Inhibition Effect of Three-Dimensional (3D) Nanostructures on the Corrosion Resistance of 1-Dodecanethiol Self-Assembled Monolayer on Copper in NaCl Solution
Abstract
1. Introduction
2. Experimental
2.1. Materials and Solutions
2.2. Fabricating 3D Nanostructures on Copper Surface
2.3. Formation of 1-Dodecanethiol SAM
2.4. Characterization
2.5. Electrochemical Measurements
3. Results
3.1. Surface Characterization
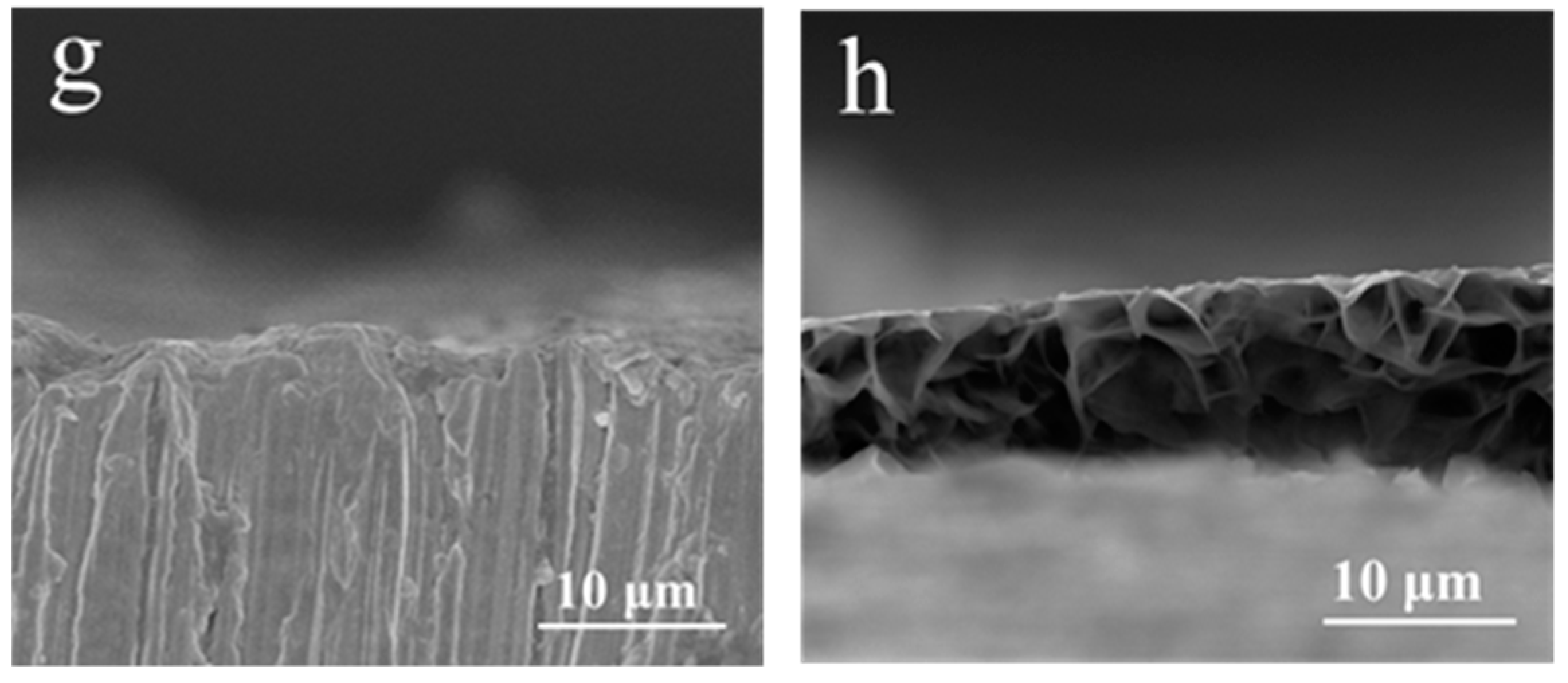
3.1.1. SEM Surface Morphologies
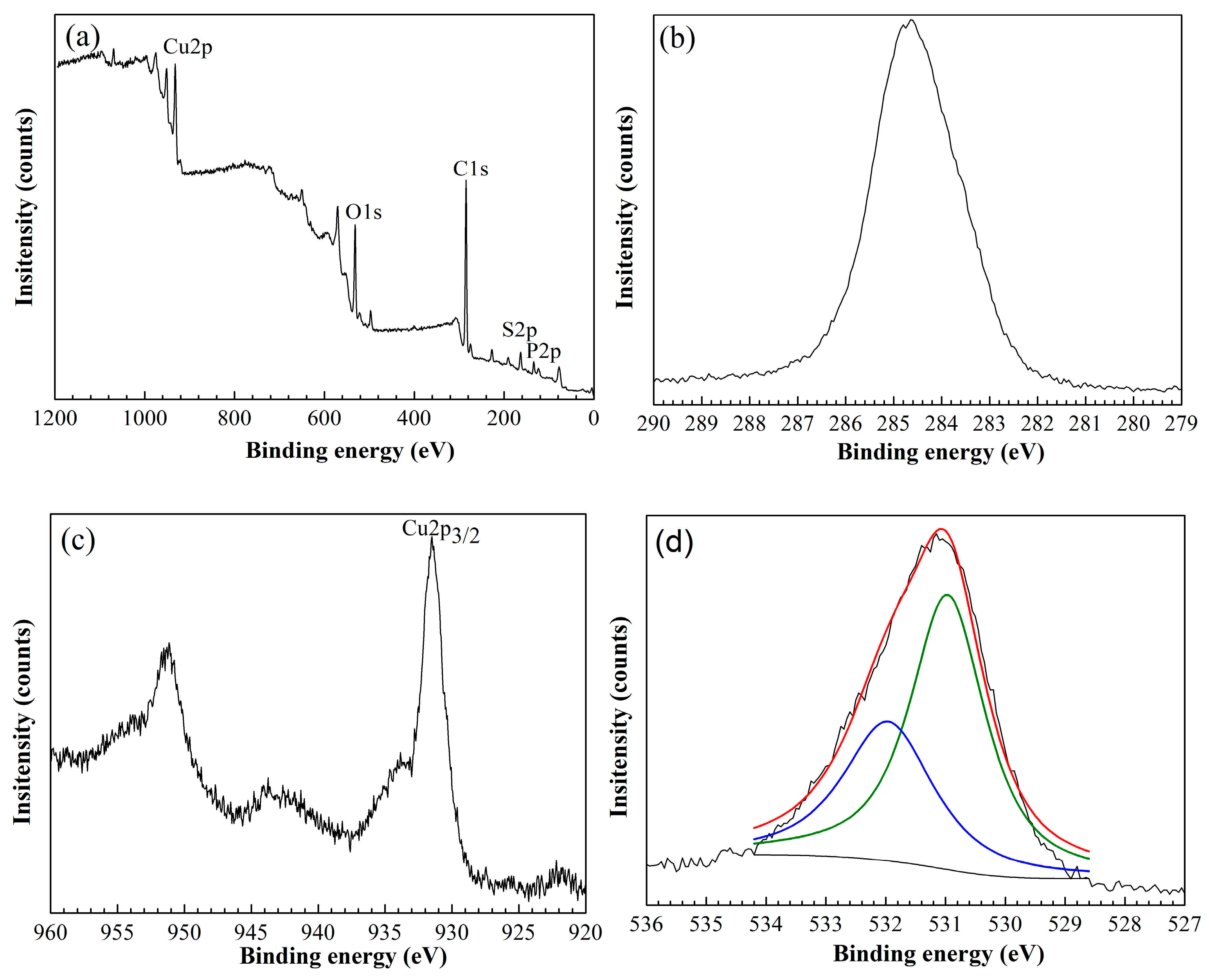
3.1.2. XPS Analysis
3.1.3. XRD Measurement
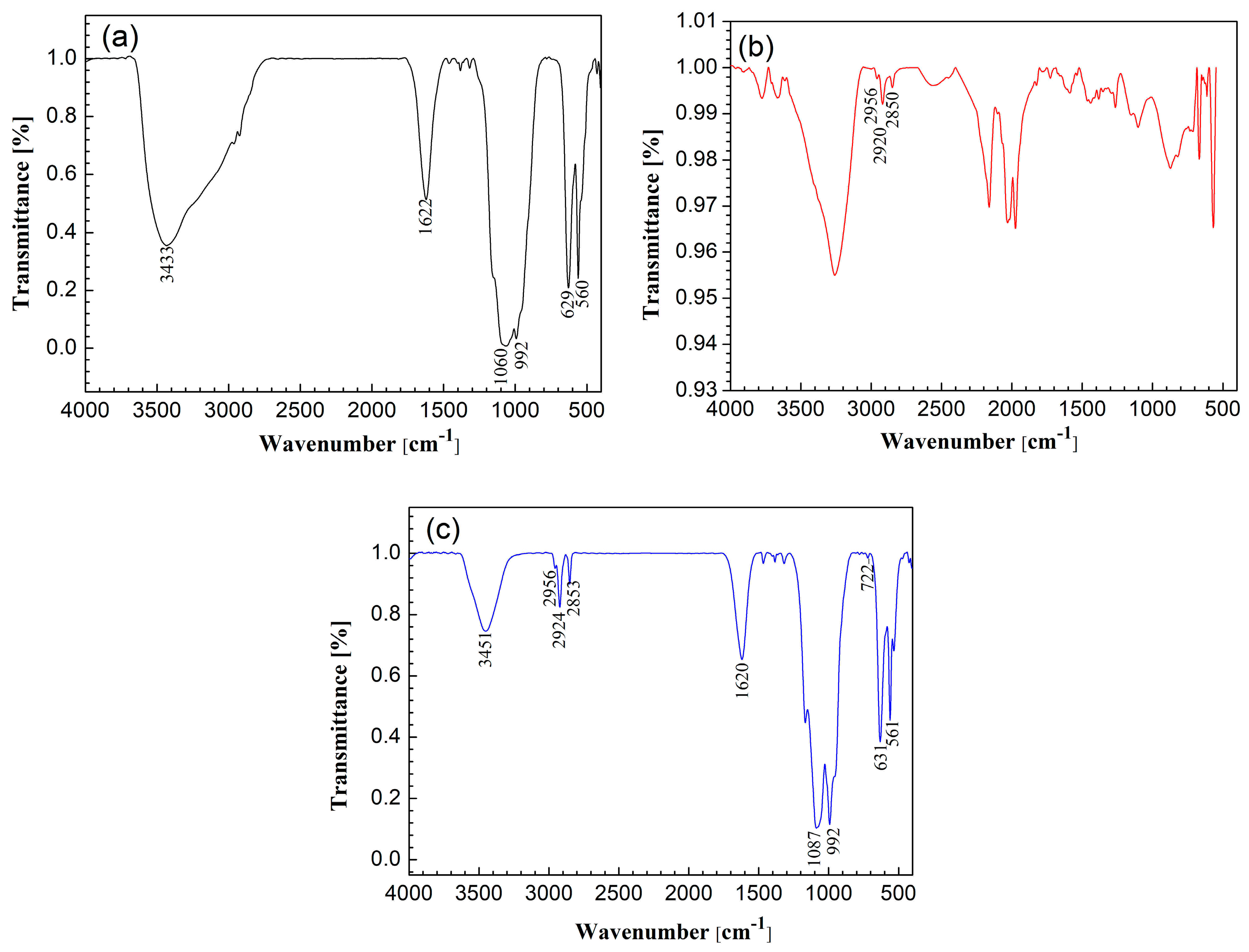
3.1.4. FTIR Measurements
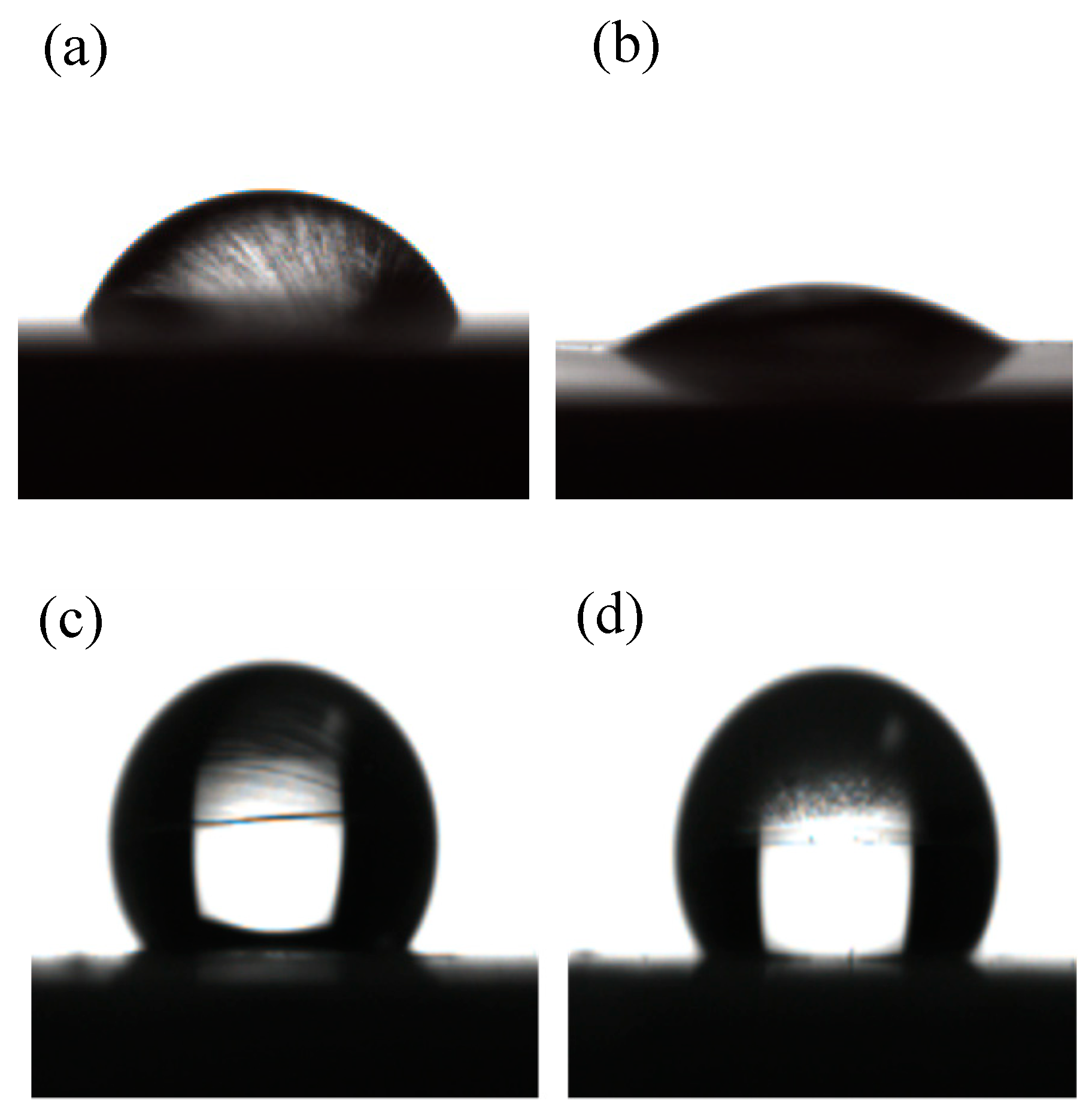
3.1.5. Contact Angle Measurements
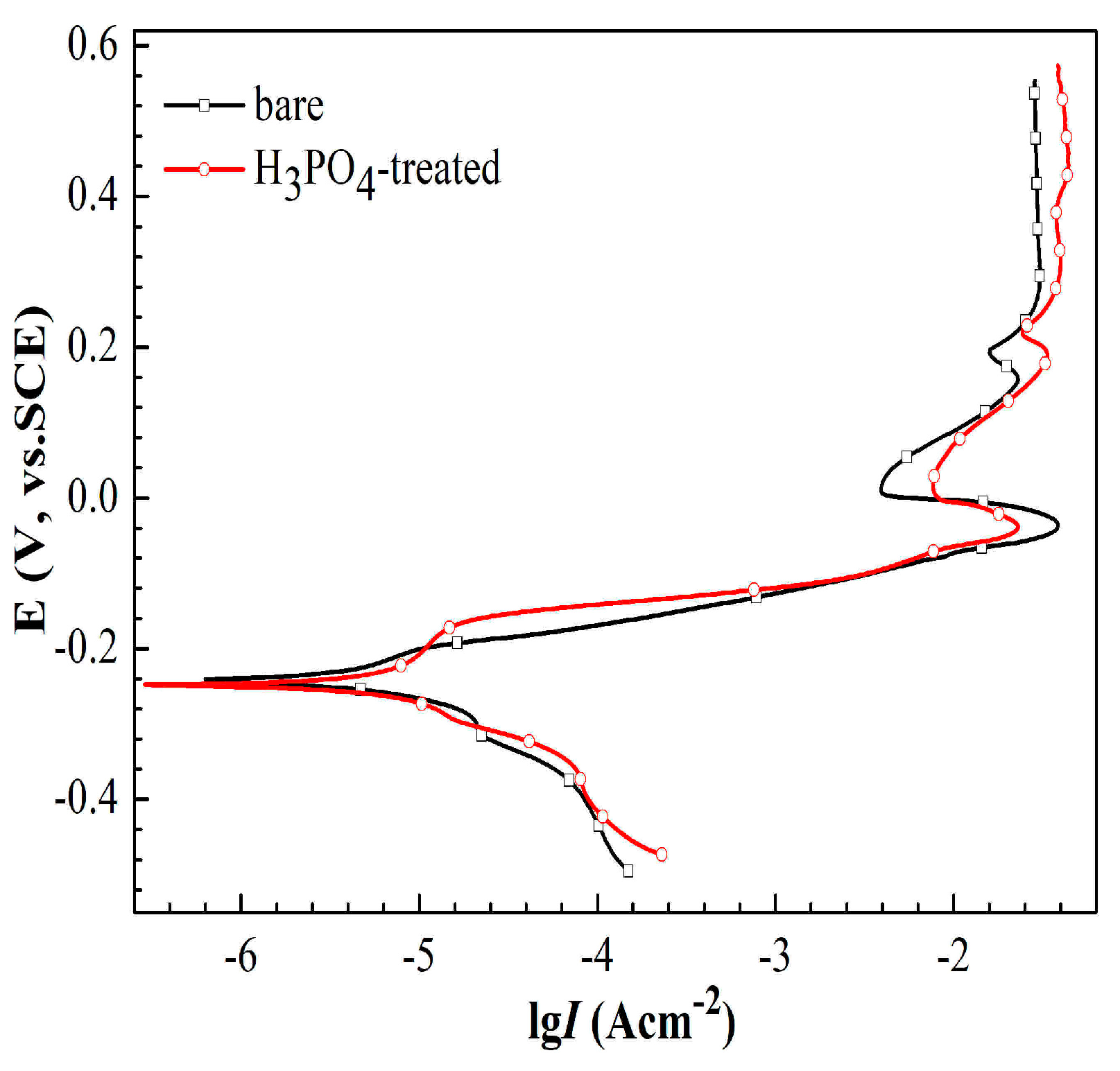
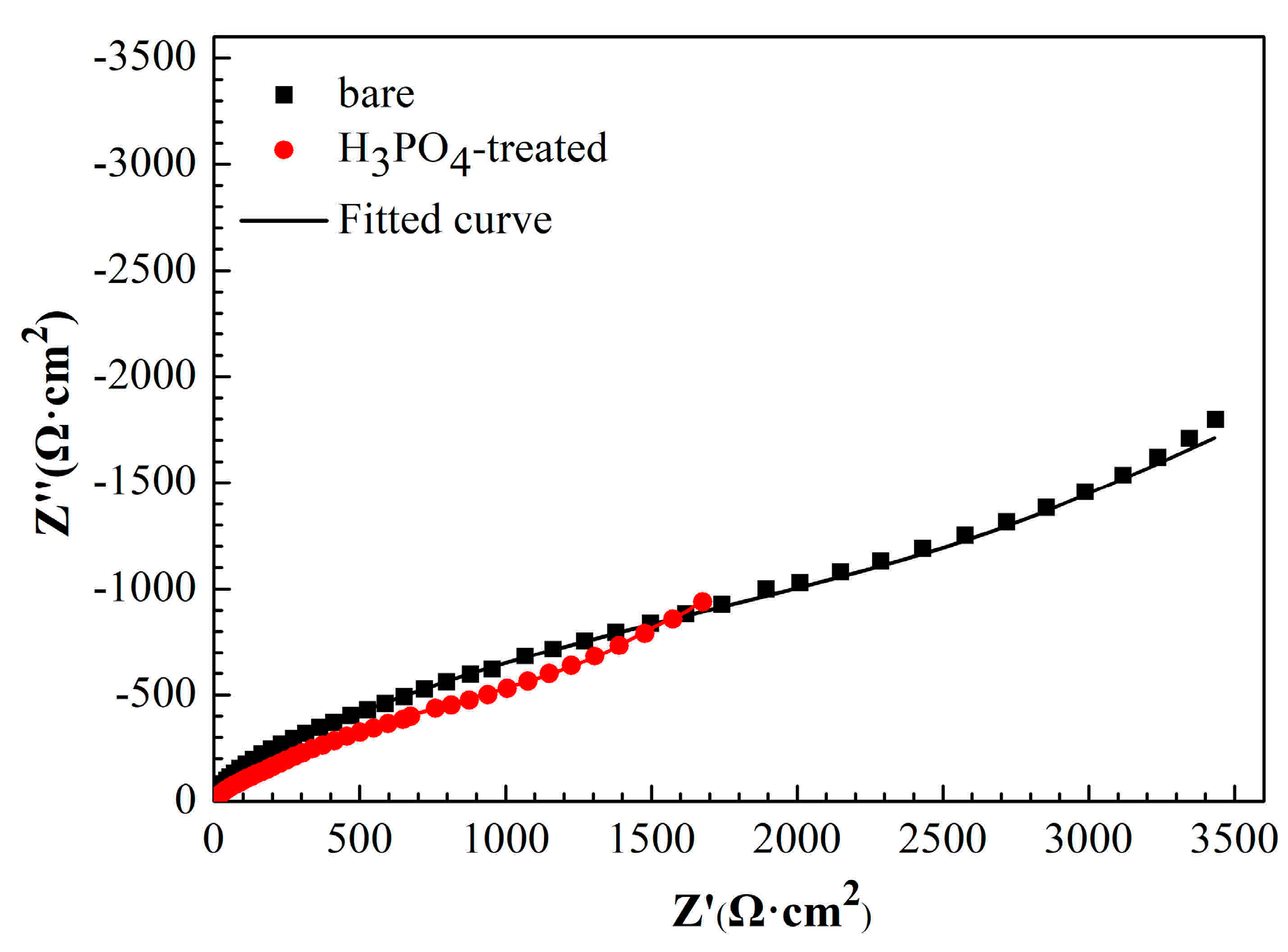
3.2. Corrosion Behaviors of Copper Treated with H3PO4 Solution
3.3. Anticorrosion Investigations of 1-Dodecanethiol SAM
3.3.1. Potentiodynamic Polarization Measurements
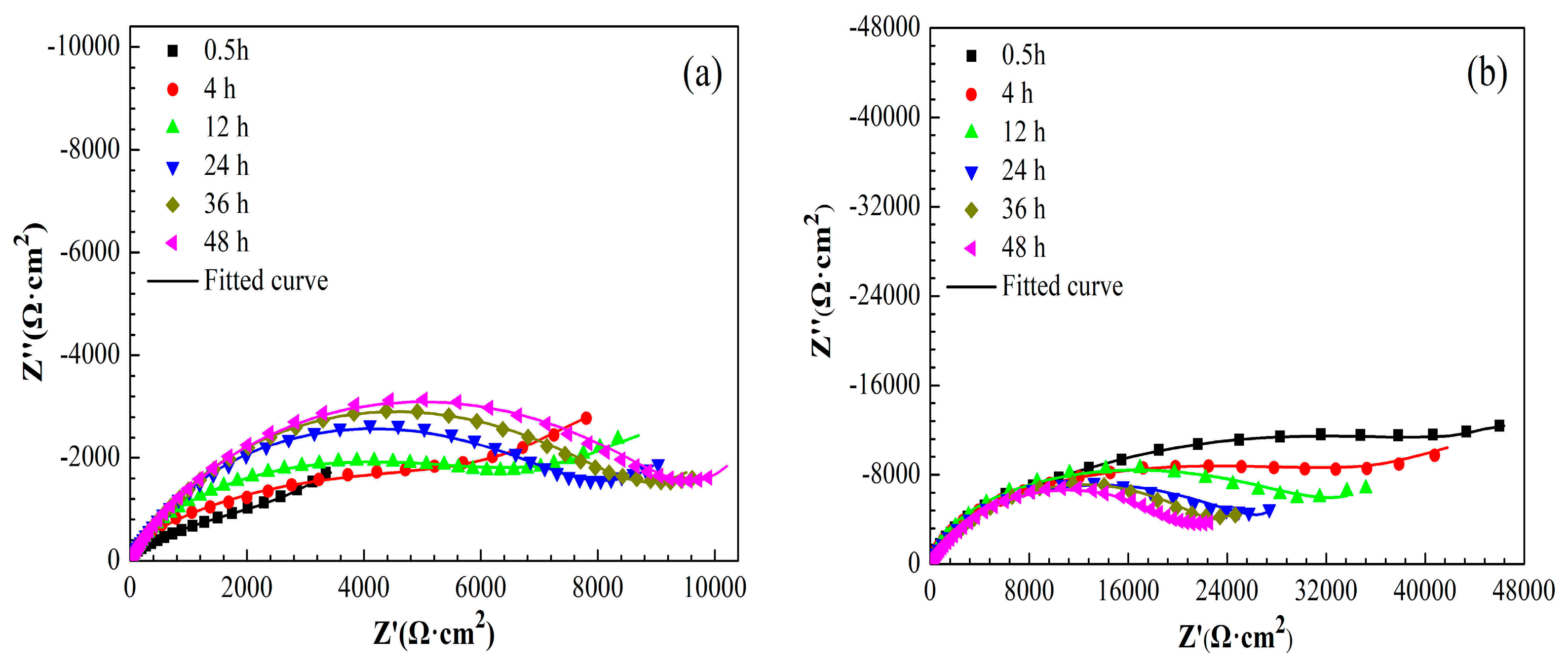
3.3.2. EIS Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ho, H.M.; Lam, W.; Stoukatch, S.; Ratchev, P.; Vath, C.J., III; Beyne, E. Direct gold and copper wires bonding on copper. Microelectron. Reliab. 2003, 43, 913–923. [Google Scholar] [CrossRef]
- Finšgar, M.; Milošev, I. Inhibition of copper corrosion by 1, 2, 3-benzotriazole: A review. Corros. Sci. 2010, 52, 2737–2749. [Google Scholar] [CrossRef]
- Zhang, D.-Q.; Gao, L.-X.; Zhou, G.-D. Inhibition of copper corrosion in aerated hydrochloric acid solution by heterocyclic compounds containing a mercapto group. Corros. Sci. 2004, 46, 3031–3040. [Google Scholar] [CrossRef]
- Finšgar, M.; Merl, D.K. An electrochemical, long-term immersion, and XPS study of 2-mercaptobenzothiazole as a copper corrosion inhibitor in chloride solution. Corros. Sci. 2014, 83, 164–175. [Google Scholar] [CrossRef]
- Finšgar, M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: Part I. Long-term immersion, 3D-profilometry, and electrochemistry. Corros. Sci. 2013, 72, 82–89. [Google Scholar] [CrossRef]
