Contribution of Local Analysis Techniques for the Characterization of Iron and Alloying Elements in Nitrides: Consequences on the Precipitation Process in Fe–Si and Fe–Cr Nitrided Alloys
Abstract
:1. Introduction
2. Experimental Procedure
3. Calculation of the Elastic Stresses and Strain Energies
4. Results and Discussion
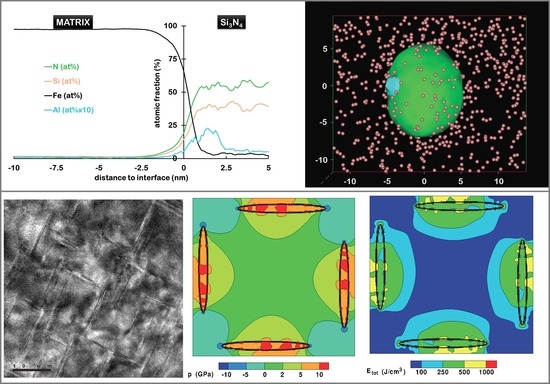
4.1. Mechanism of Amorphous Si3N4 Precipitation in Nitrided Fe–Si Alloys
4.2. Experimental Evidence of Iron in CrN in Nitrided Fe–Cr
4.2.1. Field Ion Microscopy & Transmission Electron Microscopy
4.2.2. Atom Probe Tomography
4.2.3. Analytical Transmission Electron Microscopy
4.2.4. Equilibrium Concentration of Nitrides
4.3. Excess Nitrogen in Fe–Cr–N System
4.3.1. Effects of Iron
4.3.2. Effect of Elastic Accommodation of the Misfit between Nitride and Matrix
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dalton, J.C. Thermodynamics of Paraequilibrium Carburization and Nitridation. Master Thesis, Case Western Reserve University, Cleveland, OH, USA, 2014. [Google Scholar]
- Jack, D.H. The structure of nitride Iron-Titanium alloys. Acta Metall. 1976, 24, 137–146. [Google Scholar] [CrossRef]
- Gouné, M.; Belmonte, T.; Redjaïmia, A.; Weisbecker, P.; Fiorani, J.M.; Michel, H. Thermodynamic and structural studies on nitrided Fe-1.62% Mn and Fe-0.56% V alloys. Mater. Sci. Eng. A 2003, 351, 23–30. [Google Scholar] [CrossRef]
- Van Landeghem, H.P.; Gouné, M.; Bordère, S.; Danoix, F.; Redjaïmia, A. Competitive precipitation of amorphous and crystalline silicon nitride in ferrite: Interaction between structure, morphology, and stress relaxation. Acta Mater. 2015, 93, 218–234. [Google Scholar] [CrossRef]
- Xiong, X. Elaboration et genèse des microstructures dans les aciers fer-azote. Ph.D. Thesis, University of Lorraine, Lorraine, France, 2008. [Google Scholar]
- Van Landeghem, H.P.; Gouné, M.; Redjaïmia, A. Investigation of a Ferrite/Silicon Nitride Composite Concept Aimed at Automotive Applications. Steel Res. Int. 2012, 83, 590–593. [Google Scholar] [CrossRef]
- Mittemeijer, E.J.; Biglari, M.H.; Böttger, A.J.; Van der Pers, N.M.; Sloof, W.G.; Tichelaar, F.D. Amorphous precipitates in a crystalline matrix: Precipitation of amorphous Si3N4 in α-Fe. Scr. Mater. 1999, 41, 625–630. [Google Scholar] [CrossRef]
- Meka, S.R.; Jung, K.S.; Bischoff, E.; Mittemeijer, E.J. Unusual precipitation of amorphous silicon nitride upon nitriding Fe-2at.% Si alloy. Philos. Mag. 2012, 92, 1435–1455. [Google Scholar] [CrossRef]
- Van Landeghem, H.P.; Gouné, M.; Epicier, T.; Redjaïmia, A. Unexpected low temperature crystallization of amorphous silicon nitride into α-Si3N4 in a ferritic matrix. Scr. Mater. 2013, 68, 187–190. [Google Scholar] [CrossRef]
- Cundy, S.L.; Grundy, P.J. Combined electron microscopy and energy analysis of an internally oxidized Ni + Si alloy. Philos. Mag. A 1966, 14, 1233–1242. [Google Scholar] [CrossRef]
- Nolan, P.J.; Grundy, P.J. Morphology and stability of precipitates in internally oxidised silicon-bearing nickel and cobalt alloys. J. Mater. Sci. 1971, 6, 1143–1150. [Google Scholar] [CrossRef]
- Sato, H.; Ota, I.; Fujii, T.; Onaka, S.; Kato, M. Morphological evolution of grain-boundary SiO2 in internally oxidized Cu–Si bicrystals. Mater. Trans. 2004, 45, 818–823. [Google Scholar] [CrossRef]
- Yanagihara, K.; Yamazaki, S. Characterization of oxidation behavior at Fe–Si alloy surface. Nippon Steel Tech. Rep. 2011, 2, 27–32. [Google Scholar]
