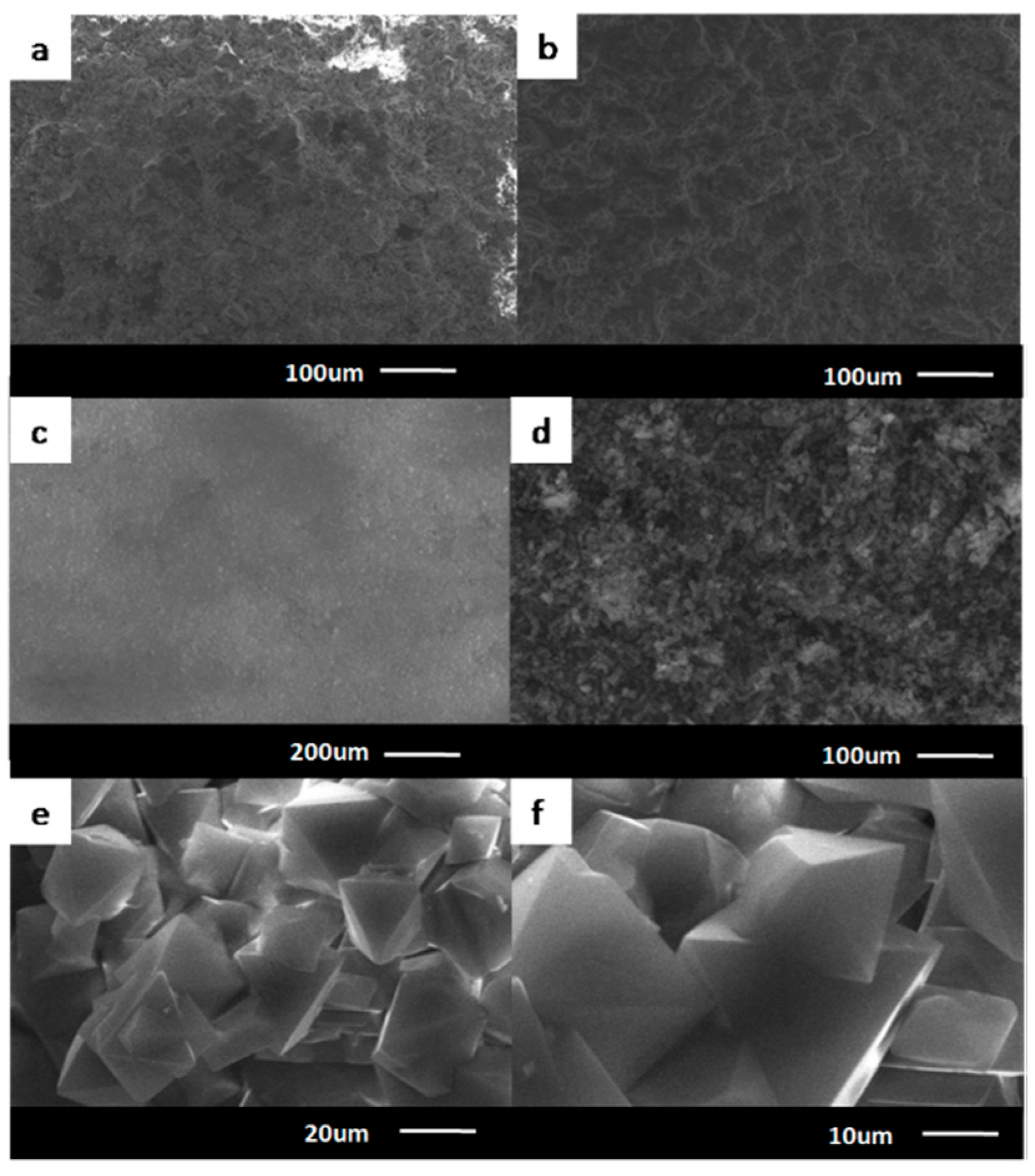
3.5. The SEM of the Cu3(BTC)2 Membranes
3.6. The Gas Separation Test of Cu3(BTC)2 Membrane
3.7. Mechanical Stability of Cu3(BTC)2 Membrane
4. Discussion
4.1. Preparation of the MOF Membrane
4.2. The Morphology and the Stability of the Cu3(BTC)2 Membrane
4.3. The Gas Separation Performance of Cu3(BTC)2 Membrane
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kraytsberg, A.; Ein-Eli, Y. Review of advanced materials for proton exchange membrane fuel cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wu, H.; Tian, Z.; Xin, Q.; He, G.; Guiver, M.D. Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Padaki, M.; Murali, R.S.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Ismail, A.F. Membrane technology enhancement in oil–water separation: A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane gas separation technologies for biogas upgrading. RSC Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Kim, S.; Lin, X.; Ou, R.; Liu, H.; Zhang, X.; Simon, G.P.; Wang, H. Highly crosslinked, chlorine tolerant polymer network entwined graphene oxide membrane for water desalination. J. Mater. Chem. A 2017, 5, 1533–1540. [Google Scholar] [CrossRef]
- Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Jiang, W.L.; Ding, L.G.; Yao, B.J.; Wang, J.C.; Chen, G.J.; Li, Y.A.; Dong, Y.B. A MOF-membrane based on the covalent bonding driven assembly of a NMOF with an organic oligomer and its application in membrane reactors. Chem. Commun. 2016, 52, 13564–13567. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Marti, A.M.; Hopkinson, D.P. Layer-by-layer assembled polymer/MOF membrane for H2/CO2 separation. J. Membr. Sci. 2018, 556, 146–153. [Google Scholar] [CrossRef]
- Lin, Y.S. Metal organic framework membranes for separation applications. Curr. Opin. Chem. Eng. 2015, 8, 21–28. [Google Scholar] [CrossRef]
- Adatoz, E.; Avci, A.K.; Keskin, S. Opportunities and challenges of MOF-based membranes in gas separations. Sep. Purif. Technol. 2015, 152, 207–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Yuan, S.; Zhou, J.; Wang, B. Challenges and recent advances in MOF–polymer composite membranes for gas separation. Inorg. Chem. Front. 2016, 3, 896–909. [Google Scholar] [CrossRef]
- Campbell, M.G.; Sheberla, D.; Liu, S.F.; Swager, T.M.; Dincă, M. Cu3(hexaiminotriphenylene)2: An electrically conductive 2D metal–organic framework for chemiresistive sensing. Angew. Chem. Int. Ed. 2015, 54, 4349–4352. [Google Scholar] [CrossRef] [PubMed]
