Synthesis and Irreversible Thermochromic Sensor Applications of Manganese Violet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization Techniques
2.3. Thermochromic Sensor Fabrication
3. Results and Discussion
3.1. Structure and Properties of Manganese Violet
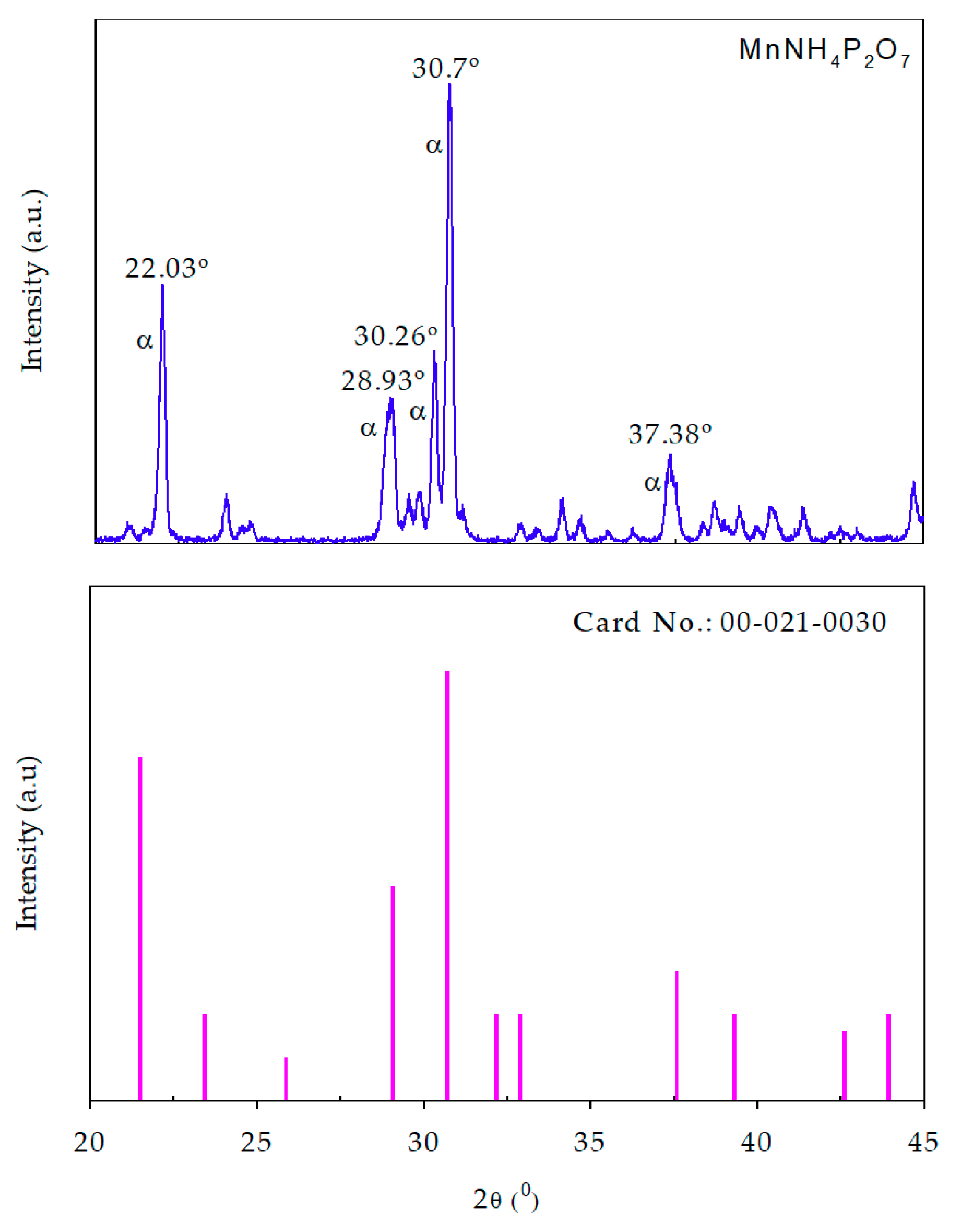
3.1.1. X-ray Diffraction Spectra
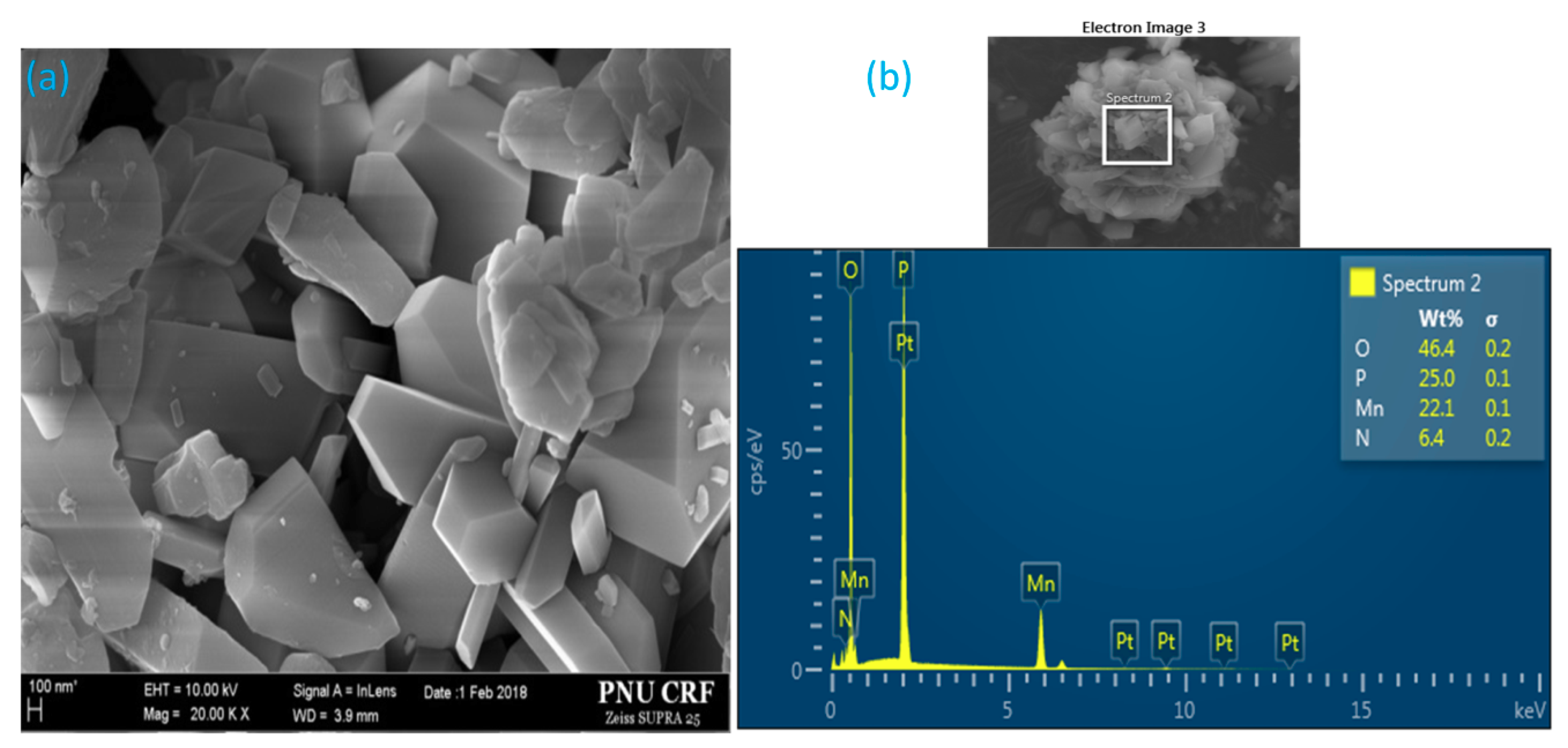
3.1.2. SEM and Spot-Chemical Analysis
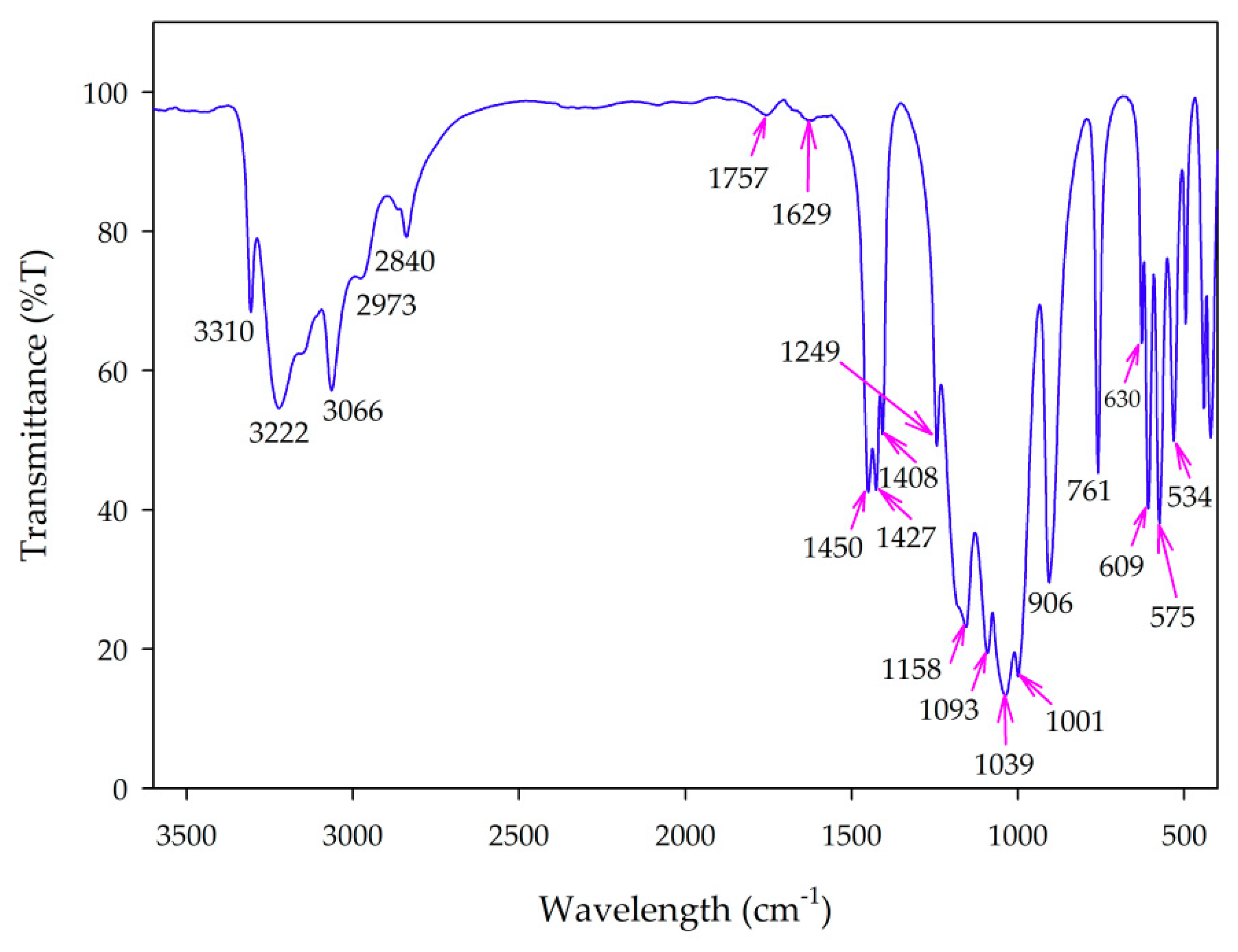
3.2.3. FT-IR Spectra
3.2. Thermal Properties
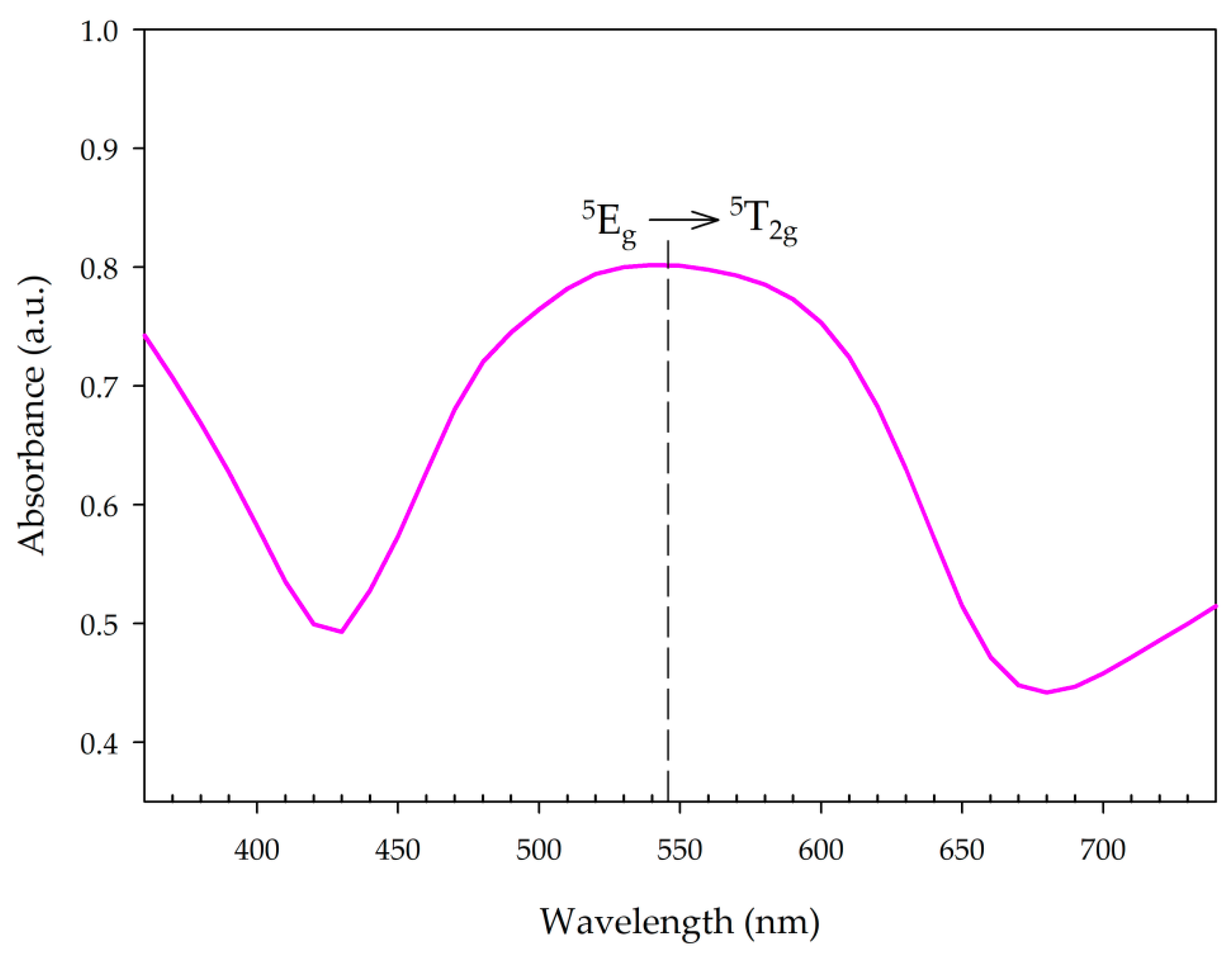
3.3. UV-Vis Spectra
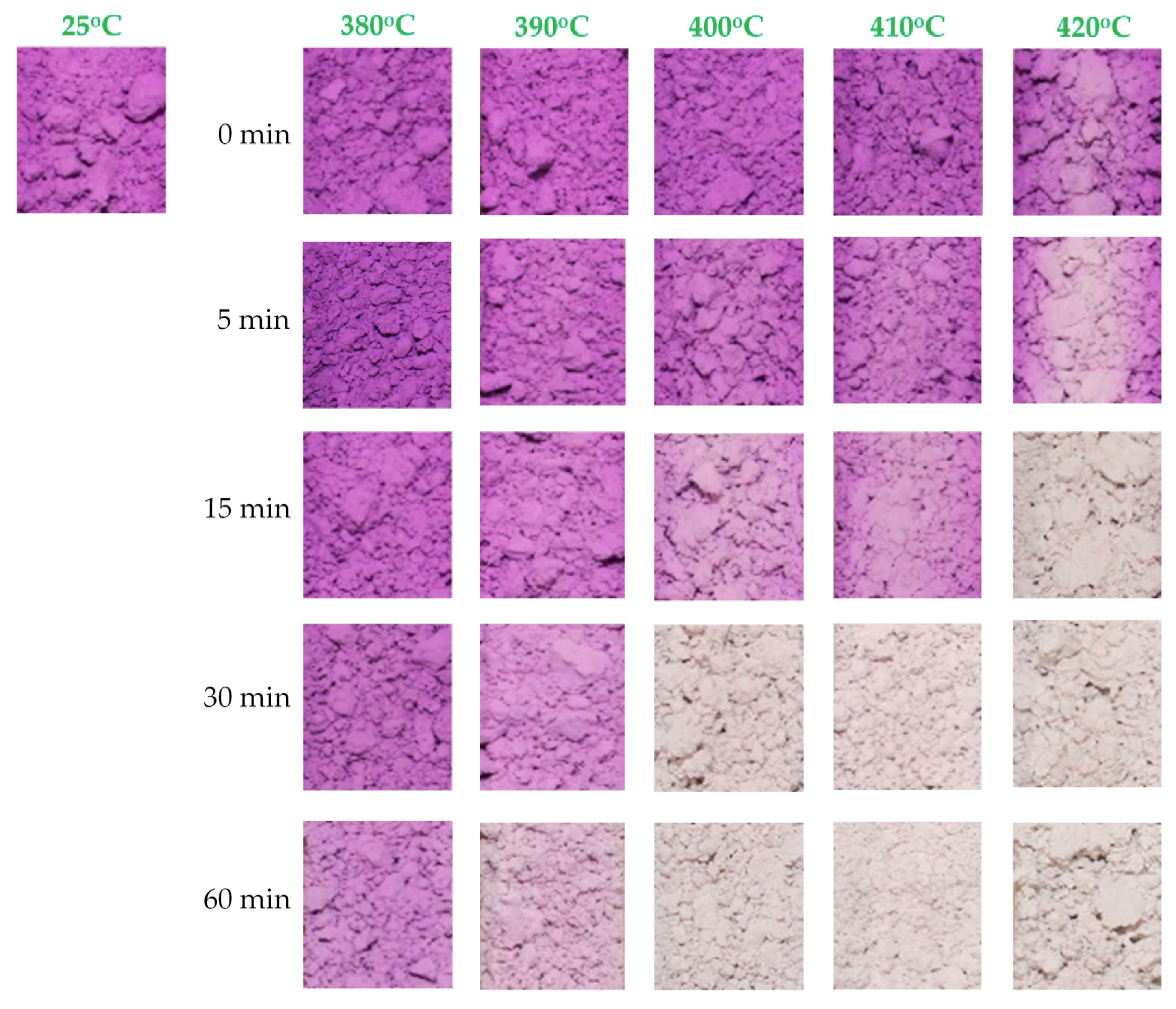
3.4. Thermochromism of Synthesized Pigment Powders
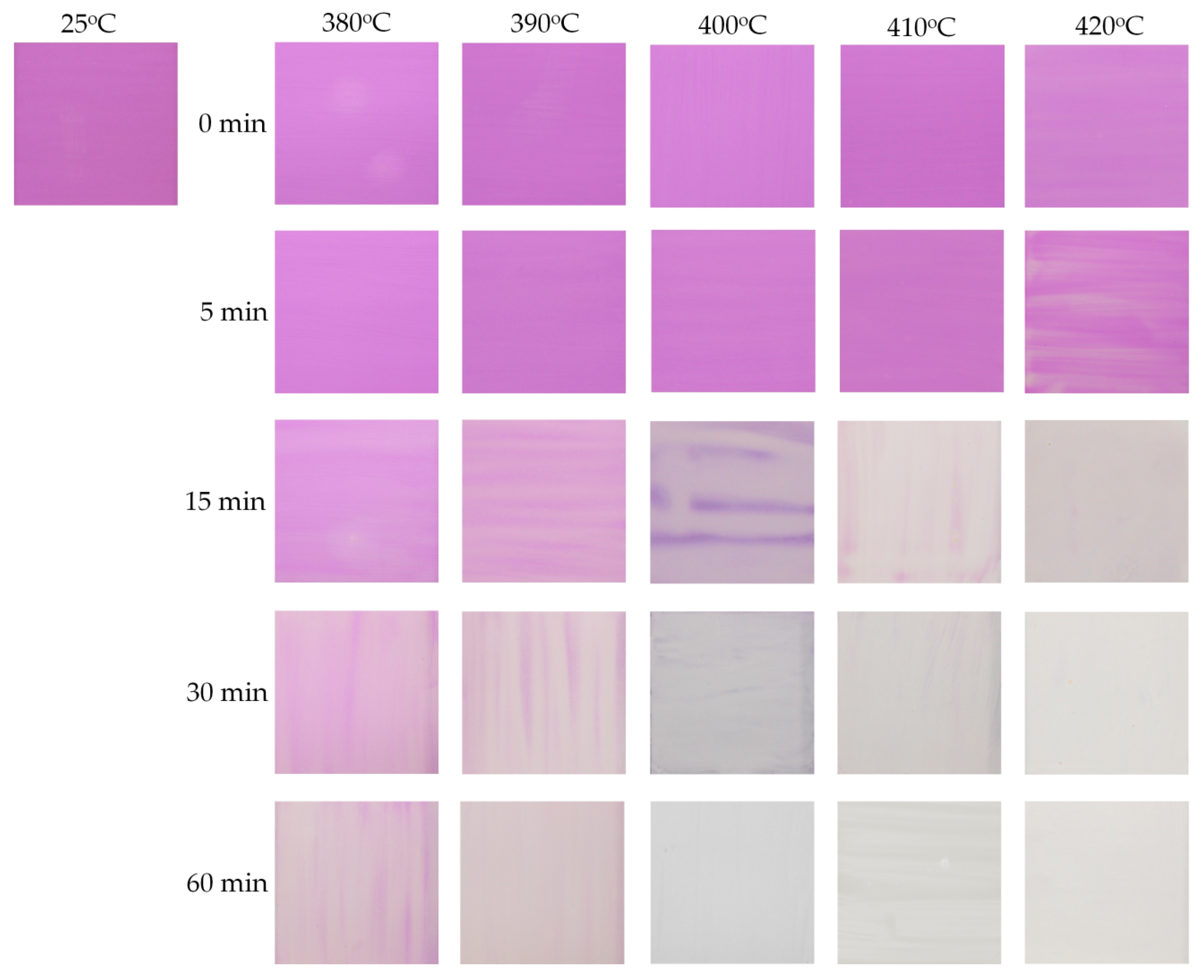
3.5. Manganese Violet-Based Irreversible