- Danoix, R.; Legras, L.; Hanoyer, B.; Dulcy, J.; Danoix, F. Atom probe tomography and transmission electron microscopy investigations of chromium nitrides in FeCr alloys. In Proceedings of the International Conference on Solid to Solid Phase Transformations in Inorganic Materials (PTM) 2005, Phoenix, AZ, USA, 29 May–3 June 2005; Howe, J.M., Laughlin, D.E., Lee, J.K., Dahmen, U., Soffa, W.A., Eds.; TMS (The Minerals, Metals and Materials Society): Warrendale, PA, USA, 2005; Volume 1, pp. 351–356. [Google Scholar]
- Ginter, C.; Torchane, L.; Dulcy, J.; Gantois, M.; Malchère, A.; Esnouf, C.; Turpin, T. A new approach to hardening mechanisms in the diffusion layer of gas nitrided α-alloyed steels. Effects of chromium and aluminium: Experimental and simulation studies. Metall. Ital. 2006, 98, 29–35. [Google Scholar]
- Hosmani, S.S.; Schacherl, R.E.; Mittemeijer, E.J. Nitrogen uptake by an Fe-V alloy: Quantitative analysis of excess nitrogen. Acta Mater. 2006, 54, 2783–2792. [Google Scholar] [CrossRef]
- Jung, K.S.; Schacherl, R.E.; Bischoff, E.; Mittemeijer, E.J. Nitriding of ferritic Fe-Cr-Al alloys. Surf. Coat. Technol. 2010, 204, 1942–1946. [Google Scholar] [CrossRef]
- Schacherl, R.E.; Graat, P.C.J.; Mittemeijer, E.J. The nitriding kinetics of Iron-Chromium Alloys; The Role of Excess Nitrogen: Experiments and Modelling. Metall. Mater. Trans. A 2004, 35A, 3388–3398. [Google Scholar] [CrossRef]
- Hekker, P.M.; Rozendall, H.C.F.; Mittemeijer, E.J. Excess nitrogen and discontinuous precipitation in nitride iron-chromium alloys. J. Mater. Sci. 1985, 20, 718–729. [Google Scholar] [CrossRef]
- Jessner, P.; Danoix, R.; Hannoyer, B.; Danoix, F. Investigations of the nitrided subsurface layers of an Fe-Cr-model alloy. Ultramicroscopy 2009, 109, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Jessner, P. Phénomène de précipitation dans les alliages nitrurés: contribution tomographique atomique et de la microscopie électronique à transmission. Ph.D. Thesis, Univ ersity of Rouen, Rouen, France, 2010. [Google Scholar]
- Martinavičius, A.; Van Landeghem, H.P.; Danoix, R.; Redjaïmia, A.; Gouné, M.; Danoix, F. Mechanism of Si3N4 precipitation in nitrided Fe-Si alloys: A novel example of particle-stimulated-nucleation. Mater. Lett. 2017, 189, 25–27. [Google Scholar] [CrossRef]
- DaCosta, G.; Vurpillot, F.; Bostel, A.; Bouet, M.; Deconihout, B. Design of a delay-line position-sensitive detector with improved performance. Rev. Sci. Instrum. 2005, 76, 013304. [Google Scholar] [CrossRef]
- Bas, P.; Bostel, A.; Deconihout, B.; Blavette, D. A general protocole for the reconstruction of 3D atom probe data. Appl. Surf. Sci. 1995, 87, 298–304. [Google Scholar] [CrossRef]
- Vaumousse, D.; Cerezo, A.; Warren, P.J. A procedure for quantification of precipitate microstructures from three-dimensional atom probe data. Ultramicroscopy 2003, 95, 215–221. [Google Scholar] [CrossRef]
- Lefebvre, W.; Danoix, F.; Da Costa, G.; De Geuser, F.; Hallem, H.; Deschamps, A. 3DAP measurements of Al content in different types of precipitates in aluminium alloys. Surf. Interface Anal. 2007, 39, 206–212. [Google Scholar] [CrossRef]
- Ayache, J.; Beaunier, L.; Boumendil, J.; Ehret, G.; Laub, D. Sample Preparation Handbook for Transmission Electron Microscopy—Techniques; Springer: New York, NY, USA, 2010. [Google Scholar]
- Egerton, R.F. Electron Energy-Loss Spectroscopy in the Electron Microscopy, 2nd ed.; Plenum Press: New York, NY, USA; London, UK, 1996. [Google Scholar]
- Mitterbauer, C.; Hébert, C.; Kothleitner, G.; Hofer, F.; Schattschneider, P.; Zandbergen, H.W. Electron energy loss-near edge structure as a fingerprint for identifying chromium nitrides. Solid State Commun. 2004, 130, 209–213. [Google Scholar] [CrossRef]