- Al-Janabi, N.; Hill, P.; Torrente-Murciano, L.; Garforth, A.; Gorgojo, P.; Siperstein, F.; Fan, X. Mapping the Cu-BTC metal–organic framework (HKUST-1) stability envelope in the presence of water vapour for CO2 adsorption from flue gases. Chem. Eng. J. 2015, 281, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Yun, W.S.; Kim, M.B.; Kim, J.Y.; Bae, Y.S.; Lee, J.; Jeong, N.C. A Chemical route to activation of open metal sites in the copper-based metal–organic framework materials HKUST-1 and Cu-MOF-2. J. Am. Chem. Soc. 2015, 137, 10009–10015. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Das, S.; Xing, G.; Ben, T.; Valtchev, V.; Qiu, S. Fabrication of COF-MOF composite membranes and their highly selective separation of H2/CO2. J. Am. Chem. Soc. 2016, 138, 7673–7680. [Google Scholar] [CrossRef] [PubMed]
- Denny, M.S.; Cohen, S.M. In situ modification of metal–organic frameworks in mixed-matrix membranes. Angew. Chem. Int. Ed. 2015, 54, 9029–9032. [Google Scholar] [CrossRef] [PubMed]
- Sorribas, S.; Kudasheva, A.; Almendro, E.; Zornoza, B.; de la Iglesia, Ó.; Téllez, C.; Coronas, J. Pervaporation and membrane reactor performance of polyimide based mixed matrix membranes containing MOF HKUST-1. Chem. Eng. Sci. 2015, 124, 37–44. [Google Scholar] [CrossRef]
- Seoane, B.; Coronas, J.; Gascon, I.; Benavides, M.E.; Karvan, O.; Caro, J.; Gascon, J. Metal–organic framework based mixed matrix membranes: A solution for highly efficient CO2 capture. Chem. Soc. Rev. 2015, 44, 2421–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef]
- Dutta, S. A review on production, storage of hydrogen and its utilization as an energy resource. J. Ind. Eng. Chem. 2014, 20, 1148–1156. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Tremel, A.; Wasserscheid, P.; Baldauf, M.; Hammer, T. Techno-economic analysis for the synthesis of liquid and gaseous fuels based on hydrogen production via electrolysis. Int. J. Hydrog. Energy 2015, 40, 11457–11464. [Google Scholar] [CrossRef]
- Singh, S.; Jain, S.; Venkateswaran, P.S.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A sustainable fuel for future of the transport sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Uusitalo, V.; Väisänen, S.; Inkeri, E.; Soukka, R. Potential for greenhouse gas emission reductions using surplus electricity in hydrogen, methane and methanol production via electrolysis. Energy Convers. Manag. 2017, 134, 125–134. [Google Scholar] [CrossRef]
- Shan, X.; Qian, Y.; Zhu, L.; Lu, X. Effects of EGR rate and hydrogen/carbon monoxide ratio on combustion and emission characteristics of biogas/diesel dual fuel combustion engine. Fuel 2016, 181, 1050–1057. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Yang, Q.; Li, L.; Tan, W.; Sun, Y.; Wang, H.; Ma, J.; Zhao, X. Exceptional high selectivity of hydrogen/methane separation on a phosphonate-based MOF membrane with exclusion of methane molecules. Chem. Commun. 