- Kühnle, A. Self-assembly of organic molecules at metal surfaces. Curr. Opin. Colloid Interface Sci. 2009, 14, 157–168. [Google Scholar] [CrossRef]
- Felhösi, I.; Kálmán, E.; Póczik, P. Corrosion protection by self-assembly. Russ. J. Electrochem. 2002, 38, 230–237. [Google Scholar] [CrossRef]
- Laibinis, P.E.; Whitesides, G.M. Self-assembled monolayers of n-alkanethiolates on copper are barrier films that protect the metal against oxidation by air. J. Am. Chem. Soc. 1992, 114, 9022–9028. [Google Scholar] [CrossRef]
- Vikholm-Lundin, I.; Rosqvist, E.; Ihalainen, P.; Munter, T.; Honkimaa, A.; Marjomäki, V.; Albers, W.M.; Peltonen, J. Assembly of citrate gold nanoparticles on hydrophilic monolayers. Appl. Surf. Sci. 2016, 378, 519–529. [Google Scholar] [CrossRef]
- She, Z.; Di Falco, A.; Hähner, G.; Buck, M. Electrodeposition of gold templated by patterned thiol monolayers. Appl. Surf. Sci. 2016, 373, 51–60. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nishihara, H.; Aramaki, K. Self-Assembled Layers of Alkanethiols on Copper for Protection against Corrosion. J. Electrochem. Soc. 1993, 140, 436–443. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Han, G.-C.; Chen, S.-L.; Chen, Z. Inhibition of copper corrosion by the formation of Schiff base self-assembled monolayers. Appl. Surf. Sci. 2016, 389, 601–608. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, Q.; Nie, K.; Zhao, L.; Yang, D.; Yue, Z.; Ge, H.; Li, Y. Self-assembled monolayers formed by ammonium pyrrolidine dithiocarbamate on copper surfaces in sodium chloride solution. Corros. Sci. 2015, 93, 201–210. [Google Scholar] [CrossRef]
- Patois, T.; Taouil, A.E.; Lallemand, F.; Carpentier, L.; Roizard, X.; Hihn, J.-Y.; Bondeau-Patissier, V.; Mekhalif, Z. Microtribological and corrosion behaviors of 1H, 1H, 2H, 2H-perfluorodecanethiol self-assembled films on copper surfaces. Surf. Coat. Technol. 2010, 205, 2511–2517. [Google Scholar] [CrossRef]
