Biomedical Porous Shape Memory Alloys for Hard-Tissue Replacement Materials
Abstract
:1. Introduction
2. Fundamentals of Shape Memory Alloys (SMAs)
3. Requirements for Hard-Tissue Replacements
4. Porous NiTi Shape Memory Alloys
4.1. Fabrication and Pore Structure of Porous NiTi SMAs
4.1.1. Homogeneous Porous Structure
4.1.2. Gradient Porous Structure
4.2. Microstructure and Properties of Porous NiTi SMAs
4.2.1. Effect of Microstructure and Pores on Martensitic Transformation (MT)
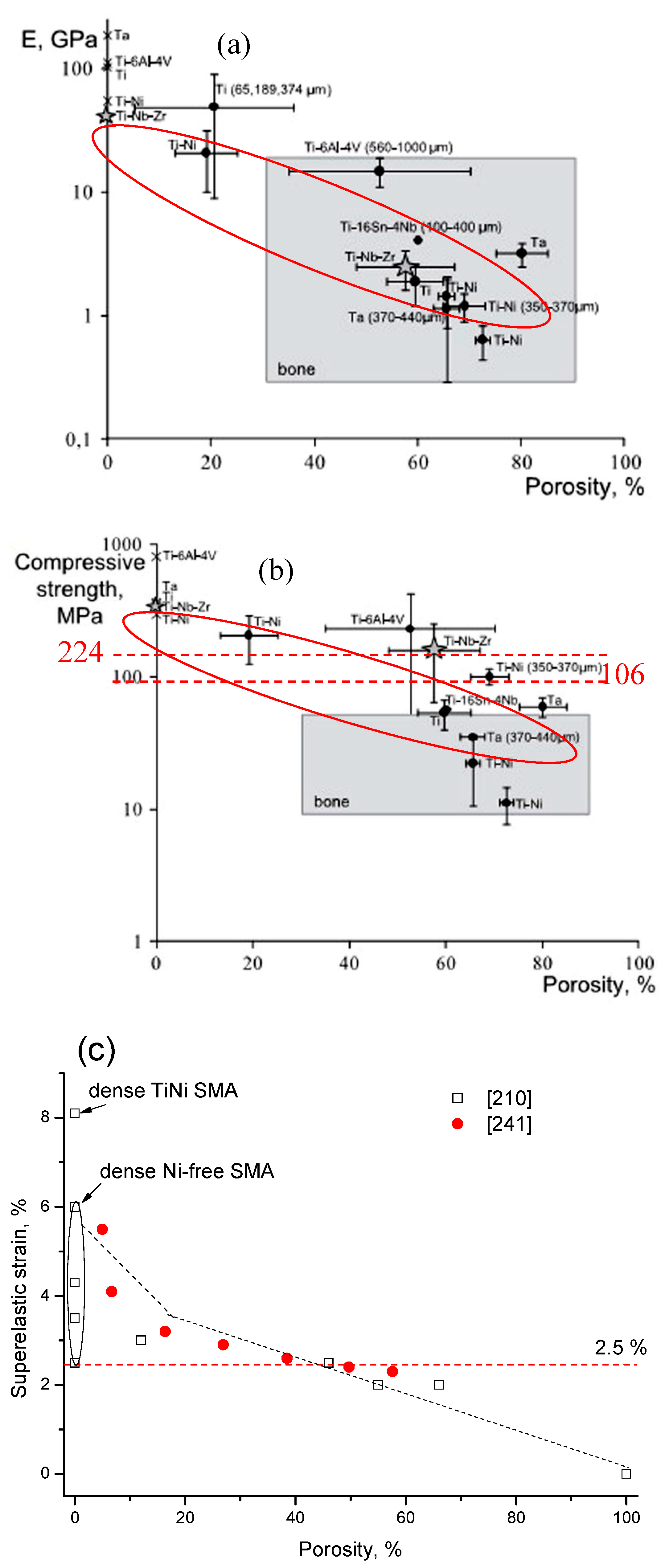
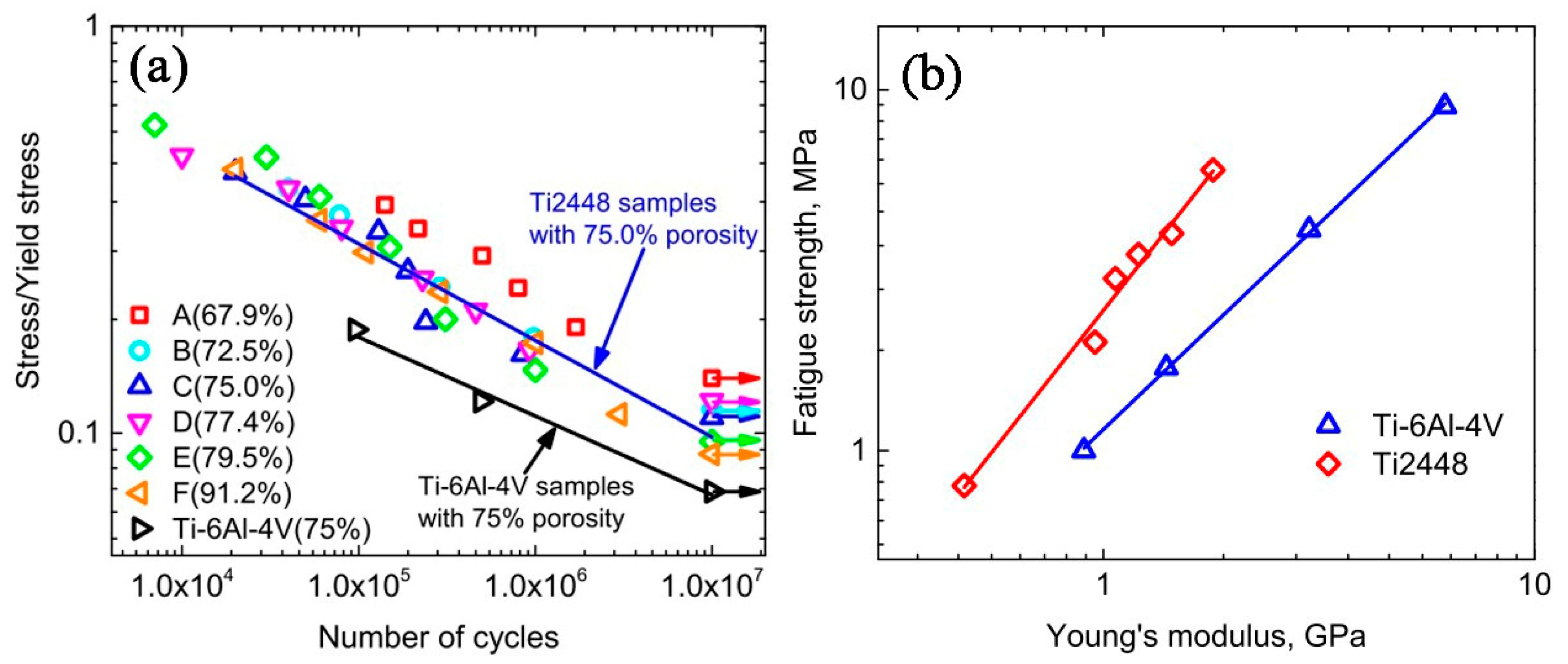
4.2.2. Effect of Pores on Mechanical Properties and Superelasticity (SE)
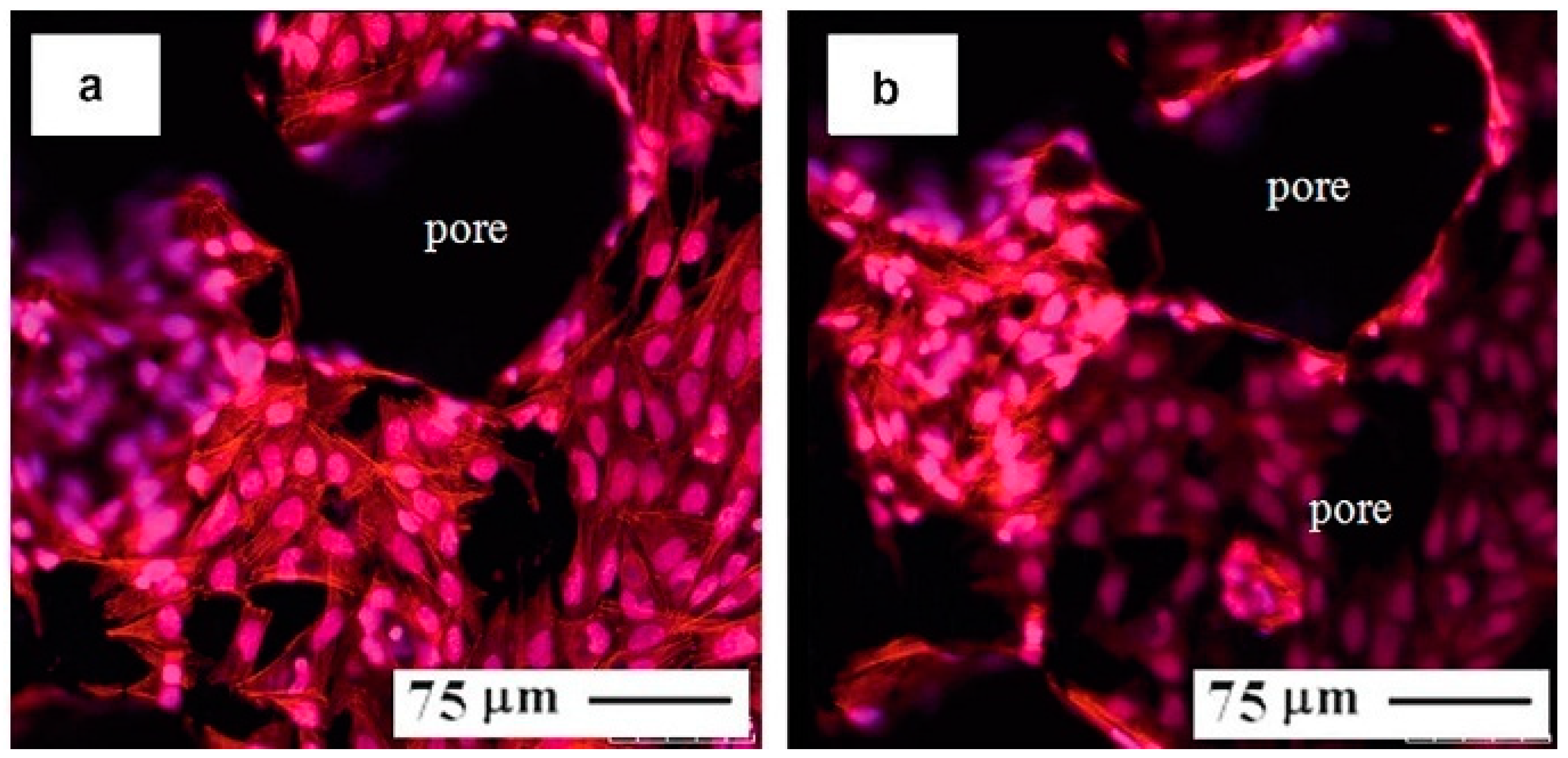
4.2.3. Effect of Pores on Biomedical Properties
4.3. Surface Modification of Porous NiTi SMAs
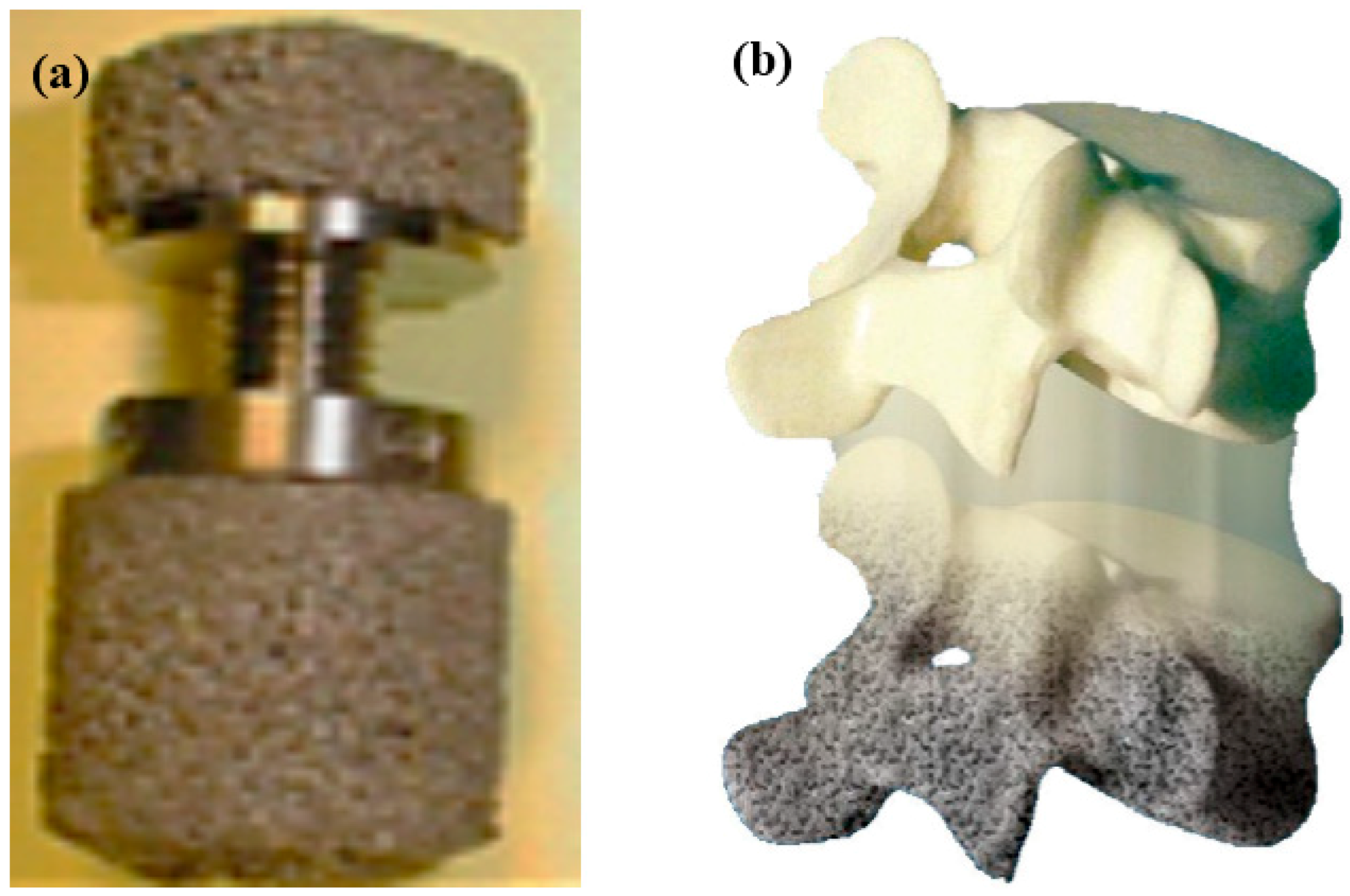
4.4. Application of Porous NiTi SMAs
5. Porous Ni-Free Shape Memory Alloys
5.1. Dense Ni-Free SMAs
5.1.1. Development History and Alloy Systems
5.1.2. MT, Microstructure and Shape Memory Effect (SME)
5.1.3. Mechanical and Biological Properties
5.2. Porous Ni-Free SMAs
5.2.1. Fabrication Method and Porous Structure
5.2.2. Mechanical and Biomedical Properties
6. Prospects and Summaries
Author Contributions
Funding
Conflicts of Interest
References
- World Population Prospects 2017; Population Division, DESA, United Nations: New York, NY, USA, 2017.