2017, 53, 9797–9800. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dong, X.; Nan, J.; Jin, W.; Ren, X.; Xu, N.; Lee, Y.M. Metal–organic framework membranes fabricated via reactive seeding. Chem. Commun. 2011, 47, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Nenoff, T.M. Hydrogen purification: MOF membranes put to the test. Nat. Chem. 2015, 7, 377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, N.; Ji, S.; Zhang, R.; Zhao, C.; Li, J.R. Metal–organic framework/poly (vinyl alcohol) nanohybrid membrane for the pervaporation of toluene/n-heptane mixtures. J. Membr. Sci. 2015, 489, 144–152. [Google Scholar] [CrossRef]
- Dolgopolova, E.A.; Brandt, A.J.; Ejegbavwo, O.A.; Duke, A.S.; Maddumapatabandi, T.D.; Galhenage, R.P.; Chandrashekhar, M. Electronic Properties of Bimetallic Metal–Organic Frameworks (MOFs): Tailoring the Density of Electronic States through MOF Modularity. J. Am. Chem. Soc. 2017, 139, 5201–5209. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.G.; Zhang, J. Epitaxial growth and applications of oriented metal–organic framework thin films. Coord. Chem. Rev. 2017. [Google Scholar] [CrossRef]
- Van Gestel, T.; Hauler, F.; Bram, M.; Meulenberg, W.A.; Buchkremer, H.P. Synthesis and characterization of hydrogen-selective sol–gel SiO2 membranes supported on ceramic and stainless steel supports. Sep. Purif. Technol. 2014, 121, 20–29. [Google Scholar] [CrossRef]
- Sun, J.; Bi, H.; Su, S.; Jia, H.; Xie, X.; Sun, L. One-step preparation of GO/SiO2 membrane for highly efficient separation of oil-in-water emulsion. J. Membr. Sci. 2018, 553, 131–138. [Google Scholar] [CrossRef]
- Huang, A.; Dou, W.; Caro, J. Steam-stable zeolitic imidazolate framework ZIF-90 membrane with hydrogen selectivity through covalent functionalization. J. Am. Chem. Soc. 2010, 132, 15562–15564. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Dong, Z.; Li, Q.; Jin, W. Growth of a ZIF-8 membrane on the inner-surface of a ceramic hollow fiber via cycling precursors. Chem. Commun. 2013, 49, 10326–10328. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yang, J.; Wang, J.; Bai, J.; Yin, H.; Yuan, B.; Duan, C. Deposition of chemically modified α-Al2O3 particles for high performance ZIF-8 membrane on a macroporous tube. Chem. Commun. 2012, 48, 5977–5979. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Fujita, K.; Shimada, T.; Tanaka, S.; Miyake, Y. Layer-by-layer aqueous rapid synthesis of ZIF-8 films on a reactive surface. Dalton Trans. 2013, 42, 11128–11135. [Google Scholar] [CrossRef] [PubMed]