- Sinapi, F.; Lejeune, I.; Delhalle, J.; Mekhalif, Z. Comparative protective abilities of organothiols SAM coatings applied to copper dissolution in aqueous environments. Electrochim. Acta 2007, 52, 5182–5190. [Google Scholar] [CrossRef]
- Denayer, J.; Delhalle, J.; Mekhalif, Z. Surface modification of copper with 2-dodecylpropane-1, 3-dithiol: The key effect of the solvent. Appl. Surf. Sci. 2009, 256, 1426–1430. [Google Scholar] [CrossRef]
- Hutt, D.A.; Liu, C. Oxidation protection of copper surfaces using self-assembled monolayers of octadecanethiol. Appl. Surf. Sci. 2005, 252, 400–411. [Google Scholar] [CrossRef]
- Itoh, M.; Nishihara, H.; Aramaki, K. Preparation and Evaluation of Two-Dimensional Polymer Films by Chemical Modification of an Alkanethiol Self-Assembled Monolayer for Protection of Copper against Corrosion. J. Electrochem. Soc. 1995, 142, 3696–3704. [Google Scholar] [CrossRef]
- Yu, H.; Li, C.; Yuan, B.; Li, L.; Wang, C. The inhibitive effects of AC-treated mixed self-assembled monolayers on copper corrosion. Corros. Sci. 2017, 120, 231–238. [Google Scholar] [CrossRef]
- Maho, A.; Denayer, J.; Delhalle, J.; Mekhalif, Z. Electro-assisted assembly of aliphatic thiol, dithiol and dithiocarboxylic acid monolayers on copper. Electrochim. Acta 2011, 56, 3954–3962. [Google Scholar] [CrossRef]
- Wang, C.; Chen, S.; Zhao, S. Inhibition effect of AC-treated, mixed self-assembled film of phenylthiourea and 1-dodecanethiol on copper corrosion. J. Electrochem. Soc. 2004, 151, B11–B15. [Google Scholar] [CrossRef]
- Rohwerder, M.; Stratmann, M. Surface modification by ordered monolayers: New ways of protecting materials against corrosion. MRS Bull. 1999, 24, 43–47. [Google Scholar] [CrossRef]
- Wang, J.; Ho, G.W. Corrosion-Mediated Self-Assembly (CMSA): Direct Writing Towards Sculpturing of 3D Tunable Functional Nanostructures. Angew. Chem. 2015, 127, 16030–16034. [Google Scholar] [CrossRef]