Thermochromic Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Freddi, S.; Sironi, L.; D’Antuono, R.; Morone, D.; Dona, A.; Cabrini, E.; D’Alfonso, L.; Collini, M.; Pallavicini, P.; Baldi, G.; et al. A molecular thermometer for nanoparticles for optical hyperthermia. Nano Lett. 2013, 13, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, Y.; Liu, T.; Zhang, G.; Liu, S. Intracellular cascade FRET for temperature imaging of living cells with polymeric ratiometric fluorescent thermometers. ACS Appl. Mater. Interfaces 2015, 7, 15551–15560. [Google Scholar] [CrossRef] [PubMed]
- Abram, C.; Fond, B.; Heyes, A.L.; Beyrau, F. High-speed planar thermometry and velocimetry using thermographic phosphor particles. Appl. Phys. B-Lasers Opt. 2013, 111, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Cellini, F.; Peterson, S.D.; Porfiri, M. Flow velocity and temperature sensing using thermosensitive fluorescent polymer seed particles in water. Int. J. Smart Nano Mater. 2017, 8, 232–252. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, M.; Cellini, F.; Cacciotti, I.; Peterson, S.D.; Porfiri, M. In situ temperature sensing with fluorescent chitosan-coated PNIPAAm/alginate beads. J. Mater. Sci. 2017, 52, 12506–12512. [Google Scholar] [CrossRef]
- Popescu, M.; Serban, L.; Popescu, M. Thermo-indicating paint for damage warning. J. Therm. Anal. 1996, 46, 317–321. [Google Scholar] [CrossRef]
- Lempereur, C.; Andral, R.; Prudhomme, J.Y. Surface temperature measurement on engine components by means of irreversible thermal coating. Meas. Sci. Technol. 2008, 19, 105501–105512. [Google Scholar] [CrossRef]
- Pelvich, C.W.; Foulk, D.L.; Polec, T.W. Method of Sensing High Surface Temperature in an Aircraft. Patent EP1959246 A2, 19 June 2008. [Google Scholar]