- Bux, H.; Feldhoff, A.; Cravillon, J.; Wiebcke, M.; Li, Y.S.; Caro, J. Oriented zeolitic imidazolate framework-8 membrane with sharp H2/C3H8 molecular sieve separation. Chem. Mater. 2011, 23, 2262–2269. [Google Scholar] [CrossRef]
- Lu, C.; Ben, T.; Xu, S.; Qiu, S. Electrochemical synthesis of a microporous conductive polymer based on a Metal-Organic Framework thin film. Angew. Chem. Int. Edit. 2014, 53, 6454–6458. [Google Scholar] [CrossRef] [PubMed]
- Ben, T.; Lu, C.; Pei, C.; Xu, S.; Qiu, S. Polymer-supported and free-standing metal-organic framework membrane. Chem. A Eur. J. 2012, 18, 10250–10253. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Guo, X.; Sun, H.; Navrotsky, A. Thermodynamics of methane adsorption on copper HKUST-1 at low pressure. J. Phys. Chem. Lett. 2015, 6, 2439–2443. [Google Scholar] [CrossRef] [PubMed]
- Hamon, L.; Jolimaitre, E.; Pirngruber, G.D. CO2 and CH4 separation by adsorption using Cu-BTC metal–organic framework. Ind. Eng. Chem. Res. 2010, 49, 7497–7503. [Google Scholar] [CrossRef] [Green Version]
- Koh, H.S.; Rana, M.K.; Wong-Foy, A.G.; Siegel, D.J. Predicting methane storage in open-metal-site metal–organic frameworks. J. Phys. Chem. C 2015, 119, 13451–13458. [Google Scholar] [CrossRef]
- Mao, Y.; Li, J.; Cao, W.; Ying, Y.; Sun, L.; Peng, X. Pressure-assisted synthesis of HKUST-1 thin film on polymer hollow fiber at room temperature toward gas separation. ACS Appl. Mater. Interfaces 2014, 6, 4473–4479. [Google Scholar] [CrossRef] [PubMed]







| Gas | Kinetic Diameter (nm) | Permeance (mol m−2 s−1 Pa−1) |
|---|---|---|
| H2 | 0.29 | 1.61 × 10−7 |
| CO2 | 0.33 | 1.69 × 10−8 |
| N2 | 0.36 | 1.84 × 10−8 |
| CH4 | 0.38 | 1.98 × 10−8 |
| Gas | Single Component Flow in Mixed Gas (10−6 mol m−2 s−1 Pa−1) | Single Component Flow (10−6 mol m−2 s−1 Pa−1) | Separation Factor | Ideal Separation Factor |
|---|---|---|---|---|
| H2 | 0.148 | 0.161 | 10.20 | 8.75 |
| N2 | 0.0145 | 0.0184 | ||
| H2 | 0.152 | 0.161 | 11.34 | 8.13 |
| CH4 | 0.0134 | 0.0198 | ||
| H2 | 0.143 | 0.161 | 10.07 | 9.53 |
| CO2 | 0.0142 | 0.0169 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Wang, G.; Wang, K.; Guo, D.; Bai, M.; Wang, Y. Modified Porous SiO2-Supported Cu3(BTC)2 Membrane with High Performance of Gas Separation. Materials 2018, 11, 1207. https://doi.org/10.3390/ma11071207
Lu C, Wang G, Wang K, Guo D, Bai M, Wang Y. Modified Porous SiO2-Supported Cu3(BTC)2 Membrane with High Performance of Gas Separation. Materials. 2018; 11(7):1207. https://doi.org/10.3390/ma11071207
Chicago/Turabian StyleLu, Chunjing, Gang Wang, Keliang Wang, Daizong Guo, Mingxing Bai, and Ying Wang. 2018. "Modified Porous SiO2-Supported Cu3(BTC)2 Membrane with High Performance of Gas Separation" Materials 11, no. 7: 1207. https://doi.org/10.3390/ma11071207