- He, G.; Hu, W.; Li, C.M. Spontaneous interfacial reaction between metallic copper and PBS to form cupric phosphate nanoflower and its enzyme hybrid with enhanced activity. Colloids Surf. B 2015, 135, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, Y.; Lei, Y.; Yin, Y. Novel strategy in enhancing stability and corrosion resistance for hydrophobic functional films on copper surfaces. Electrochem. Commun. 2009, 11, 1675–1679. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Ding, S.-J.; Wu, C.-C.; Peng, J.; Jiang, C.-T.; Chou, C.-C. Fabrication of nanostructured copper phosphate electrodes for the detection of α-amino acids. Sens. Actuators, B 2015, 206, 584–591. [Google Scholar] [CrossRef]
- Wu, X.; Shi, G.; Wang, S.; Wu, P. Formation of 3D dandelions and 2D nanowalls of copper phosphate dihydrate on a copper surface and their conversion into a nanoporous CuO film. Eur. J. Inorg. Chem. 2005, 2005, 4775–4779. [Google Scholar] [CrossRef]
- Hamada, S.; Kudo, Y.; Tojo, T. Preparation and reduction kinetics of uniform copper particles from copper (I) oxides with hydrogen. Colloids Surf. 1992, 67, 45–51. [Google Scholar] [CrossRef]
- Lo, P.H.; Tsai, W.T.; Lee, J.T.; Hung, M.P. The Electrochemical Behavior of Electroless Plated Ni-P Alloys in Concentrated NaOH Solution. J. Electrochem. Soc. 1995, 142, 91–96. [Google Scholar] [CrossRef]
- Deslouis, C.; Tribollet, B.; Mengoli, G.; Musiani, M.M. Electrochemical behaviour of copper in neutral aerated chloride solution. I. Steady-state investigation. J. Appl. Electrochem. 1988, 18, 374–383. [Google Scholar] [CrossRef]
- Wang, P.; Liang, C.; Wu, B.; Huang, N.; Li, J. Protection of copper corrosion by modification of dodecanethiol self-assembled monolayers prepared in aqueous micellar solution. Electrochim. Acta 2010, 55, 878–883. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Chattopadhyay, S.; Dey, A. The protonation state of thiols in self-assembled monolayers on roughened Ag/Au surfaces and nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 24866–24873. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Lei, J.; Zare, R.N. Protein–inorganic hybrid nanoflowers. Nat. Nanotechnol. 2012, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Kloprogge, T.; Williams, P.A.; Martens, W.; Johnson, T.E.; Leverett, P. Vibrational spectroscopy of the basic copper phosphate minerals: Pseudomalachite, ludjibaite and reichenbachite. Spectrochim. Acta Part A 2002, 58, 2861–2868. [Google Scholar] [CrossRef]