- Gage, B.F.; Birman-Deych, E.; Radford, M.J.; Nilasena, D.S.; Binder, E.F. Risk of osteoporotic fracture in elderly patients taking warfarin: Results from the National Registry of Atrial Fibrillation. Arch. Intern. Med. 2006, 166, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.L. Biomaterials Engineering and Devices; Humana Press: Berlin, Germany, 2000; pp. 205–319. [Google Scholar]
- Park, J.B.; Bronzino, J.D. Biomaterials: Principles and Applications; CRC Press: Boca Rator, FL, USA, 2003; pp. 1–241. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants–A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Morgan, N.B. Medical shape memory applications–The market and its product. Mater. Sci. Eng. A 2004, 378, 16–23. [Google Scholar] [CrossRef]
- Jani, J.M.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Des. 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- Chang, L.C.; Read, T.A. Plastic deformation and diffusionless phase changes in metals—The gold-cadmium beta phase. Trans. AIME 1951, 189, 47–52. [Google Scholar] [CrossRef]
- Buehler, W.J.; Gilfrich, J.W.; Wiley, R.C. Effect of low–Temperature phase changes on the mechanical properties of alloys near composition TiNi. J. Appl. Phys. 1963, 34, 1475–1476. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Sargeant, T.D.; Stupp, S.I.; Dunand, D.C. Porous NiTi for bone implants: A review, Acta Biomaterialia. Acta Biomater. 2008, 4, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Li, S.J.; Wang, H.L.; Hou, W.T.; Hao, Y.L.; Yang, R.; Sercombe, T.B.; Zhang, L.C. Microstructure, defects and mechanical behavior of beta-type titanium porous structures manufactured by electron beam melting and selective laser melting. Acta Mater. 2016, 113, 56–67. [Google Scholar] [CrossRef]
- Yuan, B.; Chung, C.Y.; Zhu, M. Microstructure and martensitic transformation behavior of porous NiTi shape memory alloy prepared by HIP processing. Mater. Sci. Eng. A 2004, 382, 181–187. [Google Scholar] [CrossRef]
- Yuan, B.; Chung, C.Y.; Huang, P.; Zhu, M. Superelastic properties of porous TiNi shape memory alloys prepared by hot isostatic pressing. Mater. Sci. Eng. A 2006, 438–440, 657–660. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, Y.C.; Xiong, J.Y.; Hodgson, P.D.; Wen, C.E. Porous TiNbZr alloy scaffolds for biomedical applications. Acta Biomater. 2009, 5, 3616–3624. [Google Scholar] [CrossRef] [PubMed]
- Haberland, C.; Elahinia, M.; Walker, J.M.; Meier, H.; Frenzel, J. On the development of high quality NiTi shape memory and pseudoelastic parts by additive manufacturing. Smart Mater. Struct. 2014, 23, 10. [Google Scholar]
- Andani, M.T.; Moghaddam, N.S.; Haberland, C.; Dean, D.; Miller, M.J.; Elahinia, M. Metals for bone implants. Part 1. Powder metallurgy and implant rendering. Acta Biomater. 2014, 10, 4058–4070. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Wayman, C.M. Shape Memory Materials; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Otsuka, K.; Ren, X. Physical metallurgy of Ti–Ni-based shape memory alloys. Prog. Mater. Sci. 2005, 50, 511–678. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Karaman, I.; Noebe, R.D. High temperature shape memory alloys. Int. Mater. Rev. 2010, 55, 257–315. [Google Scholar] [CrossRef]
- Otsuka, K.; Sakamoto, H.; Shimizu, K. Successive stress-induced martensitic transformations and associated transformation pseudoelasticity in Cu-Al-Ni alloys. Acta Metall. 1979, 27, 585–589. [Google Scholar] [CrossRef]
- Wollants, P.; Nonte, M.D.; Roos, J.R. Thermodynamic analysis of the stress-induced martensitic-transformation in a single-crystal. Z. Metallkd. 1979, 70, 113–117. [Google Scholar]
- Hall, S. Basic Biomechanics, 5th ed.; McGraw-Hill: New York, NY, USA, 2007; pp. 88–100. [Google Scholar]
- Bone. Available online: http://en.wikipedia.org/wiki/Bone (accessed on 30 June 2018).
- Burr, D.B.; Martin, R.B. Errors in bone remodeling: Toward a unified theory of metabolic bone disease. Am. J. Anat. 1989, 186, 186–216. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kuhn, J.L.; Ciarelli, M.J.; Goldstein, S.A. The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. J. Biomech. 1990, 23, 1103–1113. [Google Scholar] [CrossRef] [Green Version]
- Rho, J.Y.; Spearing, L.K.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Parente, M.A.; Geil, M. In the Future: Prosthetic Advances and Challenges. In Prosthetics and Patient Management: A Comprehensive Clinical Approach; Carroll, K., Edelstein, J., Eds.; SLACK Incorporated: Thorofare, NJ, USA, 2006; pp. 215–232. [Google Scholar]
- Currey, J. Cortical bone. In Handbook of Biomaterial Properties; Black, J., Hastings, G., Eds.; Chapman and Hall: London, UK, 1998; pp. 3–14. [Google Scholar]
- Keaveny, T.M. Cancellous bone. In Handbook of Biomaterial Properties; Black, J., Hastings, G., Eds.; Chapman and Hall: London, UK, 1998; pp. 15–23. [Google Scholar]
- Thomson, R.C.; Wake, M.C.; Yaszemski, M.J.; Mikos, A.G. Biodegradable polymer scaffolds to regenerate organs. Adv. Polym. Sci. 1995, 122, 245–274. [Google Scholar]
- Nouri, A.; Hodgson, P.D.; Wen, C.E. Biomimetic porous titanium scaffolds for orthopaedic and dental applications. In Biomimetics Learning from Nature; Mukherjee, A., Ed.; InTech: Rijek, Croatia, 2010; pp. 415–450. [Google Scholar]
- Wang, X.J.; Li, Y.C.; Hodgson, P.D.; Wen, C.E. Nano- and macro-scale characterisation of the mechanical properties of bovine bone. Mater. Forum 2007, 31, 156–159. [Google Scholar]
- Arola, D.D.; Reprogel, R.K. Tubule orientation and the fatigue strength of human dentin. Biomaterials 2006, 27, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Giannini, N.; Soares, C.J.; de Carvalho, R.M. Ultimate tensile strength of tooth structures. Dent. Mater. 2004, 20, 322–329. [Google Scholar] [CrossRef]
- Angker, L.; Swain, M.V.; Kilpatrick, N. Micro-mechanical characterisation of the properties of primary tooth dentine. J. Dent. 2003, 31, 261–267. [Google Scholar] [CrossRef]
- Alvarez, K.; Nakajima, H. Metallic Scaffolds for Bone Regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef] [Green Version]
- Ryan, G.E.; Pandit, A.S.; Apatsidis, D.P. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 2008, 29, 3625–3635. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Miyazaki, S. (Eds.) Shape Memory Alloys for Biomedical Application; CRC Press: Bocaton, FL, USA, 2009; pp. 194–227. [Google Scholar]
- Netti, P.A. (Ed.) Biomedical Foams for Tissue Engineering Applications; Woodhead publishing: Sawston, UK, 2014; pp. 36–78. [Google Scholar]
- Kujala, S.; Ryhanen, J.; Danilov, A.; Tuukkanen, J. Effect of porosity on the osteointegration and bone ingrowth of a weight-bearing nickel–titanium bone graft substitute. Biomaterials 2003, 24, 4691–4697. [Google Scholar] [CrossRef]
- Kang, S.B.; Yoon, K.S.; Kim, J.S.; Nam, T.H.; Gjunter, V.E. In vivo result of porous TiNi shape memory alloy: Bone response and growth. Mater. Trans. 2002, 43, 1045–1048. [Google Scholar] [CrossRef]
- Assad, M.; Jarzem, P.; Leroux, M.A.; Coillard, C.; Chernyshov, A.V.; Charette, S. Porous titanium–nickel for intervertebral fusion in a sheep model: Part 1. Histomorphometric and radiological analysis. J. Biomed. Mat. Res. Part B Appl. Biomater. 2003, 64, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Bobyn, J.D.; Pilliar, R.M.; Cameron, H.U.; Weatherly, G.C. The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone. Clin. Orthopaed. Relat. Res. 1980, 150, 263–270. [Google Scholar] [CrossRef]
- Atwater, M.A.; Guevara, L.N.; Darling, K.A.; Tschopp, M.A. Solid state porous metal production: A review of the capabilities, characteristics, and challenges. Adv. Eng. Mater. 2018, 20, 1700766. [Google Scholar] [CrossRef]
- Wen, C.E.; Mabuchi, M.; Yamada, Y.; Shimojima, K.; Chino, Y.; Asahina, T. Processing of biocompatible porous Ti and Mg. Scr. Mater. 2001, 45, 1147–1153. [Google Scholar] [CrossRef]
- Elahinia, M.H.; Hashemi, M.; Tabesh, M.; Bhaduri, S.B. Manufacturing and processing of NiTi implants: A review. Prog. Mater. Sci. 2012, 57, 911–946. [Google Scholar] [CrossRef]
- Banhart, J. Functional applications. In Handbook of Cellular Metals: Production, Processing, Applications; Degischer, H.P., Kriszt, B., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2002; pp. 313–320. [Google Scholar]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997; pp. 66–80. [Google Scholar]
- Ishizaki, K.; Komarneni, S.; Nanko, M. Porous Materials: Process Technology and Applications; Kluwer Academic Publishers: Philip Drive Norwell, MA, USA, 1998. [Google Scholar]
- Li, B.Y.; Rong, L.J.; Li, Y.Y. Porous NiTi alloy prepared from elemental powder sintering. J. Mater. Res. 1998, 13, 2847–2851. [Google Scholar] [CrossRef]
- Zhu, S.L.; Yang, X.J.; Fu, D.H.; Zhang, L.Y.; Li, C.Y.; Cui, Z.D. Stress-strain behavior of porous NiTi alloys prepared by powders sintering. Mater. Sci. Eng. A 2005, 408, 264–268. [Google Scholar] [CrossRef]
- Bertheville, B. Porous single-phase NiTi processed under Ca reducing vapor for use as a bone graft substitute. Biomaterials 2006, 27, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Lagoudas, D.C.; Vandygriff, E.L. Processing and characterization of NiTi porous SMA by elevated pressure sintering. J. Int. Mater. Syst. Struct. 2002, 13, 837–850. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, X.P.; Chung, C.Y.; Zhu, M. The effect of porosity on phase transformation behavior of porous Ti–50.8 at.% Ni shape memory alloys prepared by capsule-free hot isostatic pressing. Mater. Sci. Eng. A 2006, 438, 585–588. [Google Scholar] [CrossRef]
- Yuan, B.; Chung, C.Y.; Zhang, X.P.; Zeng, M.Q.; Zhu, M. Control of porosity and superelasticity of porous NiTi shape memory alloy prepared by hot isostatic pressing. Smart Mater. Struct. 2005, 14, S201–S206. [Google Scholar] [CrossRef]
- Li, H.; Yuan, B.; Gao, Y.; Chung, C.Y.; Zhu, M. High porosity NiTi superelastic alloys fabricated by low pressure sintering using titanium hydride as pore-forming agent. J. Mater. Sci. 2009, 44, 875–881. [Google Scholar] [CrossRef]
- Resnina, N.; Belyaev, S.; Voronkov, A. Functional properties of porous Ti-48.0 at.% Ni shape memory alloy produced by self-propagating high-temperature synthesis. J. Mater. Eng. Perform. 2018, 27, 1257–1264. [Google Scholar] [CrossRef]
- Biswas, A. Porous NiTi by thermal explosion mode of SHS: Processing, mechanism and generation of single phase microstructure. Acta Mater. 2005, 53, 1415–1425. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Superelastic behaviors of biomedical porous NiTi alloy with high porosity and large pore size prepared by spark plasma sintering. J. Alloys Compd. 2015, 644, 513–522. [Google Scholar]
- Ibrahim, M.K.; Hamzah, E.; Saud, S.N.; Nazim, E.M. Power metallurgy fabrication of porous 51(at.%)Ni-Ti shape memory alloys for biomedical applications. Shap. Mem. Superelasticity 2018, 4, 327–336. [Google Scholar] [CrossRef]
- Elahinia, M.; Moghaddam, N.S.; Andani, M.T.; Amerinatanzi, A.; Bimber, B.A.; Hamilton, R.F. Fabrication of NiTi through additive manufacturing: A review. Prog. Mater. Sci. 2016, 83, 630–663. [Google Scholar] [CrossRef] [Green Version]
- Saedi, S.; Saghaian, S.E.; Jahadakbar, A.; Moghaddam, N.S.; Andani, M.T.; Saghaian, S.M.; Lu, Y.C.; Elahinia, M.; Karaca, H.E. Shape memory response of porous NiTi shape memory alloys fabricated by selective laser melting. J. Mater. Sci. Mater. Med. 2018, 29, 40–716. [Google Scholar] [CrossRef] [PubMed]