- Watson, H.M.L. An Irreversible Temperature Indicating Paint. Patent EP1614724A2, 11 January 2006. [Google Scholar]
- Efremov, A.M.; Mikhailov, M.D. Thermochromic Material. Patent EP1405890 B1, 17 October 2012. [Google Scholar]
- Lataste, E.; Demourgues, A.; Salmi, J.; Naporea, C.; Gaudon, M. Thermochromic behaviour (400 < T °C < 1200 °C) of barium carbonate/binary metal oxide mixtures. Dyes Pigm. 2011, 91, 396–403. [Google Scholar]
- Khiem, N.D.; Heesoo, L.; In-Tae, K. Synthesis and thermochromic properties of Cr-doped Al2O3 for a reversible thermochromic sensor. Materials 2017, 10, 476. [Google Scholar] [CrossRef]
- Seeboth, A. Thermochromic and Thermotropic Materials; CRC Press: Singapore, 2014. [Google Scholar]
- Begum, Y.; Wright, A.J. Relating highly distorted Jahn-Teller MnO6 to colouration in manganese violet pigments. J. Mater. Chem. 2012, 22, 21110–21116. [Google Scholar] [CrossRef]
- Lee, J.D.; Browne, L.S. The nature and properties of manganese violet. J. Chem. Soc. A 1968. [Google Scholar] [CrossRef]
- Chemberlain, J.R. Temperature Indicating Paint and Method of Preparing a Specimen with the Same. Patent GB2204874A, 23 November 1988. [Google Scholar]
- Anselmi, C.; Vagnini, M.; Cartechini, L.; Grazia, C.; Vivani, R.; Romani, A.; Rosi, F.; Sgamellotti, A.; Miliani, C. Molecular and structure characterization of some violet phosphate pigments for their non-invasive identification in modern paintings. Spectrochim. Acta A 2017, 173, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Brouzi, K.; Ennaciri, A.; Harcharras, M.; Capitelli, F. Structure and vibrational spectra of a new trihydrate diphosphate MnNH4NaP2O7·3H2O. J. Ram. Spectr. 2004, 35, 41–46. [Google Scholar] [CrossRef]