- Laibinis, P.E.; Whitesides, G.M.; Allara, D.L.; Tao, Y.T.; Parikh, A.N.; Nuzzo, R.G. Comparison of the structures and wetting properties of self-assembled monolayers of n-alkanethiols on the coinage metal surfaces, copper, silver, and gold. J. Am. Chem. Soc. 1991, 113, 7152–7167. [Google Scholar] [CrossRef]
- Yu, N.; Wang, Z.; Zhang, J.; Liu, Z.; Zhu, B.; Yu, J.; Zhu, M.; Peng, C.; Chen, Z. Thiol-capped Bi nanoparticles as stable and all-in-one type theranostic nanoagents for tumor imaging and thermoradiotherapy. Biomaterials 2018, 161, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.D.; Bright, T.B.; Allara, D.L.; Chidsey, C.E. Spontaneously organized molecular assemblies. 4. Structural characterization of n-alkyl thiol monolayers on gold by optical ellipsometry, infrared spectroscopy, and electrochemistry. J. Am. Chem. Soc. 1987, 109, 3559–3568. [Google Scholar] [CrossRef]
- Bertilsson, L.; Liedberg, B. Infrared study of thiol monolayer assemblies on gold: Preparation, characterization, and functionalization of mixed monolayers. Langmuir 1993, 9, 141–149. [Google Scholar] [CrossRef]
- Jennings, G.K.; Munro, J.C.; Yong, T.-H.; Laibinis, P.E. Effect of chain length on the protection of copper by n-alkanethiols. Langmuir 1998, 14, 6130–6139. [Google Scholar] [CrossRef]
- Schlenoff, J.B.; Li, M.; Ly, H. Stability and self-exchange in alkanethiol monolayers. J. Am. Chem. Soc. 1995, 117, 12528–12536. [Google Scholar] [CrossRef]
- Srivastava, P.; Chapman, W.G.; Laibinis, P.E. Odd−Even Variations in the Wettability of n-Alkanethiolate Monolayers on Gold by Water and Hexadecane: A Molecular Dynamics Simulation Study. Langmuir 2005, 21, 12171–12178. [Google Scholar] [CrossRef] [PubMed]