- Gorgin Karaji, Z.; Speirs, M.; Dadbakhsh, S.; Kruth, J.-P.; Weinans, H.; Zadpoor, A.A.; Amin Yavari, S. Additively manufactured and surface biofunctionalized porous nitinol. ACS Appl. Mater. Interfaces 2017, 9, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Dadbakhsh, S.; Speirsc, M.; Kruth, J.P.; Humbeeck, J.V. Influence of SLM on shape memory and compression behavior of NiTi scaffolds. CIRP Ann. Manuf. Tecnol. 2015, 64, 209–212. [Google Scholar] [CrossRef] [Green Version]
- Gotman, I.; Ben-David, D.; Unger, R.E.; Böse, T.; Gutmanas, E.Y.; Kirkpatrick, C.J. Mesenchymal stem cell proliferation and differentiation on load-bearing trabecular Nitinol scaffolds. Acta Biomater. 2013, 9, 8440–8448. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Zhang, X.P.; Chung, C.Y.; Zeng, M.Q.; Zhu, M. A comparative study of the porous TiNi shape memory alloys fabricated by three different processes. Metal. Mater. Trans. A 2006, 37, 755–761. [Google Scholar] [CrossRef]
- Kaya, M.; Orhan, N.; Tosun, G. The effect of the combustion channels on the compressive strength of porous NiTi shape memory alloy fabricated by SHS as implant material. Curr. Opin. Solid State Mater. Sci. 2010, 14, 21–25. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Alizadeh, M.; Ghasemi, A.; Meshkot, M.A. Highly Porous NiTi with isotropic pore morphology fabricated by self-propagated high-temperature synthesis. J. Mater. Eng. Perform. 2013, 22, 405–409. [Google Scholar] [CrossRef]
- Li, B.Y.; Rong, L.J.; Li, Y.Y. The influence of addition of TiH2 in elemental powder sintering porous NiTi alloys. Mater. Sci. Eng. A 2000, 282, 169–175. [Google Scholar] [CrossRef]
- Aydogmus, T.; Bor, S. Superelasticity and compression behavior of porous TiNi alloys produced using Mg spacers. J. Mech. Behav. Biomed. Mater. 2012, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Cao, P. NiTi powder sintering from TiH2 powder: An in situ investigation. Metal. Mater. Trans. A 2013, 44, 5630–5633. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Yuan, B.; Zeng, M.Q.; Chung, C.Y.; Zhang, X.P. High porosity and large pore size shape memory alloys fabricated by using pore-forming agent (NH4HCO3) and capsule-free hot isostatic pressing. J. Mater. Proc. Technol. 2007, 192, 439–442. [Google Scholar] [CrossRef]
- Wu, S.L.; Chung, C.Y.; Liu, X.; Chu, P.K.; Ho, J.P.Y.; Chu, C.L.; Chan, Y.L.; Yeung, K.W.K.; Lu, W.W.; Cheung, K.M.C.; et al. Pore formation mechanism and characterization of porous NiTi shape memory alloys synthesized by capsule-free hot isostatic pressing. Acta Mater. 2007, 55, 3437–3451. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Li, D.S.; Zhang, X.P. Gradient porosity and large pore size NiTi shape memory alloys. Scr. Mater. 2007, 57, 1020–1023. [Google Scholar] [CrossRef]
- Ghasemi, A.; Hosseini, S.R.; Sadrnezhaad, S.K. Pore control in SMA NiTi scaffolds via space holder usage. Mater. Sci. Eng. C 2012, 32, 1266–1270. [Google Scholar] [CrossRef]
- Li, D.S.; Zhang, Y.P.; Eggeler, G.; Zhang, X.P. High porosity and high-strength porous NiTi shape memory alloys with controllable pore characteristics. J. Alloys Compd. 2009, 470, L1–L5. [Google Scholar] [CrossRef]
- Zhang, X.X.; Hou, H.W.; Wei, L.S.; Chen, Z.X.; Wei, W.T.; Geng, L. High damping capacity in porous NiTi alloy with bimodal pore architecture. J. Alloys Compd. 2013, 550, 297–301. [Google Scholar] [CrossRef]
- Tosun, G.; Ozler, L.; Kaya, M.; Orthan, N. A study on microstructure and porosity of NiTi alloy implants produced by SHS. J. Alloys Compd. 2009, 487, 605–611. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Dunand, D.C. Shape-memory NiTi foams produced by solid-state replication with NaF. Intermetallics 2007, 15, 1612–1622. [Google Scholar] [CrossRef]
- Nakaş, G.İ.; Dericioğlu, A.F.; Bor, Ş. Monotonic and cyclic compressive behavior of superelastic TiNi foams processed by sintering using magnesium space holder technique. Mater. Sci. Eng. A 2013, 582, 140–146. [Google Scholar] [CrossRef]
- Aydogmus, T.; Bor, S. Processing of porous TiNi alloys using magnesium as space holder. J. Alloys Compd. 2009, 478, 705–710. [Google Scholar] [CrossRef]
- Neurohr, A.J.; Dunand, D.C. Mechanical anisotropy of shape-memory NiTi with two-dimensional networks of micro-channels. Acta Mater. 2011, 59, 4616–4630. [Google Scholar] [CrossRef]
- Sugiyama, M.; Hyun, S.K.; Tane, M.; Nakajima, H. Fabrication of lotus-type porous NiTi shape memory alloys using the continuous zone melting method and tensile property. High Temp. Mater. Proc. 2007, 26, 297–301. [Google Scholar] [CrossRef]
- Yuan, B.; Zhu, M.; Gao, Y.; Li, X.; Chung, C.Y. Forming and control of pores by capsule-free hot isostatic pressing in NiTi shape memory alloys. Smart Mater. Struct. 2008, 17, 025013. [Google Scholar] [CrossRef]
- Gupta, S.P.; Mukherjee, K.; Johnson, A.A. Diffusion controlled solid state transformation in the near-equiatomic Ti-Ni alloys. Mater. Sci. Eng. 1973, 11, 2–5. [Google Scholar] [CrossRef]
- Chen, G.; Liss, K.D.; Cao, P. In situ observation and neutron diffraction of NiTi powder sintering. Acta Mater. 2014, 67, 32–44. [Google Scholar] [CrossRef]
- Grummon, D.S.; Shaw, J.A.; Gremillet, A. Low-density open-cell foams in the NiTi system. Appl. Phys. Lett. 2003, 82, 2727–2729. [Google Scholar] [CrossRef]
- Yuan, B. Evolution of superelasticity behavior in porous NiTi shape memory alloys. Unpublished results.
- Guo, Z.Q.; Xie, H.X.; Dai, F.L.; Qiang, H.C.; Rong, L.J.; Chen, P.W.; Huang, F.L. Compressive behavior of 64% porosity NiTi alloy: An experimental study. Mater. Sci. Eng. A 2009, 515, 117–130. [Google Scholar] [CrossRef]
- Panico, M.; Brinson, L.C. Computational modeling of porous shape memory alloys. Int. J. Solids Struct. 2008, 45, 5613–5626. [Google Scholar] [CrossRef]
- Shariat, B.S.; Liu, Y.N.; Rio, C. Pseudoelastic behaviour of perforated NiTi shape memory plates under tension. Intermetallics 2014, 50, 59–64. [Google Scholar] [CrossRef]
- Xue, L.J.; Dui, G.S.; Liu, B.F.; Xin, L.B. A phenomenological constitutive model for functionally graded porous shape memory alloy. Int. J. Eng. Sci. 2014, 78, 103–113. [Google Scholar] [CrossRef]
- Entchev, P.B.; Lagoudas, D.C. Modeling porous shape memory using micromechanical averaging techniques. Mech. Mater. 2002, 34, 1–24. [Google Scholar] [CrossRef]
- Nemat-Nasser, S.; Su, Y.; Guo, W.G.; Isaacs, J. Experimental characterization and micromechanical modeling of superelastic response of a porous NiTi shape-memory alloy. J. Mech. Phys. Solids 2005, 53, 2320–2346. [Google Scholar] [CrossRef]
- Liu, B.F.; Dui, G.S.; Xie, B.M.; Xue, L.J. A constitutive model of porous SMAs considering tensile–compressive asymmetry behaviors. J. Mech. Behav. Biomed. Mater. 2014, 32, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.F.; Dui, G.S.; Zhu, Y.P. A micromechanical constitutive model for porous shape memory alloys. Adv. Mater. Res. 2010, 29–32, 1855–1861. [Google Scholar] [CrossRef]
- Zhao, Y.; Taya, M.; Kang, Y.S.; Kawasaki, A. Compression behavior of porous NiTi shape memory alloy. Acta Mater. 2005, 53, 337–343. [Google Scholar] [CrossRef]
- Zhao, Y.; Taya, M. Analytical modeling for stress-strain curve of a porous NiTi. J. Appl. Mech. 2007, 74, 291–297. [Google Scholar] [CrossRef]
- Maîtrejean, G.; Terriault, P.; Brailovski, V. Density dependence of the superelastic behavior of porous shape memory alloys: Representative volume element and scaling relation approaches. Comp. Mater. Sci. 2013, 77, 93–101. [Google Scholar] [CrossRef]
- Qidwai, M.A.; Entchev, P.B.; Lagoudas, D.C.; DeGiorgi, V.G. Modeling of the thermomechanical behaviors of porous shape memory alloys. Int. J. Solid Struct. 2001, 38, 8635–8671. [Google Scholar] [CrossRef]
- Zhu, P.P.; Stebner, A.P.; Brinson, L.C. A numerical study of the coupling of elastic and transformation fields in pore arrays in shape memory alloy plates toadvance porous structure design andoptimization. Smart Mater. Struct. 2013, 22, 094009. [Google Scholar] [CrossRef]
- Sayed, T.E.; Gurses, E.; Siddiq, A. A phenomenological two-phase constitutive model for porous shape memory alloys. Comp. Mater. Sci. 2012, 60, 44–52. [Google Scholar] [CrossRef]
- Bormann, T.; Schulz, G.; Deyhle, H.; Beckmann, F.; Wild, M.D.; Kuffer, J.; Munch, C.; Hoffmann, W.; Muller, B. Combining micro computed tomography and three-dimensional registration to evaluate local strains in shape memory scaffolds. Acta Biomater. 2014, 10, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.S.; Zhang, Z.L. Effect of spherical micro-voids in shape memory alloys subjected to uniaxial loading. Int. J. Solids Struct. 2012, 49, 1947–1960. [Google Scholar] [CrossRef]
- Nakas, G.I.; Dericioglu, A.F.; Bor, S. Fatigue behavior of TiNi foams processed by the magnesium space holder technique. J. Mech. Behav. Biomed. Mater. 2011, 4, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Balla, V.K.; Bose, S.; Bandyopadhyay, A. Compression fatigue behavior of laser processed porous NiTi alloy. J. Mech. Behav. Biomed. Mater. 2012, 13, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Köhl, M.; Bram, M.; Moser, A.; Buchkremer, H.P.; Beck, T.; Stöver, D. Characterization of porous, net-shaped NiTi alloy regarding its damping and energy-absorbing capacity. Mater. Sci. Eng. A 2011, 528, 2454–2462. [Google Scholar] [CrossRef]
- Zhang, X.P.; Liu, H.Y.; Yuan, B.; Zhang, Y.P. Superelasticity decay of porous NiTi shape memory alloys under cyclic strain-controlled fatigue conditions. Mater. Sci. Eng. A 2008, 481–482, 170–173. [Google Scholar] [CrossRef]
- Rhalmi, S.; Charette, S.; Assad, M.; Coillard, C.; Rivard, C.H. The spinal cord dura mater reaction to nitinol and titanium alloy particles: A 1-year study in rabbits. Eur. Spine J. 2007, 16, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Itin, V.; Gyunter, V.; Khodorenko, V.; Chobanyan, M.; Mirgazizov, M.; Korsteleva, E.; Belyalova, M. Dynamic of ingrowth of living tissues in porous permeable implant. Mechanical behavior of the Nitinol-living tissue composite. Lett. J. Tech. Phys. 1996, 22, 37–42. [Google Scholar]
- Yuan, B.; Lai, M.; Gao, Y.; Chung, C.Y.; Zhu, M. The effect of pore characteristics on Ni suppression of porous NiTi shape memory alloys modified by surface treatment. Thin Solid Film 2011, 519, 5297–5301. [Google Scholar] [CrossRef]
- Menne, T. Prevention of nickel allergy by regulation of specific exposures. Ann. Clin. Lab. Sci. 1996, 26, 133–138. [Google Scholar] [PubMed]
- Schrooten, J.; Assad, M.; Van Humbeeck, J.; Leroux, M.A. In vitro corrosion resistance of porous NiTi intervertebral fusion devices. Smart Mater. Struct. 2007, 16, S145–S154. [Google Scholar] [CrossRef]
- Wu, S.L.; Liu, X.M.; Chan, Y.L.; Ho, J.P.Y. Nickel release behavior, cytocompatibility, and superelasticity of oxidized porous single-phase NiTi. J. Biomed. Mat. Res. A 2007, 81A, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Prymak, O.; Bogdanski, D.; Koller, M.; Esenwein, S.A.; Muhr, G.; Beckmann, F.; Donath, T.; Assad, M.; Epple, M. Morphological characterization and in vitro biocompatibility of a porous nickel–titanium alloy. Biomaterials 2005, 26, 5801–5817. [Google Scholar] [CrossRef] [PubMed]
- Kohl, M.; Bram, M.; Buchkremer, P.; Stover, D.; Habijan, T.; Koller, M. Production of highly porous near-net-shape NiTi components for biomedical applications. In Proceedings of the Metfoam Conference, Montreal, ON, Canada, 5–7 September 2007. [Google Scholar]
- Assad, M.; Chernyshov, A.; Leroux, M.A.; Rivard, C.H. A new porous titanium–nickel alloy: Part 1. Cytotoxicity and genotoxicity evaluation. Bio-Med. Mater. Eng. 2002, 12, 225–237. [Google Scholar]
