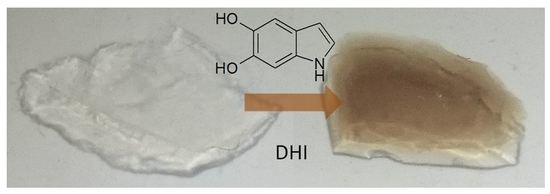
Eumelanin Coating of Silica Aerogel by Supercritical Carbon Dioxide Deposition of a 5,6-Dihydroxyindole Thin Film
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Apparatus
2.3. Adsorption Experiments
2.4. Analytical Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Sarna, T. Chemical and structural diversity in eumelanins: Unexplored bio-optoelectronic materials. Angew. Chem. Int. Ed. 2009, 48, 3914–3921. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K.; D’Ischia, M.; Napolitano, A.; Pezzella, A. Structure of Melanins. In Melanins and Melanosomes: Biosynthesis, Biogenesis, Physiological, and Pathological Functions; Wiley-VCH: Chichester, UK, 2011; pp. 167–185. [Google Scholar]
- D’Ischia, M.; Napolitano, A.; Pezzella, A. 5,6-Dihydroxyindole chemistry: Unexplored opportunities beyond eumelanin. Eur. J. Org. Chem. 2011, 28, 5501–5516. [Google Scholar] [CrossRef]
- Pezzella, A.; Barra, M.; Musto, A.; Navarra, A.; Alfè, M.; Manini, P.; Parisi, S.; Cassinese, A.; Criscuolo, V.; D’Ischia, M. Stem cell-compatible eumelanin biointerface fabricated by chemically controlled solid state polymerization. Mater. Horiz. 2015, 2, 212–220. [Google Scholar] [CrossRef]
- Wünsche, J.; Deng, Y.; Kumar, P.; Di Mauro, E.; Josberger, E.; Sayago, J.; Pezzella, A.; Soavi, F.; Cicoira, F.; Rolandi, M.; et al. Protonic and electronic transport in hydrated thin films of the pigment eumelanin. Chem. Mater. 2015, 27, 436–442. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U. Aerogels-Airy Materials: Chemistry, Structure, and Properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Igarashi, K.; Nishio, T. Effects of supercritical drying media on structure and properties of silica aerogel. J. Non-Cryst. Solids 1995, 186, 83–87. [Google Scholar] [CrossRef]
- Gesser, H.D.; Goswami, P.C. Aerogels and related porous materials. Chem. Rev. 1989, 89, 765–788. [Google Scholar] [CrossRef]
- Panzella, L.; Melone, L.; Pezzella, A.; Rossi, B.; Pastori, N.; Perfetti, M.; D’Errico, G.; Punta, C.; D’Ischia, M. Surface-Functionalization of Nanostructured Cellulose Aerogels by Solid State Eumelanin Coating. Biomacromolecules 2016, 17, 564–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finore, I.; Poli, A.; Di Donato, P.; Lama, L.; Trincone, A.; Fagnano, M.; Mori, M.; Nicolaus, B.; Tramice, A. The hemicellulose extract from Cynara cardunculus: A source of value-added biomolecules produced by xylanolytic thermozymes. Green Chem. 2016, 18, 2460–2472. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Ma, H.S.; Roberts, A.P.; Prévost, J.H.; Jullien, R.; Scherer, G.W. Mechanical structure-property relationship of aerogels. J. Non Cryst. Solids 2000, 277, 127–141. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Martín, L.; Osso, J.O.; Ricart, S.; Roig, A.; García, O.; Sastre, R. Organo-modified silica aerogels and implications for material hydrophobicity and mechanical properties. J. Mater. Chem. 2008, 18, 207–213. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. Development of Mechanically Strong Ambient Pressure Dried Silica Aerogels with Optimized Properties. J. Phys. Chem. C 2015, 119, 7689–7703. [Google Scholar] [CrossRef]
- Moner-Girona, M.; Martínez, E.; Roig, A.; Esteve, J.; Molins, E. Mechanical properties of silica aerogels measured by microindentation: Influence of sol-gel processing parameters and carbon addition. J. Non Cryst. Solids 2001, 285, 244–250. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring mechanical properties of aerogels for aerospace applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Hu, Z.; Cao, B.; Chen, X.; Song, H. Enhancements of thermal insulation and mechanical property of silica aerogel monoliths by mixing graphene oxide. Mater. Chem. Phys. 2017, 187, 183–190. [Google Scholar] [CrossRef]
- Caputo, G.; Scognamiglio, M.; De Marco, I. Nimesulide adsorbed on silica aerogel using supercritical carbon dioxide. Chem. Eng. Res. Des. 2012, 90, 1082–1089. [Google Scholar] [CrossRef]
- Gargiulo, V.; Alfè, M.; Capua, R.D.; Togna, A.R.; Cammisotto, V.; Fiorito, S.; Musto, A.; Navarra, A.; Parisi, S.; Pezzella, A. Supplementing π-systems: Eumelanin and graphene-like integration towards highly conductive materials for the mammalian cell culture bio-interface. J. Mater. Chem. B 2015, 3, 5070–5079. [Google Scholar] [CrossRef]
- Dastorian Jamnani, B.; Hosseini, S.; Shavandi, A.; Hassan, M.R. Thermochemical Properties of Glass Wool/Maerogel Composites. Adv. Mater. Sci. Eng. 2016, 2016. [Google Scholar] [CrossRef]
- Stein, A.; Melde, B.J.; Schroden, R.C. Hybrid inorganic-organic mesoporous silicates-nanoscopic reactors coming of age. Adv. Mater. 2000, 12, 1403–1419. [Google Scholar] [CrossRef]
- Yokoi, T.; Karouji, T.; Ohta, S.; Kondo, J.N.; Tatsumi, T. Synthesis of Mesoporous Silica Nanospheres Promoted by Basic Amino Acids and their Catalytic Application. Chem. Mater. 2010, 22, 3900–3908. [Google Scholar] [CrossRef]
- Vitiello, G.; Pezzella, A.; Calcagno, V.; Silvestri, B.; Raiola, L.; D’Errico, G.; Costantini, A.; Branda, F.; Luciani, G. 5,6-Dihydroxyindole-2-carboxylic Acid-TiO2 Charge Transfer Complexes in the Radical Polymerization of Melanogenic Precursor(s). J. Phys. Chem. C 2016, 120, 6262–6268. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caputo, G.; Bonadies, I.; Migliaccio, L.; Caso, M.F.; Pezzella, A. Eumelanin Coating of Silica Aerogel by Supercritical Carbon Dioxide Deposition of a 5,6-Dihydroxyindole Thin Film. Materials 2018, 11, 1494. https://doi.org/10.3390/ma11091494
Caputo G, Bonadies I, Migliaccio L, Caso MF, Pezzella A. Eumelanin Coating of Silica Aerogel by Supercritical Carbon Dioxide Deposition of a 5,6-Dihydroxyindole Thin Film. Materials. 2018; 11(9):1494. https://doi.org/10.3390/ma11091494
Chicago/Turabian StyleCaputo, Giuseppe, Irene Bonadies, Ludovico Migliaccio, Maria Federica Caso, and Alessandro Pezzella. 2018. "Eumelanin Coating of Silica Aerogel by Supercritical Carbon Dioxide Deposition of a 5,6-Dihydroxyindole Thin Film" Materials 11, no. 9: 1494. https://doi.org/10.3390/ma11091494