- Folkers, J.P.; Laibinis, P.E.; Whitesides, G.M. Self-assembled monolayers of alkanethiols on gold: Comparisons of monolayers containing mixtures of short-and long-chain constituents with methyl and hydroxymethyl terminal groups. Langmuir 1992, 8, 1330–1341. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.; Walsh, F. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Feng, Y.; Siow, K.-S.; Teo, W.-K.; Tan, K.-L.; Hsieh, A.-K. Corrosion mechanisms and products of copper in aqueous solutions at various pH values. Corrosion 1997, 53, 389–398. [Google Scholar] [CrossRef]
- Brug, G.; Van Den Eeden, A.; Sluyters-Rehbach, M.; Sluyters, J. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Fiaud, C. Electrochemical Behaviour of Cu in Alkaline Solution and the Inhibitive Action of Cyclohexylamine. Corros. Sci. 1974, 14, 261–277. [Google Scholar] [CrossRef]
- Abrantes, L.; Castillo, L.; Norman, C.; Peter, L. A photoelectrochemical study of the anodic oxidation of copper in alkaline solution. J. Electroanal. Chem. Interfacial Electrochem. 1984, 163, 209–221. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Chen, S.; Yu, R.; Lei, S.; Ma, H.; Liu, D.X. Some aspects of quantum chemical calculations for the study of Schiff base corrosion inhibitors on copper in NaCl solutions. Corros. Sci. 1999, 41, 1769–1782. [Google Scholar] [CrossRef]
- Ma, H.; Yang, C.; Chen, S.; Jiao, Y.; Huang, S.; Li, D.; Luo, J. Electrochemical investigation of dynamic interfacial processes at 1-octadecanethiol-modified copper electrodes in halide-containing solutions. Electrochim. Acta 2003, 48, 4277–4289. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Babić, R.; Petrović, Ž.; Posavec, D. Copper Protection by a Self-Assembled Monolayer of Alkanethiol Comparison with Benzotriazole. J. Electrochem. Soc. 2007, 154, C138–C143. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Johnson, C.M.; Leygraf, C. Alkanethiols as inhibitors for the atmospheric corrosion of copper induced by formic acid: Effect of chain length. J. Electrochem. Soc. 2013, 160, C270–C276. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Forslund, M.; Johnson, C.M.; Pan, J.; Leygraf, C. Atmospheric corrosion of Cu, Zn, and Cu–Zn alloys protected by self-assembled monolayers of alkanethiols. Surf. Sci. 2016, 648, 170–176. [Google Scholar] [CrossRef]