- Assad, M.; Chernyshov, A.; Leroux, M.A.; Rivard, C.H. A new porous titanium–nickel alloy: Part 2. Sensitization, irritation and acute systemic toxicity evaluation. Bio-Med. Mater. Eng. 2002, 12, 339–346. [Google Scholar]
- Ayers, R.A.; Simske, S.J.; Bateman, T.A.; Petkus, A.; Sachdeva, R.L.C.; Gyunter, V.E. Effect of nitinol implant porosity on cranial bone ingrowth and apposition after 6 weeks. J. Biomed. Mater. Res. 1999, 45, 42–47. [Google Scholar] [CrossRef]
- Cempel, M.; Nikel, G. Nickel: A review of its sources and environmental toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- Heintz, C.; Riepe, G.; Birken, L.; Kaiser, E.; Chakfe, N.; Morlock, M.; Delling, G.; Imig, H. Corroded nitinol wires in explanted aortic endografts: An important mechanism of failure. J. Endovasc. Ther. 2001, 8, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C.; O’Dell, N.L.; Singh, B.B.; Ghazi, M.; Whitford, G.M.; Lockwood, P.E. Relatingnickel-induced tissue inflammation to nickel release in vivo. J. Biomed. Mater. Res. 2001, 58, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Lin, S.J.; Chen, Y.L.; Su, Y.Y.; Lai, S.T.; Wu, G.J.; Kwok, C.F.; Chung, K.H. The cytotoxicity of corrosion products of nitinol stent wire on cultured smooth muscle cells. J. Biomed. Mater. Res. 2000, 52, 395–403. [Google Scholar] [CrossRef]
- Li, Y.H.; Rao, G.B.; Rong, L.J.; Li, Y.Y.; Ke, W. Effect of pores on corrosion characteristics of porous NiTi alloy in simulated body fluid. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Proc. 2003, 363, 356–359. [Google Scholar] [CrossRef]
- Peitsch, T.; Klocke, A.; Kahl-Nieke, B.; Prymak, O.; Epple, M. The release of nickel from orthodontic NiTi wires is increased by dynamic mechanical loading but not constrained by surface nitridation. J. Biomed. Mater. Res. A 2007, 82, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Trigwell, S.; Hayden, R.D.; Nelson, K.F.; Selvaduray, G. Effects of surface treatment on the surface chemistry of NiTi alloy for biomedical applications. Surf. Interface Anal. 1998, 26, 483–489. [Google Scholar] [CrossRef]
- Shabalovskaya, S.; Rondelli, G.; Anderegg, J.; Xiong, J.P.; Wu, M. Comparative corrosion performance of black oxide, sandblasted, and fine-drawn nitinol wires in potentiodynamic and potentiostatic tests: Effects of chemical etching and electropolishing. J. Biomed. Mater. Res. B 2004, 69, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; van Humbeeck, J.; de Scheerder, I. Surface conditioning of nickel–titanium alloy stents for improving biocompatibility. Surf. Eng. 2001, 17, 451–458. [Google Scholar] [CrossRef]
- Cissé, O.; Savadogo, O.; Wu, M.; Yahia, L.H. Effect of surface treatment of NiTi alloy on its corrosion behaviour in Hanks’ solution. J. Biomed. Mater. Res. 2002, 61, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Shabalovskaya, S.; Rondelli, G.; Anderegg, J.; Simpson, B.; Budko, S. Effect of chemical etching and aging in boiling water on the corrosion resistance of Nitinol wires with black oxide resulting from manufacturing process. J. Biomed. Mater. Res. B 2003, 66, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Plant, S.D.; Grant, D.M.; Leach, L. Behaviour of human endothelial cells on surface modified NiTi alloy. Biomaterials 2005, 26, 5359–5367. [Google Scholar] [CrossRef] [PubMed]
- Michiardi, A.; Aparicio, C.; Planell, J.A.; Gil, F.J. New oxidation treatment of NiTi shape memory alloys to obtain Ni-free surfaces and to improve biocompatibility. J. Biomed. Mater. Res. B 2006, 77, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.D.; Man, H.C.; Yang, X.J. The corrosion and nickel release behavior of laser surface melted NiTi shape memory alloy in Hanks’ solution. Surf. Coat. Technol. 2005, 192, 347–353. [Google Scholar] [CrossRef]
- Choi, J.; Bogdanski, D.; Köller, M.; Esenwein, S.A.; Müller, D.; Muhr, G.; Epple, M. Calcium phosphate coating of nickel titanium shape-memory alloys. Coating procedure and adherence of leukocytes and platelets. Biomaterials 2003, 24, 3689–3696. [Google Scholar] [CrossRef]
- Bogdanski, D.; Esenwein, S.A.; Prymak, O.; Epple, M.; Muhr, G.; Köller, M. Inhibition of PMN apoptosis after adherence to dip-coated calcium phosphate surfaces on a NiTi shape memory alloy. Biomaterials 2004, 25, 4627–4632. [Google Scholar] [CrossRef] [PubMed]
- Mändl, S.; Sader, R.; Thorwarth, G.; Krause, D.; Zeilhofer, H.F.; Horch, H.H.; Rauschenbach, B. Investigation on plasma immersion ion implantation treated medical implants. Biomol. Eng. 2002, 19, 129–132. [Google Scholar] [CrossRef]
- Maitz, M.F.; Shevchenko, N. Plasma immersion ion implanted nitinol surface with depressed nickel concentration for implants in blood. J. Biomed. Mater. Res. A 2005, 76, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.W.Y.; Ho, J.P.Y.; Liu, X.; Chung, C.Y.; Chu, P.K.; Yeung, K.W.K.; Lu, W.W.; Cheung, K.M.C. Anti-corrosion performance of oxidized and oxygen plasma-implanted NiTi alloys. Mater. Sci. Eng. A 2005, 390, 444–451. [Google Scholar] [CrossRef]
- Starosvetsky, D.; Gotman, I. TiN coating improves the corrosion behavior of superelastic NiTi surgical alloy. Surf. Coat. Technol. 2001, 148, 268–276. [Google Scholar] [CrossRef]
- Shevchenko, N.; Pham, M.T.; Maitz, M.F. Studies of surface modified NiTi alloy. Appl. Surf. Sci. 2004, 235, 126–131. [Google Scholar] [CrossRef]
- Espinós, J.P.; Fernández, A.; González-Elipe, A.R. Oxidation and diffusion processes in nickel–titanium oxide systems. Surf. Sci. 1993, 295, 402–410. [Google Scholar] [CrossRef]
- Tepe, G.; Schmehl, J.; Wendel, H.P.; Schaffner, S.; Heller, S.; Gianotti, M.; Claussen, C.D.; Duda, S.H. Reduced thrombogenicity of nitinol stents–in vitro evaluation of different surface modifications and coatings. Biomaterials 2006, 27, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Huan, Z.; Fratila-Apachitei, L.E.; Apachitei, I.; Duszczyk, J. Porous NiTi surfaces for biomedical applications. Appl. Surf. Sci. 2012, 258, 5244–5249. [Google Scholar] [CrossRef]
- Shabalovskaya, S.A. Surface, corrosion and biocompatibility aspects of Nitinol as an implant material. Biomed. Mater. Eng. 2002, 12, 69–109. [Google Scholar] [PubMed]
- Liu, J.X.; Yang, D.Z.; Shi, F.; Cai, Y.J. Sol–gel deposited TiO2 film on NiTi surgical alloy for biocompatibility improvement. Thin Solid Films 2003, 429, 225–230. [Google Scholar] [CrossRef]
- Polonchuk, L.; Elbel, J.; Eckert, L.; Blum, J.; Wintermantel, E.; Eppenberger, H.M. Titanium dioxide ceramics control the differentiated phenotype of cardiac muscle cells in culture. Biomaterials 2000, 21, 539–550. [Google Scholar] [CrossRef]
- Pätsi, M.E.; Hautaniemi, J.A.; Rahiala, H.M.; Peltola, T.O.; Kangasniemi, I.M.O. Bondingstrengths of titania sol–gel derived coatings on titanium. J. Sol. Gel. Sci. Technol. 1998, 11, 55–66. [Google Scholar] [CrossRef]
- Cheng, Y.; Zheng, Y.F. Effect of N2/Ar gas flow ratio on the deposition of TiN/Ti coatings on NiTi shape memory alloy by PIIID. Mater. Lett. 2006, 60, 2243–2247. [Google Scholar] [CrossRef]
- Chu, P.K. Enhancement of surface properties of biomaterials using plasma-based technologies. Surf. Coat. Technol. 2007, 201, 8076–8082. [Google Scholar] [CrossRef]
- Chu, P.K. Plasma surface treatment of artificial orthopedic and cardiovascular biomaterials. Surf. Coat. Technol. 2007, 201, 5601–5606. [Google Scholar] [CrossRef]
- Poon, R.W.Y.; Yeung, K.W.K.; Liu, X.Y.; Chu, P.K.; Chung, C.Y.; Lu, W.W.; Cheung, K.M.C.; Chan, D. Carbon plasma immersion ion implantation of nickel–titanium shape memory alloys. Biomaterials 2005, 26, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; de Groot, K. The role of hydrated silica, titania, and alumina in inducing apatite on implants. J. Biomed. Mater. Res. 1994, 28, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.W.; Tay, B.Y.; Lim, C.S.; Yong, M.S. Biomimetic deposition of apatite coating on surface-modified NiTi alloy. Biomaterials 2005, 26, 6916–6923. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Chang, M.; Lee, C.Y.; Castner, D.G.; Sukavaneshvar, S.; Ratner, B.D.; Horbett, T.A. Plasma-deposited tetraglyme surfaces greatly reduce total blood protein adsorption, contact activation, platelet adhesion, platelet procoagulant activity, and in vitro thrombus deposition. J. Biomed. Mater. Res. A 2007, 81, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, J.; Tong, S. Surface properties of bio-implant Nitinol modified by ECR cold plasma. Mater. Sci. Technol. 2004, 20, 1427–1430. [Google Scholar] [CrossRef]
- Thierry, B.; Merhi, Y.; Silver, J.; Tabrizian, M. Biodegradable membrane-covered stent from chitosan-based polymers. J. Biomed. Mater. Res. A 2005, 75, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Kidane, A.G.; Salacinski, H.; Tiwari, A.; Bruckdorfer, K.R.; Seifalian, A.M. Anticoagulant and antiplatelet agents: Their clinical and device application(s) together with usages to engineer surfaces. Biomacromolecules 2004, 5, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Thierry, B.; Tabrizian, M.; Trepanier, C.; Savadogo, O.; Yahia, L.H. Effect of surface treatment and sterilization processes on the corrosion behavior of NiTi shape memory alloy. J. Biomed. Mater. Res. 2000, 51, 685–693. [Google Scholar] [CrossRef]
- Gu, Y.W.; Li, H.; Tay, B.Y.; Lim, C.S.; Yong, M.S.; Khor, K.A. In vitro bioactivity and osteoblast response of porous NiTi synthesized by SHS using nanocrystalline Ni–Ti reaction agent. J. Biomed. Mater. Res. A 2006, 78A, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.L.; Liu, X.M.; Hu, T.; Chu, P.K.; Ho, J.P.Y.; Chan, Y.L.; Yeung, Y.W.Y.; Chu, C.L.; Hung, T.F.; Huo, K.F.; et al. A biomimetic hierarchical scaffold: Natural growth of nanotitanates on three-dimensional microporous Ti-based metals. Nano Lett. 2008, 8, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.P.Y.; Chu, P.K. Oxygen plasma treatment to restrain nickel out-diffusion from porous nickel titanium orthopedic materials. Surf. Coat. Technol. 2007, 201, 4893–4896. [Google Scholar] [CrossRef]
- Wu, S.L.; Liu, X.M.; Hu, T.; Chu, P.K.; Ho, J.P.Y.; Chan, Y.L.; Yeung, Y.W.Y.; Chu, C.L.; Hung, T.F.; Huo, K.F.; et al. Surface characteristics, mechanical properties, and cytocompatibility of oxygen plasma-implanted porous nickel titanium shape memory alloy. J. Biomed. Mater. Res. A 2006, 79A, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.C.; Rong, L.J. Effect of hydroxyapatite coating on nickel releaseof the porous NiTi shape memory alloy fabricated by SHS method. Surf. Coat. Technol. 2006, 201, 1017–1021. [Google Scholar] [CrossRef]
- Gu, Y.W.; Tay, B.Y.; Lim, C.S.; Yong, M.S. Nanocrystallite apatite formation and its growth kinetics on chemically treated porous NiTi. Nanotechnology 2006, 17, 2212–2218. [Google Scholar] [CrossRef]
- Li, H.; Yuan, B.; Gao, Y.; Chung, C.Y.; Zhu, M. Remarkable biocompatibility enhancement of porous NiTi alloys by a new surface modification approach: In-situ nitriding and in vitro and in vivo evaluation. J. Biomed. Mater. Res. Part A 2011, 99, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Holton, A.; Walsh, E.; Anayiotos, A.; Pohost, G.; Venugopalan, R. Comparative MRI compatibility of 316L stainless steel alloy and nickel–titanium alloy stents. J. Cardiovas. Magn. Reson. 2002, 4, 423–430. [Google Scholar] [CrossRef]
- Available online: http://www.biorthex.com/index.htmls (accessed on 29 September 2004).