- Capitelli, F.; Khaoulaf, R.; Harcharras, M.; Ennaciri, A.; Habyby, S.H.; Valentini, V.; Mattei, G.; Bertolasi, V. Crystal structure and vibrational spectroscopy of the new acidic diphosphate (NH4)2Zn(H2P2O7)2·2H2O. Z. Kristallogr. 2005, 220, 25–30. [Google Scholar]
- Brouzi, K.; Ennaciri, A.; Capitelli, F.; Valentini, V.; Mattei, G.; Harcharas, M. Vibrational study of manganese ammonium dihydrogendiphosphate hydrated Mn0.5NH4H2P2O7·H2O. Phosphorus Sulfur Silicon 2005, 180, 545–553. [Google Scholar] [CrossRef]
- Jegatheesan, A.; Murugan, J.; Neelakantaprasad, B.; Rajarajan, G. FTIR, XRD, SEM, TGA investigations of ammonium dihydrogen phosphate (ADP) single crystal. Int. J. Comput. Appl. 2012, 53, 15–18. [Google Scholar] [CrossRef]
- Philip, D.B.; Lizbeth, B.; Aruldhas, G. IR and polarized raman spectra of Na4P2O7.10 H2O. J. Raman Spectrosc. 1990, 21, 523–524. [Google Scholar] [CrossRef]
- Harcharras, M.; Ennaciri, A.; Rulmont, A.; Gilbert, B. Vibrational spectra and structures of double diphosphates M2CdP2O7 (M = Li, Na, K, Rb, Cs). Spectrochim. Acta A 1997, 53, 345–352. [Google Scholar] [CrossRef]
- Sherman, D.M.; Vergo, N. Optical spectrum, site occupancy, and oxidation state of Mn in montmorillonite. Am. Mineral. 1988, 73, 140–144. [Google Scholar]




| Thermochromic Materials | Color Change | Transition Temperature (°C) |
|---|---|---|
| [NH2(C2H5)2]2CuCl4 | deep green ↔ yellow | 38 |
| Ag2(HgI4) | yellow ↔ orange | 50 |
| CuI | gray-tan → orange | 60–62 |
| Cu2(HgI4) | red ↔ brown | 70 |
| HgI2 | red ↔ yellow | 127 |
| 2Cu(CNS)2·2pyridine | green → yellow | 135 |
| yellow → black | 220 | |
| NH4VO3 | white → brown | 150 |
| brown → black | 170 | |
| CoCO2 | violet → black | 330 |
| MnNH4P2O7 | violet → white | 400 |
| NiC2O4 | light blue → black | 410 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.K.; Bach, Q.-V.; Lee, J.-H.; Kim, I.-T. Synthesis and Irreversible Thermochromic Sensor Applications of Manganese Violet. Materials 2018, 11, 1693. https://doi.org/10.3390/ma11091693
Nguyen DK, Bach Q-V, Lee J-H, Kim I-T. Synthesis and Irreversible Thermochromic Sensor Applications of Manganese Violet. Materials. 2018; 11(9):1693. https://doi.org/10.3390/ma11091693
Chicago/Turabian StyleNguyen, Duy Khiem, Quang-Vu Bach, Jong-Han Lee, and In-Tae Kim. 2018. "Synthesis and Irreversible Thermochromic Sensor Applications of Manganese Violet" Materials 11, no. 9: 1693. https://doi.org/10.3390/ma11091693