| Sample | C | Cu | O | P | S |
|---|---|---|---|---|---|
| Cu/H3PO4 | 73.25 | 8.19 | 14.36 | 4.19 | - |
| Cu/1-dodecanethiol | 75.07 | 15.31 | 7.58 | - | 2.03 |
| Cu/H3PO4/1-dodecanethiol | 81.19 | 5.01 | 3.51 | 3.61 | 6.67 |
| Sample | Ecorr (mV) | ba (mV/dec) | bc (mV/dec) | icorr (A/cm2) |
|---|---|---|---|---|
| Cu | −243 | 116 | −60 | 6.60 × 10−6 |
| Cu/H3PO4 | −248 | 98 | −66 | 7.81 × 10−6 |
| Sample | Rs (Ω·cm2) | CPEf (Sn·Ω−1·cm−2) | n | Rf (Ω·cm2) | CPEdl (Sn·Ω−1·cm−2) | n | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|
| Cu | 3 | 1.96 × 10−5 | 0.96 | 81 | 2.94 × 10−4 | 0.47 | 3762 |
| Cu/H3PO4 | 4 | 4.64 × 10−5 | 0.87 | 53 | 8.50 × 10−4 | 0.45 | 2649 |
| Sample | Ecorr (mV) | ba (mV/dec) | bc (mV/dec) | icorr (A/cm2) | IE% |
|---|---|---|---|---|---|
| Cu | −247 | 97 | −66 | 4.75 × 10−6 | - |
| Cu/1-dodecanethiol SAM | −235 | 199 | −49 | 1.28 × 10−6 | 73.1 |
| Cu/H3PO4/1-dodecanethiol SAM | −187 | 48 | −205 | 1.32 × 10−7 | 97.2 |
| Sample | Time (h) | Rs (Ω·cm2) | CPEf (Sn·Ω−1·cm−2) | n | Rf (Ω·cm2) | CPEdl (Sn·Ω−1·cm−2) | n | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|
| Cu | 0.5 | 3 | 1.96 × 10−5 | 0.96 | 81 | 2.92 × 10−4 | 0.47 | 3762 |
| 4 | 4 | 2.62 × 10−6 | 0.98 | 293 | 8.18 × 10−5 | 0.46 | 7292 | |
| 12 | 4 | 4.23 × 10−6 | 0.98 | 109 | 5.98 × 10−5 | 0.51 | 7533 | |
| 24 | 4 | 4.18 × 10−6 | 0.98 | 137 | 4.20 × 10−5 | 0.68 | 7832 | |
| 36 | 4 | 4.04 × 10−6 | 0.95 | 155 | 4.09 × 10−5 | 0.71 | 8654 | |
| 48 | 4 | 3.73 × 10−6 | 0.92 | 185 | 3.51 × 10−5 | 0.69 | 9625 |
| Sample | Time (h) | Rs (Ω·cm2) | CPEf (Sn·Ω−1·cm−2) | n | Rf (Ω·cm2) | CPEdl (Sn·Ω−1·cm−2) | n | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|
| Cu/ 1-dodecanethiol SAM | 0.5 | 6 | 1.69 × 10−6 | 0.88 | 6573 | 1.93 × 10−5 | 0.47 | 50,575 |