- Zhao, Y.; Taya, M.; Izui, H. Study on energy absorbing composite structure made of concentric NiTi spring and porous NiTi. Int. J. Solid Struct. 2006, 43, 2497–2512. [Google Scholar] [CrossRef]
- Silberstei, B.M.; Gyunther, V.E. Shape-memory implants in spinal surgery: Long-term results. In Shape Memory Implants; Springer: Berlin, Germany, 2000; pp. 147–152. [Google Scholar]
- Shishkovsky, A. Porous biocompatible implants and tissue scaffolds synthesized by selective laser sintering from Ti and NiTi. J. Mater. Chem. 2008, 18, 1309–1317. [Google Scholar] [CrossRef]
- Moffat, D.L.; Larbalestier, D.C. The composition between martensite and omega in quenched Ti-Nb alloys. MTA 1988, 19, 1677–1686. [Google Scholar] [CrossRef]
- Miyazaki, S.; Kima, H.Y.; Hosoda, H. Development and characterization of Ni-free Ti-base shape memory and superelastic alloys. Mater. Sci. Eng. A 2006, 438–440, 18–24. [Google Scholar] [CrossRef]
- Sun, B.; Meng, X.L.; Gao, Z.Y.; Cai, W. Martensite structure and mechanical property of Ti-Nb-Ag shape memory alloys for biomedical applications. Vacuum 2018, 156, 181–186. [Google Scholar] [CrossRef]
- Fukui, Y.; Inamura, T.; Hosoda, H.; Wakashima, K.; Miyazaki, S. Mechanical properties of a Ti-Nb-Al shape memory alloy. Mater. Trans. 2004, 45, 1077–1082. [Google Scholar] [CrossRef]
- Ping, D.H.; Mitarai, Y.; Yin, F.X. Microstructure and shape memory behavior of a Ti–30Nb–3Pd alloy. Scr. Mater. 2005, 52, 1287–1291. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, H.Y.; Inamura, T.; Hosoda, H.; Miyazaki, S. Shape memory characteristics of Ti–22Nb–(2–8) Zr (at.%) biomedical alloys. Mater. Sci. Eng. A 2005, 403, 334–339. [Google Scholar] [CrossRef]
- Kim, H.Y.; Ohmatsu, Y.; Kim, J.I.; Hosoda, H.; Miyazaki, S. Mechanical properties and shape memory behavior of Ti-Mo-Ga alloys. Mater. Trans. 2004, 45, 1090–1095. [Google Scholar] [CrossRef]
- Zhou, T.; Aindow, M.; Alpay, S.P.; Blackburn, M.J.; Wu, M.H. Pseudo-elastic deformation behavior in a Ti/Mo-based alloy. Scr. Mater. 2004, 50, 343–348. [Google Scholar] [CrossRef]
- Duerig, T.W.; Albrecht, J.; Richter, D.; Fischer, P. Formation and reversion of stress induced martensite in Ti-10V-2Fe-3Al. Acta Metall. 1982, 30, 2161–2172. [Google Scholar] [CrossRef]
- Ehtemam-Haghighia, S.; Lu, H.B.; Jian, G.Y.; Cao, G.H.; Habibi, D.; Zhang, L.C. Effect of α″ martensite on the microstructure and mechanical properties of beta-type Ti-Fe-Ta alloys. Mater. Des. 2015, 76, 47–54. [Google Scholar] [CrossRef]
- Ehtemam-Haghighia, S.; Liu, Y.J.; Cao, G.H.; Zhang, L.C. Influence of Nb on the β → α″ martensitic phase transformation and properties of the newly designed Ti-Fe-Nb alloys. Mater. Sci. Eng. C 2016, 60, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Hashimoto, S.; Kim, J.I.; Inamura, T.; Hosoda, H.; Miyazaki, S. Effect of Ta addition on shape memory behavior of Ti–22Nb alloy. Mater. Sci. Eng. A 2006, 417, 120–128. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, H.Y.; Inamura, T.; Hosoda, H.; Miyazaki, S. Shape memory behavior of Ti–22Nb–(0.5–2.0)O (at.%) biomedical alloy. Mater. Trans. 2005, 46, 852–857. [Google Scholar] [CrossRef]
- Ehtemam-Haghighia, S.; Liu, Y.J.; Cao, G.H.; Zhang, L.C. Phase transition, microstructural evolution and mechanical properties of Ti-Nb-Fe alloys induced by Fe addition. Mater. Des. 2016, 97, 279–286. [Google Scholar] [CrossRef]
- Maeshima, T.; Nishida, M. Shape memory properties of biomedical Ti-Mo-Ag and Ti-Mo-Sn alloys. Mater. Trans. 2004, 45, 1096–1100. [Google Scholar] [CrossRef]
- Furuhara, T.; Annaka, S.; Tomio, Y.; Maki, T. Superelasticity in Ti–10V–2Fe–3Al alloys with nitrogen addition. Mater. Sci. Eng. A 2006, 438, 825–829. [Google Scholar] [CrossRef]
- Okulov, I.V.; Bönisch, M.; Okulov, A.V.; Volegov, A.S.; Attar, H.; Ehtemam-Haghighi, S.; Calin, M.; Wang, Z.; Hohenwarter, A.; Kaban, I.; et al. Phase formation, microstructure and deformation behavior of heavily alloyed TiNb- and TiV-based titanium alloys. Mater. Sci. Eng. A 2018, 733, 80–86. [Google Scholar] [CrossRef]
- Konopatsky, A.S.; Dubinskiy, S.M.; Zhukova, Y.S.; Sheremetyev, V.; Brailovski, V.; Prokoshkina, S.D. Ternary Ti-Zr-Nb and quaternary Ti-Zr-Nb-Ta shape memory alloys for biomedical applications: Structural features and cyclic mechanical properties. Mater. Sci. Eng. A 2017, 702, 301–311. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Muller, M.; Thull, R.; Breme, J. Biocompatibility of β-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5709. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and health care goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef]
- Song, Y.; Xu, D.S.; Yang, R.; Li, D.; Wu, W.T.; Guo, Z.X. Theoretical study of the effects of alloying elements on the strength and modulus of β-type bio-titanium alloys. Mater. Sci. Eng. A 1999, 260, 269–274. [Google Scholar] [CrossRef]
- Baker, C. The shape-memory effect in a titanium-35 wt.% niobium alloy. Met. Sci. J. 1971, 5, 92–100. [Google Scholar] [CrossRef]