- Courtois, E.; Epicier, T.; Scott, C. EELS study of niobium carbo-nitride nano-precipitates in ferrite. Micron 2006, 37, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Bordère, S.; Caltagirone, J.-P. A unifying model for fluid flow and elastic solid deformation: A novel approach for fluid–structure interaction. J. Fluids Struct. 2017, 51, 344–353. [Google Scholar] [CrossRef]
- Miller, M.K.; Hetherington, M.G. Local magnification effects in the atom probe. Surf. Sci. 1991, 246, 442–449. [Google Scholar] [CrossRef]
- Jessner, P.; Gouné, M.; Danoix, R.; Hannoyer, B.; Danoix, F. Atom probe tomography evidence of nitrogen excess in the matrix of nitrided Fe-Cr. Philos. Mag. Lett. 2010, 90, 793–800. [Google Scholar] [CrossRef]
- Larson, D.J.; Gault, B.; Geiser, B.P.; De Geuser, F.; Vurpillot, F. Atom probe tomography spatial reconstruction: Status and directions. Curr. Opin. Solid State Mater. Sci. 2013, 17, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Danoix, F.; Grancher, G.; Bostel, A.; Blavette, D. Standard deviations of composition measurements in atom probe analyses–Part II: 3D atom probe. Ultramicroscopy 2007, 107, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Hellman, O.C.; Vandenbroucke, J.A.; Rusing, J.; Isheim, D.; Seidman, D.N. Analysis of three-dimensional atom-probe data by the proximity histogram. Microsc. Microanal. 2000, 6, 437–444. [Google Scholar] [PubMed]
- Schwarz, B.; Rossi, P.J.; Strassberger, L.; Jörg, F.; Meka, S.R.; Bischoff, E.; Schacherl, R.E.; Mittemeijer, E.J. Coherency strain and precipitation kinetics: Crystalline and amorphous nitride formation in ternary Fe–Ti/Cr/V–Si alloys. Philos. Mag. 2014, 94, 3098–3119. [Google Scholar] [CrossRef]
- Van Landeghem, H.P.; Gouné, M.; Redjaïmia, A. Nitride precipitation in compositionally heterogeneous alloys: Nucleation, growth and coarsening during nitriding. J. Cryst. Growth 2012, 341, 53–60. [Google Scholar] [CrossRef]
- Phillips, V.A.; Seybolt, A.U. A Transmission Electron Microscopic Study of some Iron-Nitrided Binary Iron Alloys and Steels. Trans. Metall. Soc. AIME 1968, 242, 2415–2422. [Google Scholar]
- Locquet, J.-N.; Soto, R.; Barrallier, L.; Charaï, A. Complete investigation of a nitrided layer for Cr alloy steel. Microsc. Microanal. Microstruct. 1998, 8, 335–352. [Google Scholar] [CrossRef]
- Sennour, M.; Jacq, C.; Esnouf, C. TEM and EBSD investigation of continuous and discontinuous precipitation of CrN in nitrided pure Fe-Cr alloys. J. Mater. Sci. 2004, 39, 4521–4531. [Google Scholar] [CrossRef]
- Vollstädt, H.; Ito, E.; Akaishi, M.; Akimoto, S.; Fukunaga, O. High Pressure synthesis of Rocksalt type of AlN. Proc. Jpn. Acad. 1990, 66, 7–9. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys, 2nd ed.; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Vurpillot, F.; Danoix, F.; Gilbert, M.; Koelling, S.; Dagan, M.; Seidman, D.N. True atomic-scale imaging in three-dimensions: A review of the rebirth of field-ion microscopy. Microsc. Microanal. 2017, 23, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Akré, J.; Danoix, F.; Leitner, H.; Auger, P. The morpholology of secondary-hardening carbides in a martensitic steel at the peak hardness by 3D-FIM. Ultramicroscopy 2009, 109, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Blum, I.; Portavoce, A.; Chow, L.; Hoummada, K.; Mangelinck, D. Diffusion and redistribution of boron in nickel silicides. Defect Diffus. Forum 2012, 322, 129–150. [Google Scholar] [CrossRef]
- Massardier, V.; Voron, L.; Esnouf, C.; Merlin, J. Identification of the nitrides formed during the annealing of a low-carbon low-aluminium steel. J. Mater. Sci. 2001, 36, 1363–1371. [Google Scholar] [CrossRef]
- Sennour, M.; Jacq, C.; Esnouf, C. Mechanical and microstructural investigations of nitrided Fe-Cr layers. J. Mater. Sci. 2004, 39, 4533–4541. [Google Scholar] [CrossRef]