| 4 | 5 | 2.00 × 10−6 | 0.88 | 6398 | 2.04 × 10−5 | 0.45 | 35,949 | |
| 12 | 7 | 2.46 × 10−6 | 0.98 | 4597 | 2.17 × 10−5 | 0.50 | 31,803 | |
| 24 | 6 | 3.08 × 10−6 | 0.94 | 3291 | 2.23 × 10−5 | 0.53 | 25,286 | |
| 36 | 4 | 3.96 × 10−6 | 0.89 | 1277 | 2.32 × 10−5 | 0.65 | 22,670 | |
| 48 | 4 | 5.04 × 10−6 | 0.92 | 931 | 2.52 × 10−5 | 0.64 | 21,026 |
| Sample | Time (h) | Rs (Ω·cm2) | CPEf (Sn·Ω−1·cm−2) | n | Rf (Ω·cm2) | CPEdl (Sn·Ω−1·cm−2) | n | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|
| Cu/H3PO4/ 1-dodecanethiol SAM | 0.5 | 4 | 1.21 × 10−6 | 0.57 | 1086 | 1.04 × 10−7 | 0.88 | 302,811 |
| 4 | 8 | 1.33 × 10−6 | 0.52 | 429 | 9.82 × 10−7 | 0.80 | 239,891 | |
| 12 | 5 | 1.41 × 10−6 | 0.52 | 347 | 1.21 × 10−6 | 0.84 | 214,319 | |
| 24 | 5 | 1.68 × 10−6 | 0.55 | 237 | 1.54 × 10−6 | 0.82 | 181,316 | |
| 36 | 5 | 1.86 × 10−6 | 0.50 | 202 | 1.79 × 10−6 | 0.81 | 172,380 | |
| 48 | 6 | 2.15 × 10−6 | 0.60 | 153 | 2.83 × 10−6 | 0.82 | 153,320 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Chen, Z.; Guo, X. Inhibition Effect of Three-Dimensional (3D) Nanostructures on the Corrosion Resistance of 1-Dodecanethiol Self-Assembled Monolayer on Copper in NaCl Solution. Materials 2018, 11, 1225. https://doi.org/10.3390/ma11071225
Hu S, Chen Z, Guo X. Inhibition Effect of Three-Dimensional (3D) Nanostructures on the Corrosion Resistance of 1-Dodecanethiol Self-Assembled Monolayer on Copper in NaCl Solution. Materials. 2018; 11(7):1225. https://doi.org/10.3390/ma11071225
Chicago/Turabian StyleHu, Shuai, Zhenyu Chen, and Xingpeng Guo. 2018. "Inhibition Effect of Three-Dimensional (3D) Nanostructures on the Corrosion Resistance of 1-Dodecanethiol Self-Assembled Monolayer on Copper in NaCl Solution" Materials 11, no. 7: 1225. https://doi.org/10.3390/ma11071225
APA StyleHu, S., Chen, Z., & Guo, X. (2018). Inhibition Effect of Three-Dimensional (3D) Nanostructures on the Corrosion Resistance of 1-Dodecanethiol Self-Assembled Monolayer on Copper in NaCl Solution. Materials, 11(7), 1225. https://doi.org/10.3390/ma11071225