- Arciniegas, M.; Manero, J.M.; Espinar, E.; Lamas, J.M.; Barrer, J.M.; Gil, F.L. New Ni-free superelastic alloy for orthodontic applications. Mater. Sci. Eng. C 2013, 33, 3325–3328. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Gao, Y.; Yuan, B.; Zhu, M. Remarkable superelasticity enhancement of sintered Ti-Nb alloys by Ms adjustment via oxygen content regulation. Mater. Des. 2015, 87, 466–472. [Google Scholar] [CrossRef]
- Dubinskiy, S.; Prokoshkin, S.; Brailovski, V.; Inaekyan, K.; Korotitskiy, A. In situ X-ray diffraction strain-controlled study of Ti–Nb–Zr and Ti–Nb–Ta shape memory alloys: Crystal lattice and transformation features. Mater. Charater. 2014, 88, 127–142. [Google Scholar] [CrossRef]
- Tahara, M.; Kim, H.Y.; Hosoda, H.; Miyazaki, S. Cyclic deformation behavior of a Ti–26 at.% Nb alloy. Acta Mater. 2009, 57, 2461–2469. [Google Scholar] [CrossRef]
- Hickman, B.S. Omega phase precipitation in alloys of titanium with transition metals. Trans. Metall. Soc. AIME 1969, 245, 1329–1336. [Google Scholar]
- Hickman, B.S. The formation of omega phase in titanium and zirconium alloys: A review. J. Mater. Sci. 1969, 4, 554–563. [Google Scholar] [CrossRef]
- Bowen, A.W. Omega phase embrittlement in aged Ti-15%Mo. Scr. Metall. 1971, 5, 709–716. [Google Scholar] [CrossRef]
- Kim, H.Y.; Ikehara, Y.; Kim, J.I.; Miyazaki, S. Martensitic transformation, shape memory effect and superelasticity of Ti-Nb binary alloys. Acta Mater. 2006, 54, 2419–2429. [Google Scholar] [CrossRef]
- Elmay, W.; Prima, F.; Gloriant, T. Effects of thermomechanical process on the microstructure and mechanical properties of a fully martensitic titanium-based biomedical alloy. J. Mech. Behav. Biomed. Mater. 2013, 18, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Akahori, T.; Niinomi, M.; Fukui, H.; Suzuki, A.; Hattori, Y.; Niwa, S. Titanium 2003 Science and Technology; Wiley VCH Verlag, GMBH and Co. KGaA: Weinhem, Germany, 2003; pp. 100–145. [Google Scholar]
- Li, Q.; Niinomi, M.; Nakai, M.; Cui, Z.; Zhu, S.; Yang, X. Improvements in the super-elasticity and change in deformation mode of β-type TiNb24Zr2 alloys caused by aging treatments. Metall. Mater. Trans. A 2011, 42, 2843–2849. [Google Scholar] [CrossRef]
- Al-Zain, Y.; Kim, H.Y.; Hosoda, H.; Nam, T.H.; Miyazki, S. Shape memory properties of Ti-Nb-Mo biomedical alloys. Acta Mater. 2010, 58, 4212–4223. [Google Scholar] [CrossRef]
- Kim, H.Y.; Oshika, N.; Kim, J.I.; Miyazki, S. Martensitic transformation and superelasticity of Ti-Nb-Pt alloys. Mater. Trans. 2007, 48, 400–406. [Google Scholar] [CrossRef]
- Tahara, M.; Kim, H.Y.; Hosoda, H.; Miyazki, S. Shape memory effect and cyclic deformation behavior of Ti-Nb-N alloys. Funct. Mater. Lett. 2009, 2, 79–82. [Google Scholar] [CrossRef]
- Hao, Y.L.; Li, S.J.; Sun, S.Y. Effect of Zr and Sn on Young’s modulus and superelasticity of Ti-Nb-based alloys. Mater. Sci. Eng. A 2006, 441, 112–118. [Google Scholar] [CrossRef]
- Zhang, D.C.; Mao, Y.F.; Li, Y.L.; Yuan, M.; Lin, J.G. Effect of ternary alloying elements on microstructure and superelastictity of Ti–Nb alloys. Mater. Sci. Eng. A 2013, 559, 706–710. [Google Scholar] [CrossRef]
- Ma, L.W.; Cheng, H.S.; Chung, C.Y. Effect of thermo-mechanical treatment on superelastic behavior of Ti-19Nb-14Zr(at.%) shape memory alloy. Intermetallics 2013, 32, 44–50. [Google Scholar] [CrossRef]
- Brailovski, V.; Prokoshkin, S.; Gauthier, M.; Inaekyan, I.; Dubinskiy, S. Mechanical properties of porous metastable beta Ti–Nb–Zr alloys for biomedical applications. J. Alloys Compd. 2013, 577, S413–S417. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.; Gao, Y.; Yuan, B.; Zhu, M. Indirect determination of martensitic transformation temperature of sintered porous nickel-free Ti-22Nb-6Zr alloy by low temperature compressive test. Mater. Des. 2014, 60, 193–197. [Google Scholar] [CrossRef]
- Yuan, B.; Yang, B.; Gao, Y.; Lai, M.; Chen, X.H.; Zhu, M. Achieving ultra-high superelasticity and cyclic stability of biomedical Ti-11Nb-4O (at.%) alloys by controlling Nb and oxygen content. Mater. Des. 2016, 92, 978–982. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, M.; Kato, M.; Kamimura, T.; Fukumoto, M.; Harada, I.; Kubo, K. Proceeding of 6th World Conference on Titanium, Vol.2, Cannes; Lacombe, P., Tricot, R., Beranger, G., Eds.; Les Ulis Cedex: Cedex, France, 1988; pp. 1601–1606. [Google Scholar]
- Sakaguch, N.; Niimori, M.; Akahori, T. Tensile deformation behavior of Ti-Nb-Ta-Zr biomedical alloys. Mater. Trans. 2004, 45, 1113–1119. [Google Scholar] [CrossRef]
- Arciniegas, M.; Manero, J.M.; Pena, J.; Gil, F.J.; Planell, J.A. Study of new multifunctional shape memory and low elastic modulus Ni-free Ti alloys. Metal. Mater. Trans. A 2008, 39, 742–751. [Google Scholar] [CrossRef]
- Brailovski, V.; Prokoshkin, S.; Gauthier, M.; Inaekyan, K.; Dubinskiy, S.; Petrzhik, M.; Filonov, M. Bulk and porous metastable beta Ti–Nb–Zr(Ta) alloys for biomedical applications. Mater. Sci. Eng. C 2011, 31, 643–657. [Google Scholar] [CrossRef] [Green Version]