- Catteau, S.D.; Van Landeghem, H.P.; Teixeira, J.; Dulcy, J.; Dehmas, M.; Denis, S.; Redjaïmia, A.; Courteaux, M. Carbon and nitrogen effects on microstructure and kinetics associated with bainitic transformation in a low-alloyed steel. Journal of Alloys and Compounds 2016, 658, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Frisk, K. A Thermodynamic evaluation of the Cr-N, Fe-N, Mo-N and Cr-Mo-N systems. Calphad 1991, 15, 79–106. [Google Scholar] [CrossRef]
- Hillert, M.; Staffansson, L.I. The regular solution model for stoeichiometric phases and ionic melts. Acta Chem. Scand. 1970, 24, 3618–3626. [Google Scholar] [CrossRef]
- Tessier, F.; Navrotsky, A.; Niewa, R.; Leineweber, A.; Jacobs, H.; Kikkawa, S.; Takahashi, M.; Kanamaru, F.; DiSalvo, F.S. Energetic of binary iron nitrides. Solid State Sci. 2000, 2, 457–462. [Google Scholar] [CrossRef]
- Cahn, J.W.; Larché, F. A simple model for coherent equilibrium. Acta Metall. 1984, 32, 1915–1923. [Google Scholar] [CrossRef]
- Williams, R. The calculation of coherent phase equilibria. Calphad 1984, 8, 1–14. [Google Scholar] [CrossRef]
- Maugis, P.; Gouné, M. Kinetics of vanadium carbonitride precipitation in steel: A computer model. Acta Mater. 2005, 53, 3359–3367. [Google Scholar] [CrossRef] [Green Version]
- Somers, M.A.J.; Lankreijer, R.M.; Mittemeijer, E.J. Excess nitrogen in ferrite matrix of nitride binary iron-based alloys. Philos. Mag. A 1989, 59, 353–378. [Google Scholar] [CrossRef]
- Hazotte, A. Transformations et contraintes de cohérence dans les superalliages et les intermétalliques de base TiAl. Matér. Tech. 2010, 97, 23–31. [Google Scholar] [CrossRef]














| Alloying Elements | Cold Rolled | As-Nitrided | Annealed | |
|---|---|---|---|---|
| Matrix | Si3N4 | |||
| Fe | 96.77 ± 0.01 | 96.82 ± 0.01 | 97.98 ± 0.01 | 0.10 ± 0.02 |
| Si | 3.10 ± 0.01 | 2.91 ± 0.01 | 1.79 ± 0.01 | 42.03 ± 0.35 |
| 15N | - | 0.18 ± 0.01 | 0.13 ± 0.01 | 55.71 ± 0.35 |
| Al | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.01 | 1.75 ± 0.09 |
| Ti | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.27 ± 0.04 |
| Mn | 0.01 ± 0.01 | 0.01 ± 0.01 | - | 0.14 ± 0.03 |
| C | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.03 ± 0.01 | - |
| Physical Properties | α-Fe | CrN |
|---|---|---|
| Young modulus (GPa) | 178 | 230 |
| Poisson coefficient | 0.3 | 0.29 |
| Molar volume (cm3/mol) | 7.16 | 11.30 |
| Volume weight (g/cm3) | 7.8 | 5.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Landeghem, H.P.; Danoix, R.; Gouné, M.; Bordère, S.; Martinavičius, A.; Jessner, P.; Epicier, T.; Hannoyer, B.; Danoix, F.; Redjaïmia, A. Contribution of Local Analysis Techniques for the Characterization of Iron and Alloying Elements in Nitrides: Consequences on the Precipitation Process in Fe–Si and Fe–Cr Nitrided Alloys. Materials 2018, 11, 1409. https://doi.org/10.3390/ma11081409
Van Landeghem HP, Danoix R, Gouné M, Bordère S, Martinavičius A, Jessner P, Epicier T, Hannoyer B, Danoix F, Redjaïmia A. Contribution of Local Analysis Techniques for the Characterization of Iron and Alloying Elements in Nitrides: Consequences on the Precipitation Process in Fe–Si and Fe–Cr Nitrided Alloys. Materials. 2018; 11(8):1409. https://doi.org/10.3390/ma11081409
Chicago/Turabian StyleVan Landeghem, Hugo P., Raphaële Danoix, Mohamed Gouné, Sylvie Bordère, Andrius Martinavičius, Peter Jessner, Thierry Epicier, Béatrice Hannoyer, Frédéric Danoix, and Abdelkrim Redjaïmia. 2018. "Contribution of Local Analysis Techniques for the Characterization of Iron and Alloying Elements in Nitrides: Consequences on the Precipitation Process in Fe–Si and Fe–Cr Nitrided Alloys" Materials 11, no. 8: 1409. https://doi.org/10.3390/ma11081409