- Jun, C.O.; Yun, E.; Lee, S. Correlation of microstructure with the hardness and wear resistance of (TiC, SiC)/Ti-6Al-4V surface composites fabricated by high-energy electron-beam irradiation. Metall. Mater. Trans. A 2004, 35, 525–534. [Google Scholar]
- Long, M.; Qazi, J.I.; Rack, H.J. Titanium 2003 Science and Technology; Wiley VCH Verlag, GMBH and Co.KGaA: Weinhem, Germany, 2003; pp. 691–698. [Google Scholar]
- Peterson, M.B.; Calabrese, S.J.; Li, S.Z.; Jiang, X.X. In Proceedings of the 16th Leeds-Lyon Symposium on Tribology; Dowson, D., Taylor, C.M., Godet, M., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1990; pp. 15–26. [Google Scholar]
- Ehtemam-Haghighia, S.; Prashanth, K.G.; Attar, H.; Chaubey, A.K.; Cao, G.H.; Zhang, L.C. Evaluation of mechanical and wear properties of Ti-xNb-7Fe alloys designed for biomedical applications. Mater. Des. 2016, 111, 592–599. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Islamgaliev, R.; Alexandrov, I.V. Bulk nanostructured materials fromsevere plastic deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Nakai, M.; Niinomi, M.; Oneda, T. Improvement in fatigue strength of biomedical β-type Ti–Nb–Ta–Zr alloy while maintaining low Young’s modulus through optimizing x-phase precipitation. Metall. Mater. Trans. A 2011, 43, 294–302. [Google Scholar] [CrossRef]
- Breme, H.J.; Helsen, J.A. Selection of Materials. In Metals as Biomaterials; Breme, H.J., Helsen, J.A., Eds.; John Wiley & Sons: Chichester, UK, 1998; pp. 1–35. [Google Scholar]
- Biesiekierski, A.; Wang, J.; Gepreel, M.A.-H.; Wen, C.E. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Wang, T.J.; Doi, H.; Yoneyama, T.; Hamanaka, H. Mechanical properties and corrosion resistance of Ti–6Al–7Nb alloy dental castings. Mater. Sci. Mater. Med. 1998, 9, 567–574. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T.; Fukui, H.; Toda, H. Corrosion resistance and biocompatibility of Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. A 2005, 398, 28–36. [Google Scholar] [CrossRef]
- Khan, A.; Williams, R.L.; Williamss, D.L. The corrosion behaviour of Ti–6Al–4V, Ti–6Al–7Nb and Ti–13Nb–13Zr in protein solutions. Biomaterils 1999, 20, 631–637. [Google Scholar] [CrossRef]
- Matsuno, H.; Yokoyama, A.; Watari, F.; Uo, M.; Kawaski, T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterils 2001, 22, 1253–1262. [Google Scholar] [CrossRef]
- Arciniegas, M.; Pena, J.; Gil, F.J.; Manero, J.M. In vitro response of preosteoblastic MG63 cells on Ni-free Ti shape memory substrates. J. Biomed. Mater. Res. Part B 2013, 101, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.J. Characterization, corrosion behavior, cellular response and in vivo bone tissue compatibility of titanium–niobium alloy with low Young’s modulus. Mater. Sci. Eng. C 2016, 59, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Dragan-Raileanu, L.A.; Chelariu, R.; Mareci, D.; Munteanu, C. Electrochemical behavior of new experimental TiNbZrAl alloys for dental applications. Mater. Corros. 2014, 65, 828–836. [Google Scholar] [CrossRef]
- Taddei, E.B.; Henriques, V.A.R.; Silva, C.R.M.; Cairo, C.A.A. Production of new titanium alloy for orthopedic implants. Mater. Sci. Eng. C 2004, 24, 683–687. [Google Scholar] [CrossRef]
- Ma, L.W.; Chung, C.Y.; Tong, Y.X.; Zheng, Y.F. Properties of porous TiNbZr shape memory alloy fabricated by mechanical alloying and hot isostatic pressing. J. Mater. Eng. Perform. 2011, 20, 783–786. [Google Scholar] [CrossRef]
- Rao, X.; Chu, C.L.; Zheng, Y.Y. Phase composition, microstructure, and mechanical properties of porous Ti–Nb–Zr alloys prepared by a two-step foaming powder metallurgy method. J. Mech. Behav. Biomed. Mater. 2014, 34, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.K.; Hamzah, E.; Saud, S.N.; Nazim, E.M. Parameter optimization of microwave sintering porous Ti-23%Nb shape memory alloys for biomedical applications. Trans. Nonferrous Metals Soc. China 2018, 28, 700–710. [Google Scholar] [CrossRef]
- Xiong, J.Y.; Li, Y.C.; Wang, X.J.; Hodgson, P.; Wen, C.E. Mechanical properties and bioactive surface modification via alkali-heat treatment of a porous Ti–18Nb–4Sn alloy for biomedical applications. Acta Biomater. 2008, 4, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.C.; Liu, Y.J.; Li, S.J.; Hao, Y.L. Additive manufacturing of titanium alloys by electron beam melting: A review. Adv. Eng. Mater. 2018, 20, 1700842. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, H.L.; Li, S.J.; Wang, S.G.; Wang, W.J.; Hou, W.T.; Hao, Y.L.; Yang, R.; Zhang, L.C. Compressive and fatigue behavior of beta-type titanium porous structures fabricated by electron beam melting. Acta Mater. 2017, 126, 58–66. [Google Scholar] [CrossRef]
- Wen, C.E.; Yamada, Y.; Hodgson, P.D. Fabrication of novel TiZr alloy foams for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1439–1444. [Google Scholar] [CrossRef]
- Lai, M.; Gao, Y.; Yuan, B.; Zhu, M. Effect of pore structure regulation on the properties of porous TiNbZr shape memory alloys for biomedical application. J. Mater. Eng. Perform. 2015, 24, 136–142. [Google Scholar] [CrossRef]
- Ruan, J.M.; Yang, H.L.; Weng, X.J.; Miao, J.L.; Zhou, K.C. Preparation and characterization of biomedical highly porous Ti–Nb alloy. J. Mater. Sci. Mater. Med. 2016, 27, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Weng, X.J.; Wang, X.; Huang, J.Z.; Zhang, C.; Muhammad, H.; Ma, X.; Liao, Q.-D. Potential use of porous titanium–niobium alloy in orthopedic implants: Preparation and experimental study of its biocompatibility in vitro. PLoS ONE 2013, 8, 79289. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Zhang, E.; Li, M.; Zeng, S. Preparation, microstructure and mechanical properties of porous titanium sintered by Ti fibres. J. Mater. Sci. Mater. Med. 2008, 19, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J. The mechanical behaviour of cancellous bone. J. Biomech. 1985, 18, 317–321. [Google Scholar] [CrossRef]
- Hrabe, N.W.; Heinl, P.; Flinn, B.; Korner, C.; Bordia, R.K. Compression-compression fatigue of selective electron beam melted cellular titanium (Ti–6Al–4V). J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Leuders, S.; Thöne, M.; Riemer, A.; Niendorf, T.; Tröster, T.; Richard, H.A.; Maier, H.J. On the mechanical behaviour of titanium alloy TiAl6V4 manufactured by selective laser melting: Fatigue resistance and crack growth performance. Int. J. Fatigue 2013, 48, 300–307. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Nishiguchi, S.; Kato, H.; Neo, M.; Oka, M.; Kim, H.M.; Kokubo, T. Alkali- and heat-treated porous titanium for orthopedic implants. J. Biomed. Mater. Res. 2001, 54, 198–208. [Google Scholar] [CrossRef]
- Li, Y.X.; Cui, Z.D.; Yang, X.J. Corrosion behavior of porous Ti-24Nb-4Zr alloy in different simulated body fluids. Adv. Mater. Res. 2012, 399–401, 1577–1581. [Google Scholar] [CrossRef]
- Zheng, Y.F. Corrosion behaviour of Ti–Nb–Sn shape memory alloys in different simulated body solutions. Mater. Sci. Eng. A 2006, 438–440, 891–895. [Google Scholar] [CrossRef]
- Hsu, J.C.; Hsu, S.K.; Tsou, H.K.; Wu, S.C.; Lai, T.H.; Ho, W.F. Fabrication and characterization of porous Ti–7.5Mo alloys scaffolds for biomedical applications. J. Mater. Sci. Mater. Med. 2013, 24, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Bucholtz, R.W. Nonallograft osteoconductive bone graft substitutes. Clin. Orthopaed. Relat. Res. 2002, 395, 44–52. [Google Scholar] [CrossRef]
- Laptev, A.; Bram, M.; Buchkremer, H.P.; Stöver, D. Study of production route for titanium parts combining very high porosity and complex shape. Powder Metal. 2004, 47, 85–92. [Google Scholar] [CrossRef]
- Clemow, A.J.T.; Weinstein, A.M.; Klawitter, J.J.; Koeneman, J.; Anderson, J. Interface mechanics of porous titanium implants. J Biomed. Mater. Res. 2004, 15, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.; Toksvig-Larsen, S.; Aspenberg, P. Ingrowth of bone into pores in titanium chambers implanted in rabbits: Effect of pore cross-sectional shape in the presence of dynamic shear. J. Biomed. Mater. Res. 1993, 27, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Oppenheimer, S.M.; Stupp, S.I.; Dunand, D.C.; Brinson, L.C. Effects of pore morphology and bone ingrowth on mechanical properties of microporous titanium as an orthopaedic implant material. Mater. Trans. 2004, 45, 1124–1131. [Google Scholar] [CrossRef]
- Tuchinskiy, L.; Loutfy, R. Titanium Foams for Medical Applications. Materials & Processes for Medical Devices; ASM International: Anaheim, CA, USA, 2003; pp. 377–381. [Google Scholar]
- Tan, X.P.; Tan, Y.J.; Chow, C.S.L.; Tor, S.B.; Yeong, W.Y. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C 2017, 76, 1328–1343. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhatnagar, N. A survey of fabrication and application of metallic foams. J. Porous Mater. 2018, 25, 537–554. [Google Scholar] [CrossRef]
- Netti, P.A. Biomedical Foams for Tissue Engineering Applications; Woodhead Publishing Limited: Waltham, MA, USA, 2014; pp. 40–46. [Google Scholar]
- Brooks, C.R. Heat Treatment, Structure and Properties of Nonferrous Alloys; American Society for Metals: Metals Park, OH, USA, 1982; pp. 120–150. [Google Scholar]
- Kim, B.S.; Nikolovski, J.; Bonadio, J.; Mooney, D.J. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat. Biotechnol. 1999, 17, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Yung, Y.C.; Fischbach, C.; Kong, H.J.; Nakaoka, R.; Mooney, D.J. Mechanical strain regulates endothelial cell patterning in vitro. Tissue Eng. 2007, 13, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Sumanasinghe, R.D.; Bernacki, S.H.; Loboa, E.G. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: Effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2)mRNA expression. Tissue Eng. 2006, 12, 3459–3465. [Google Scholar] [CrossRef] [PubMed]



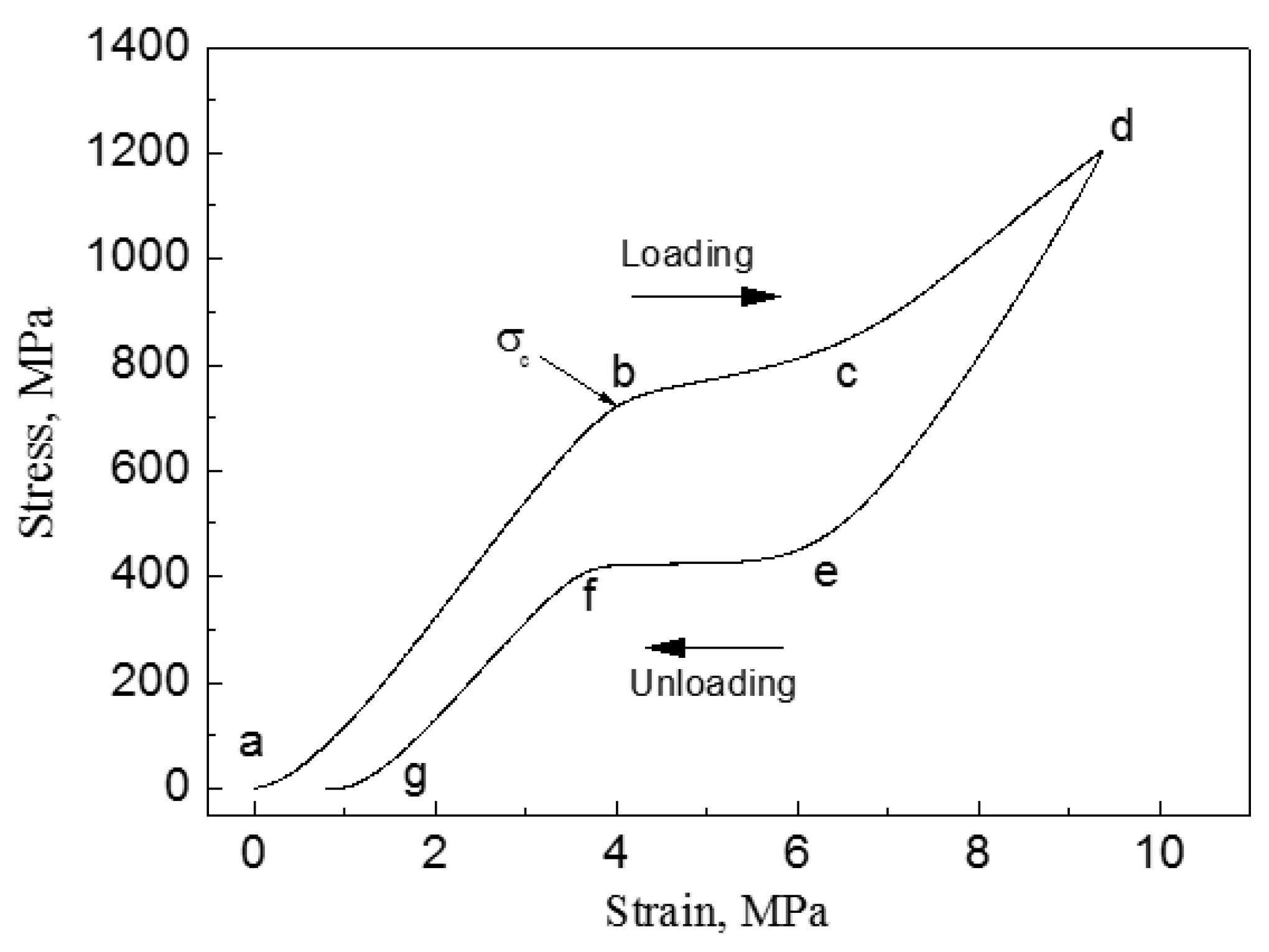

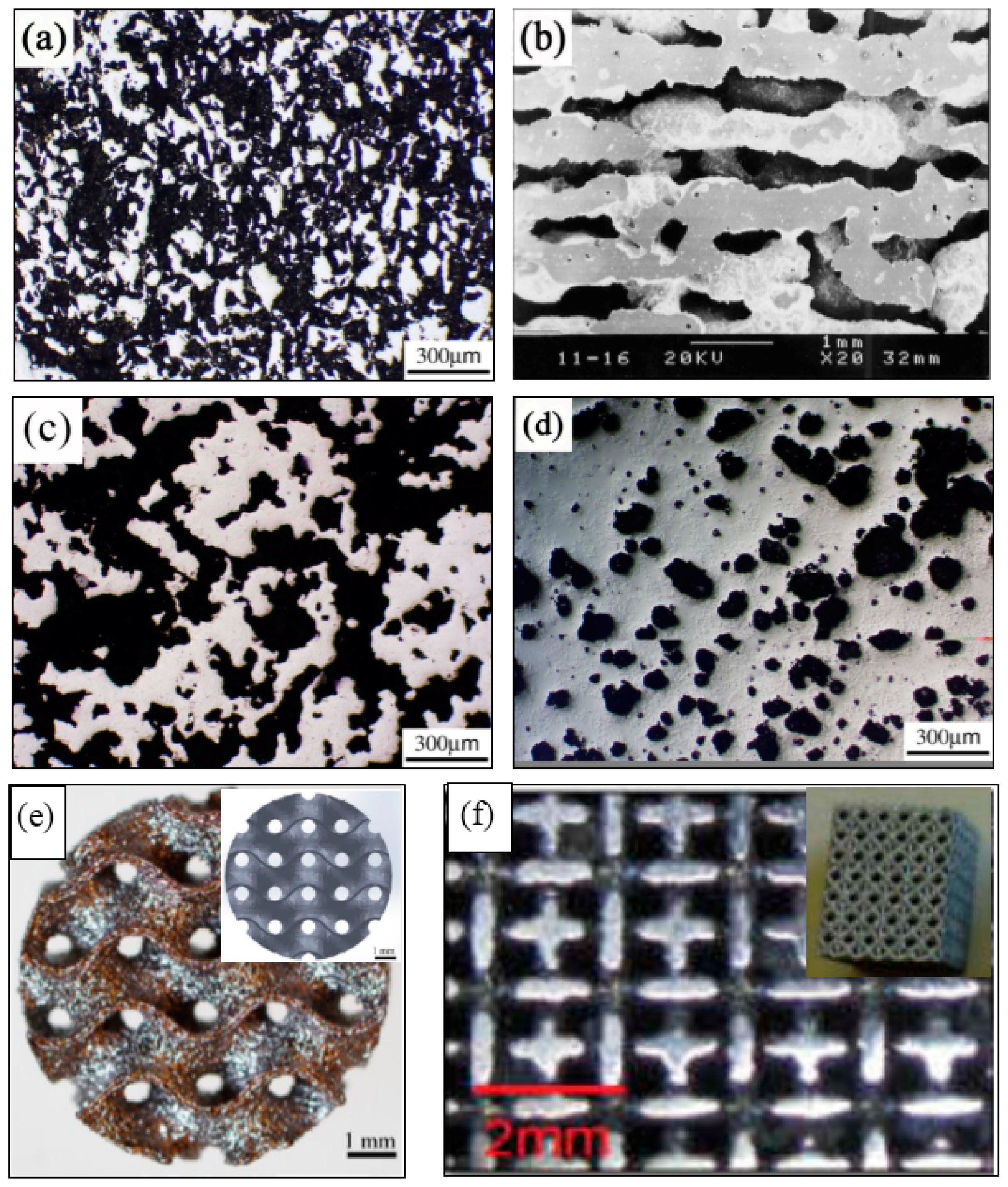






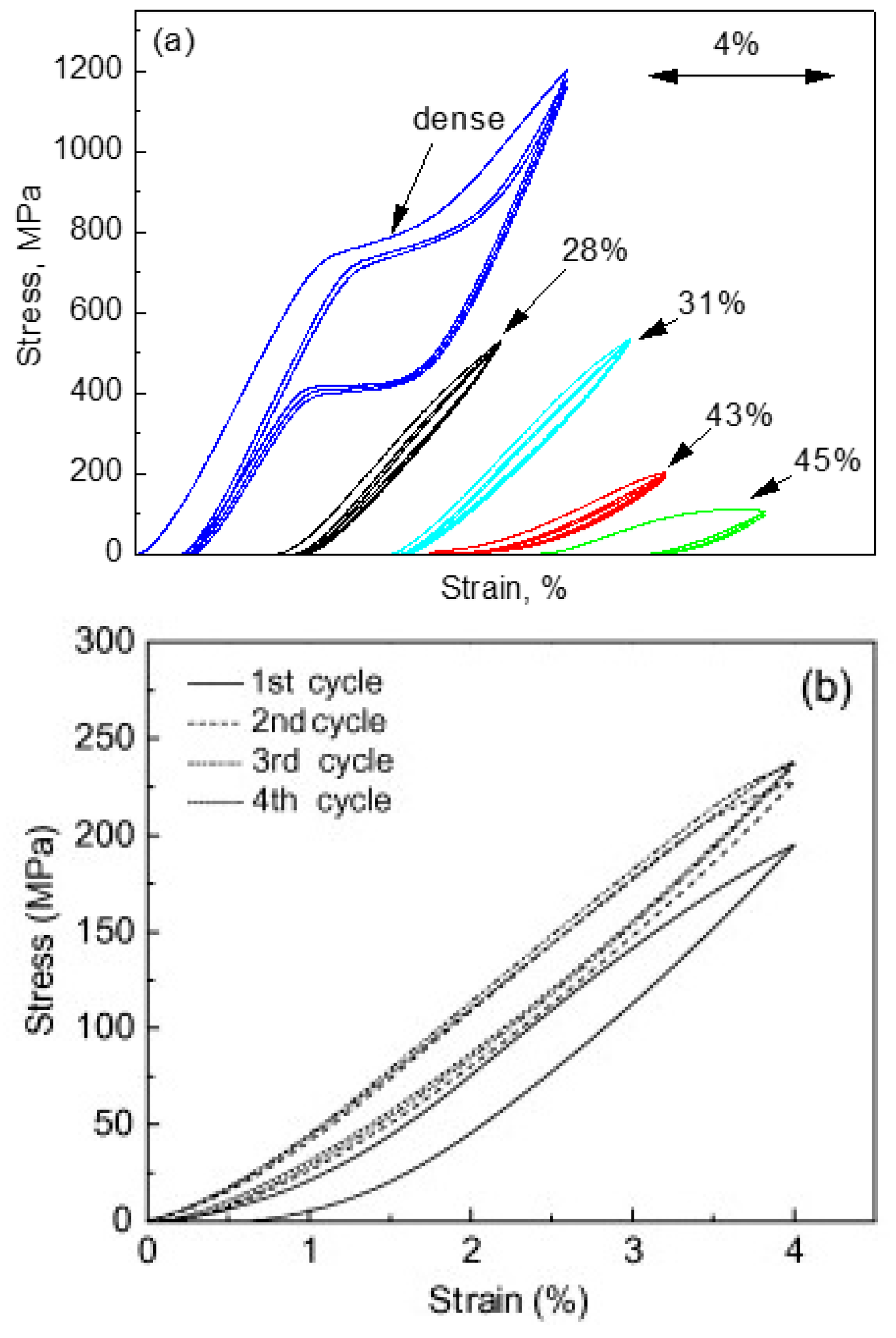

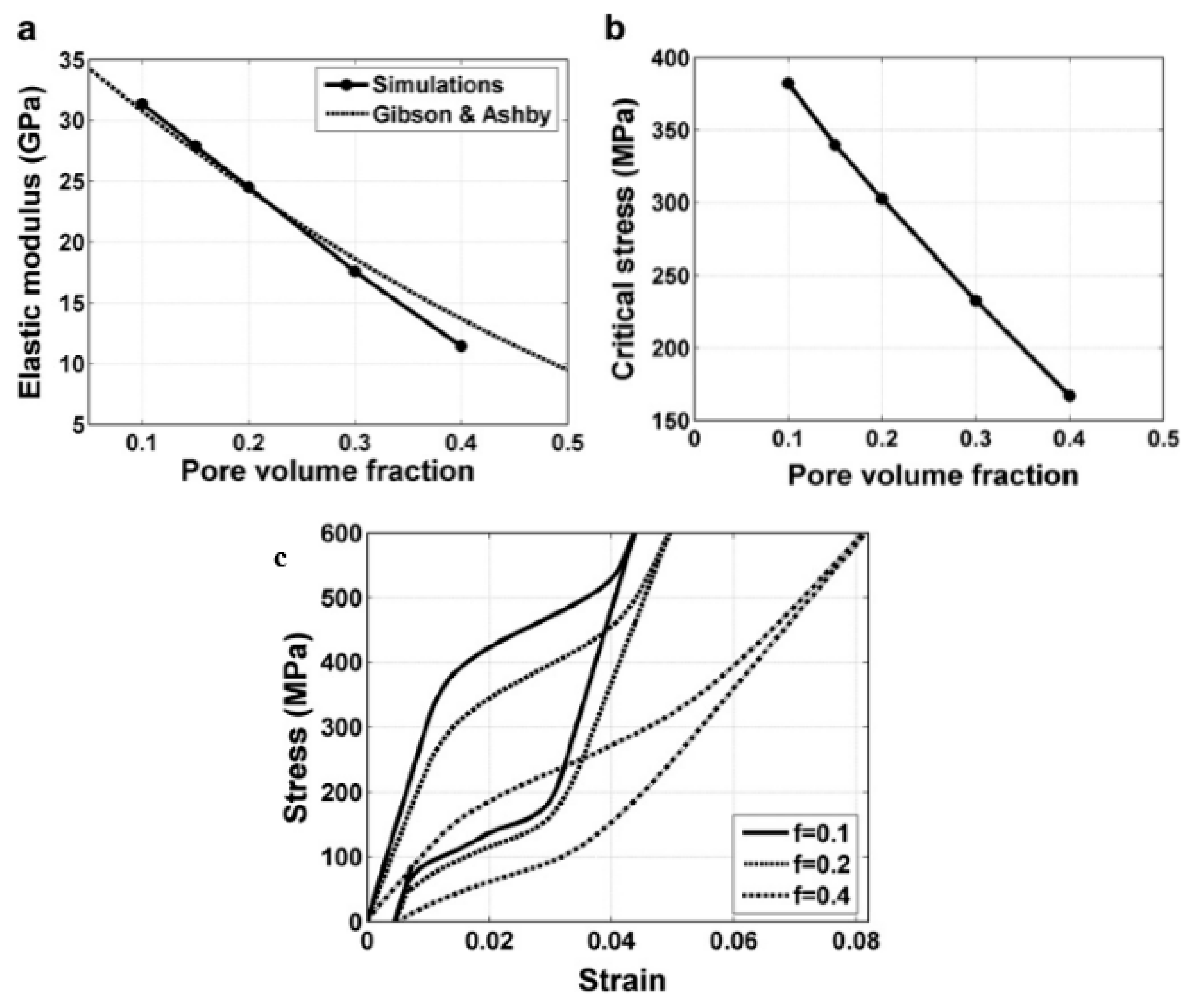


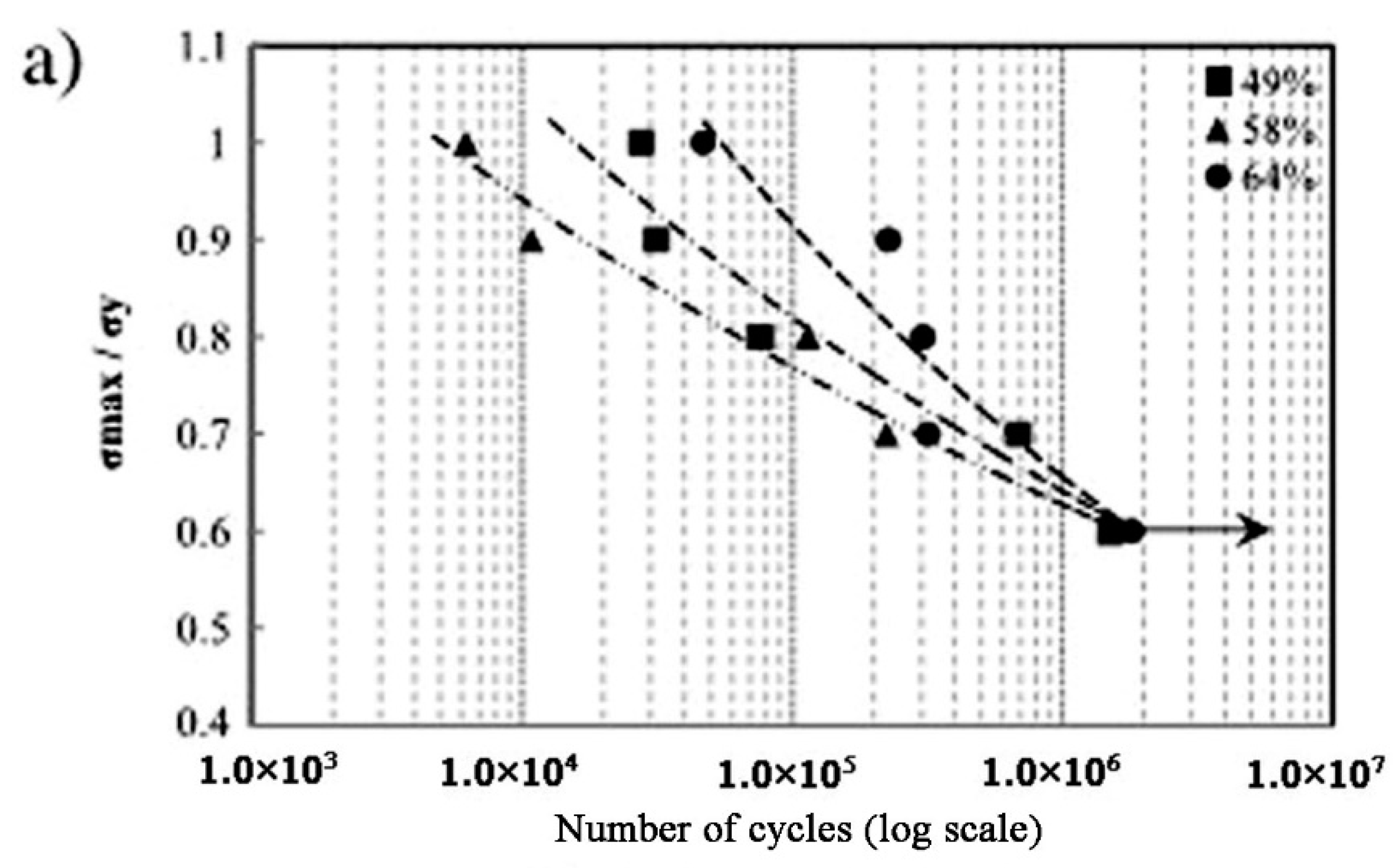
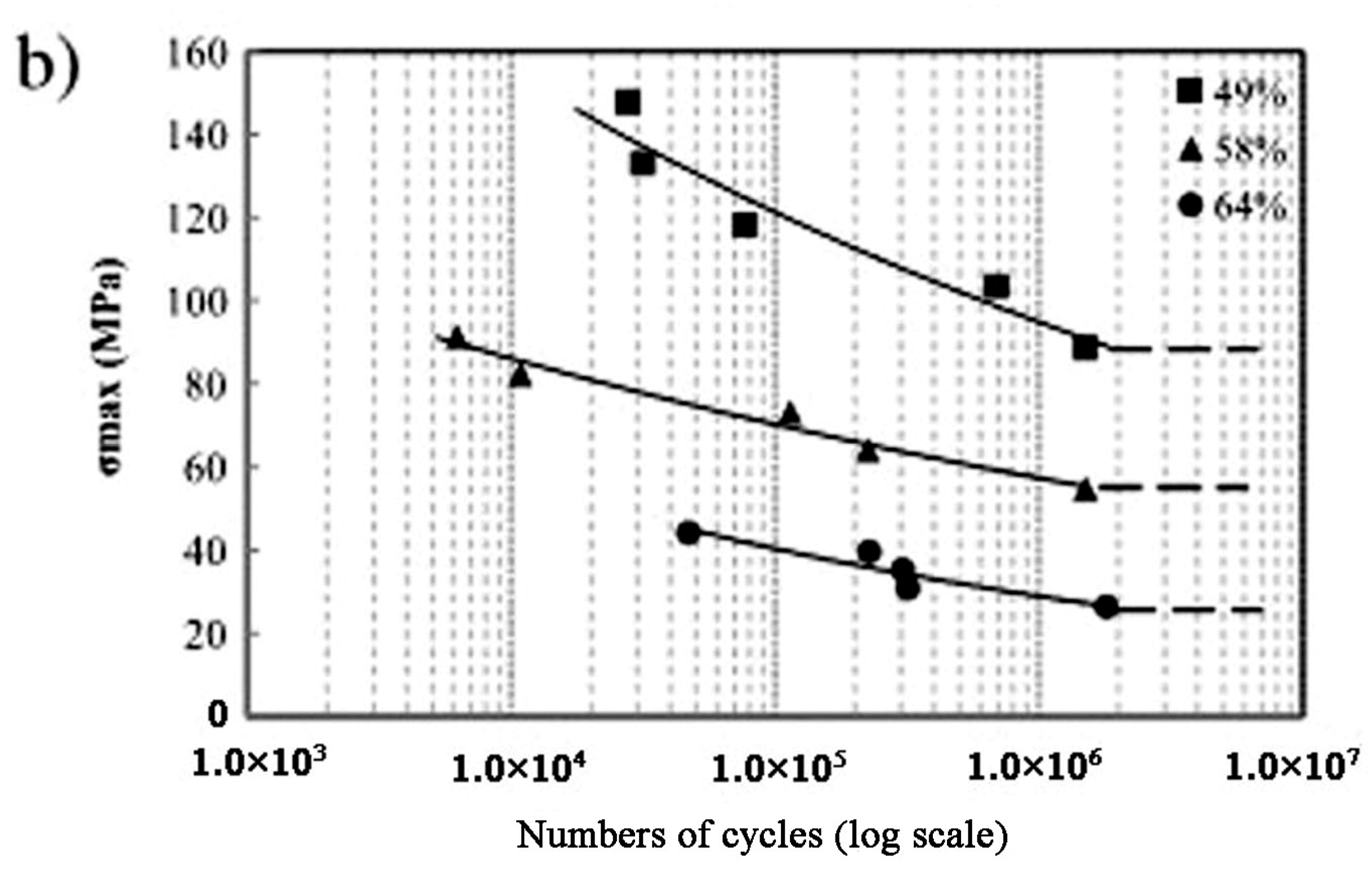


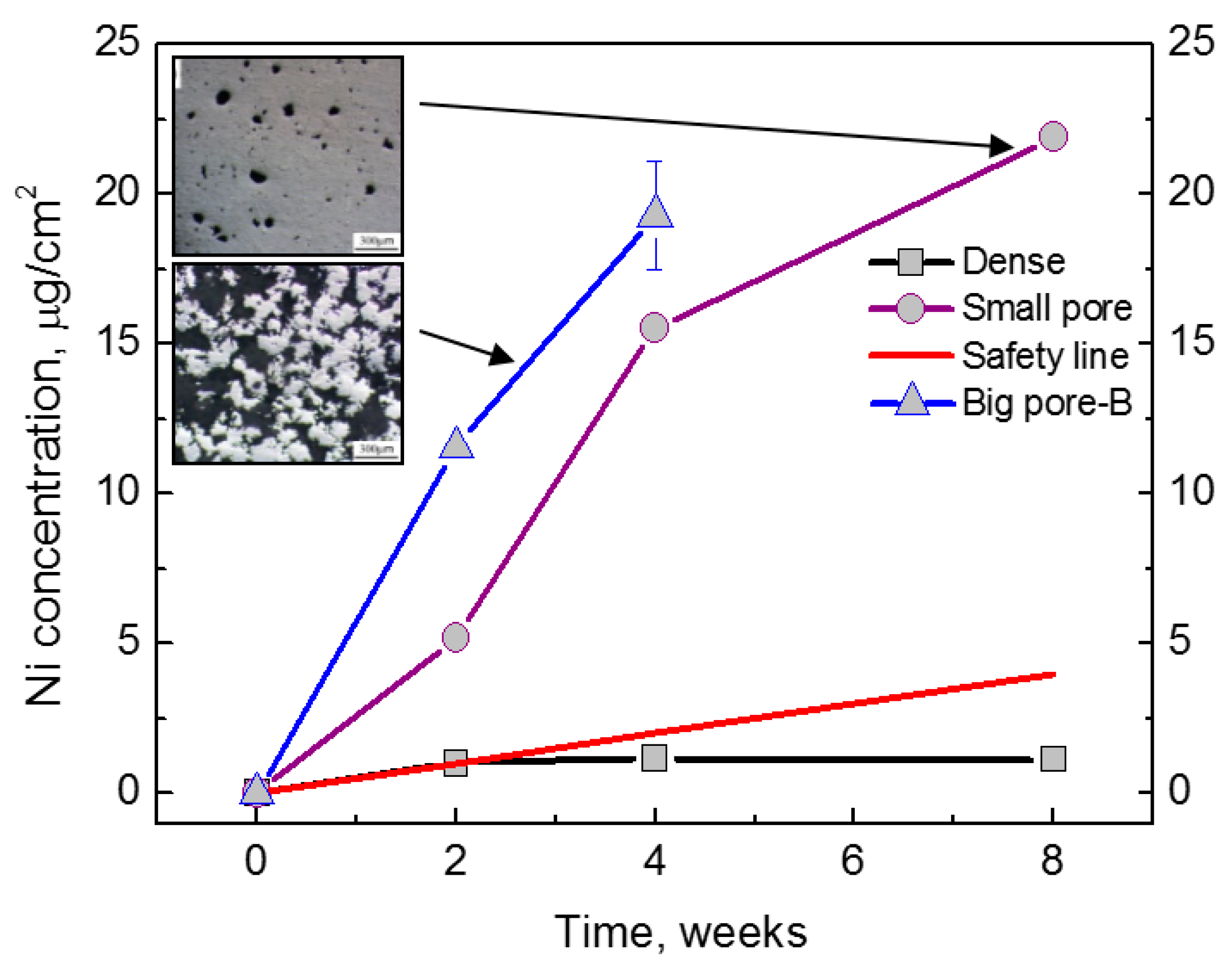
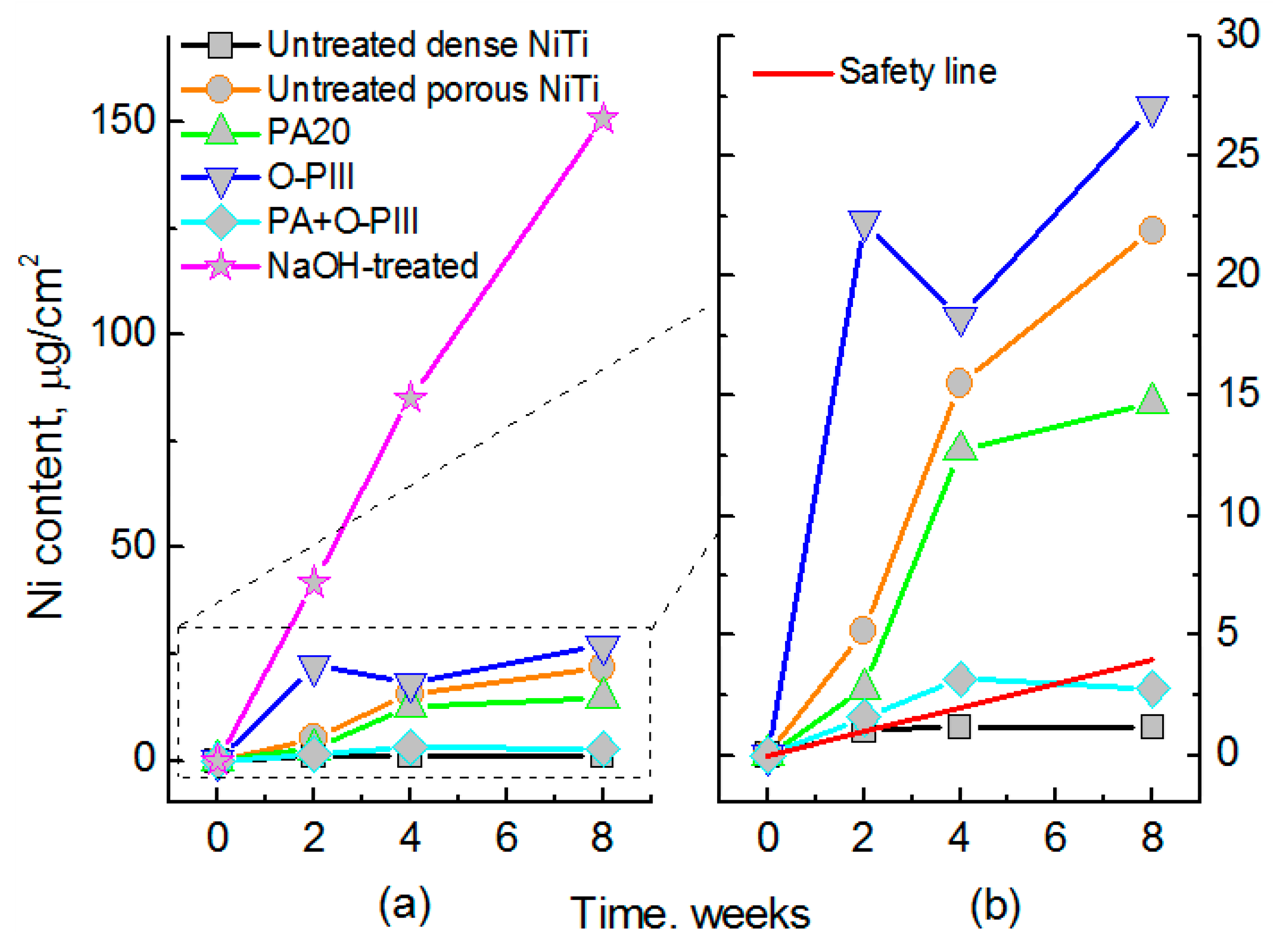


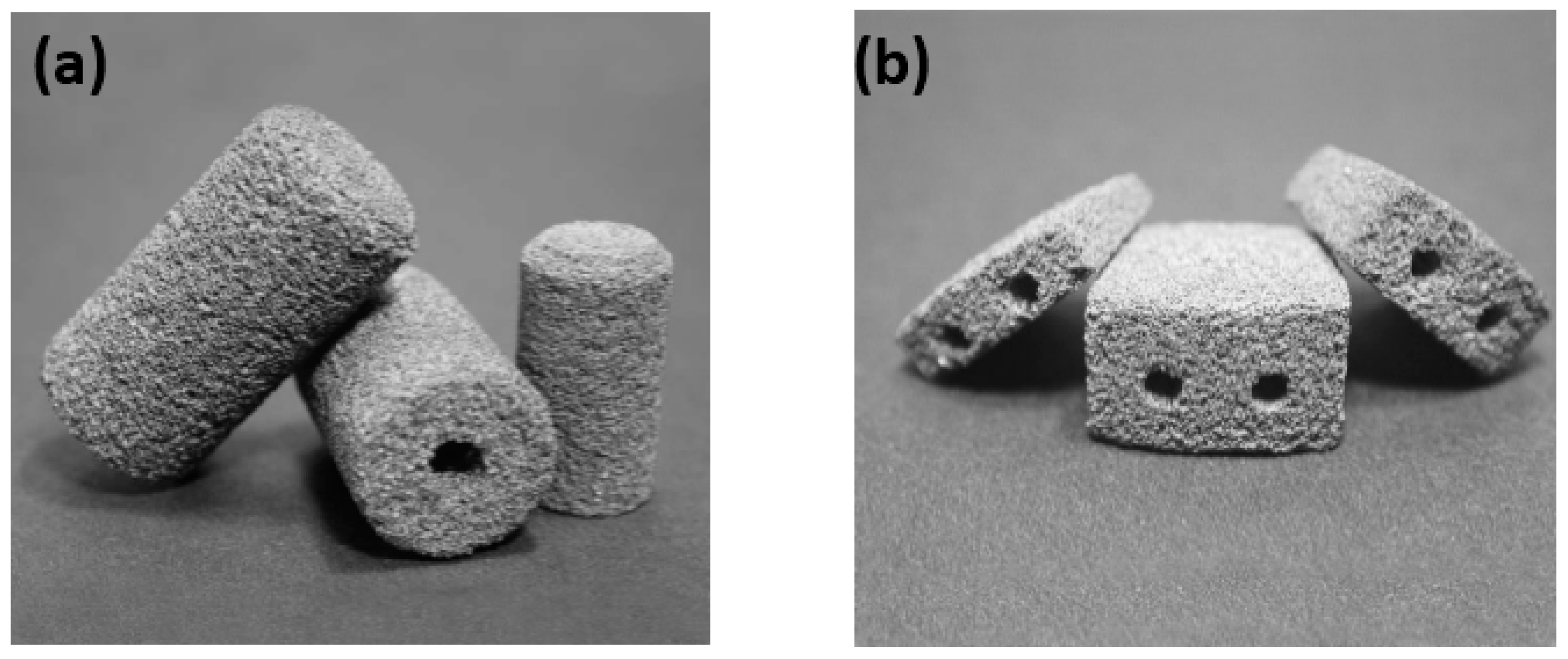
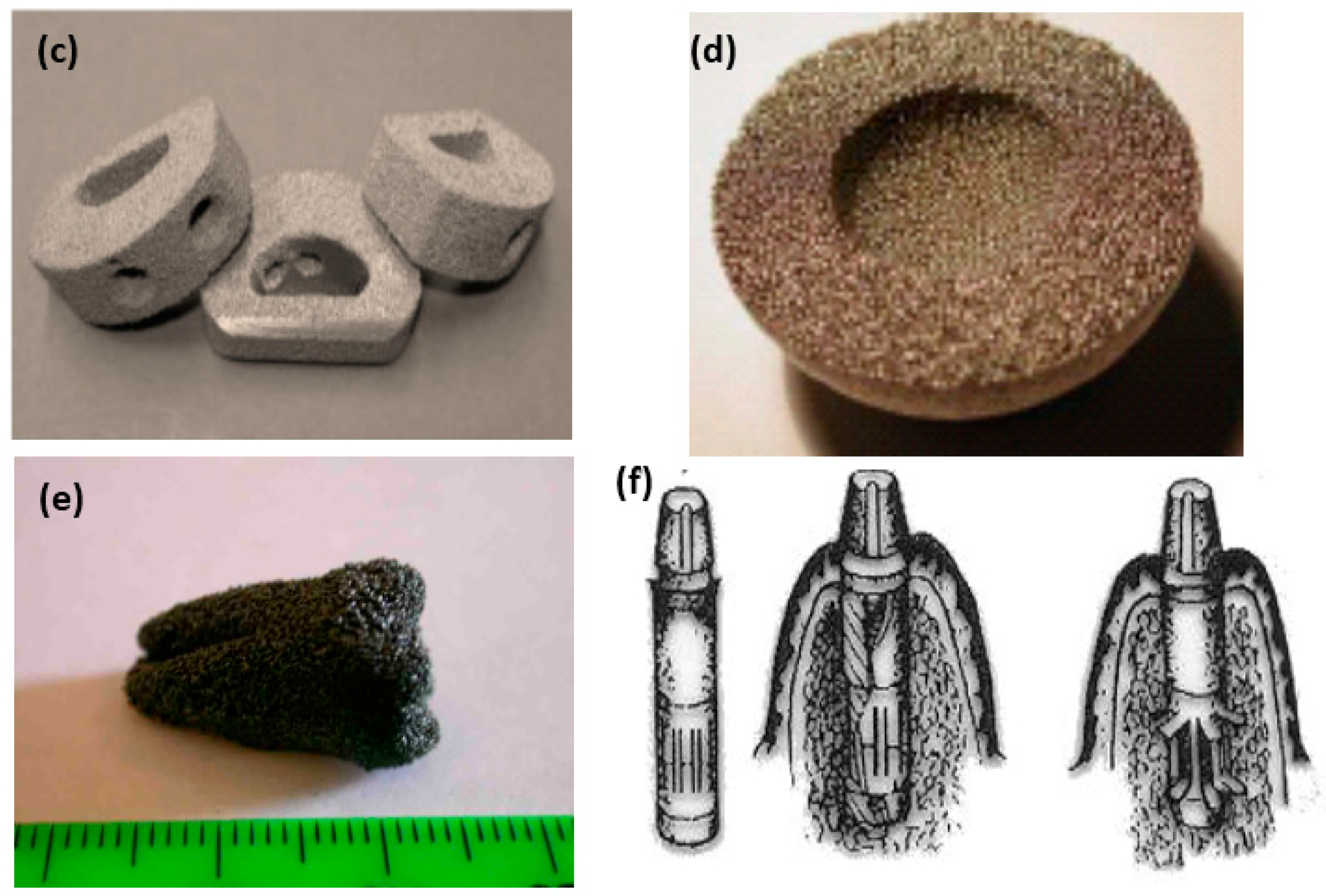



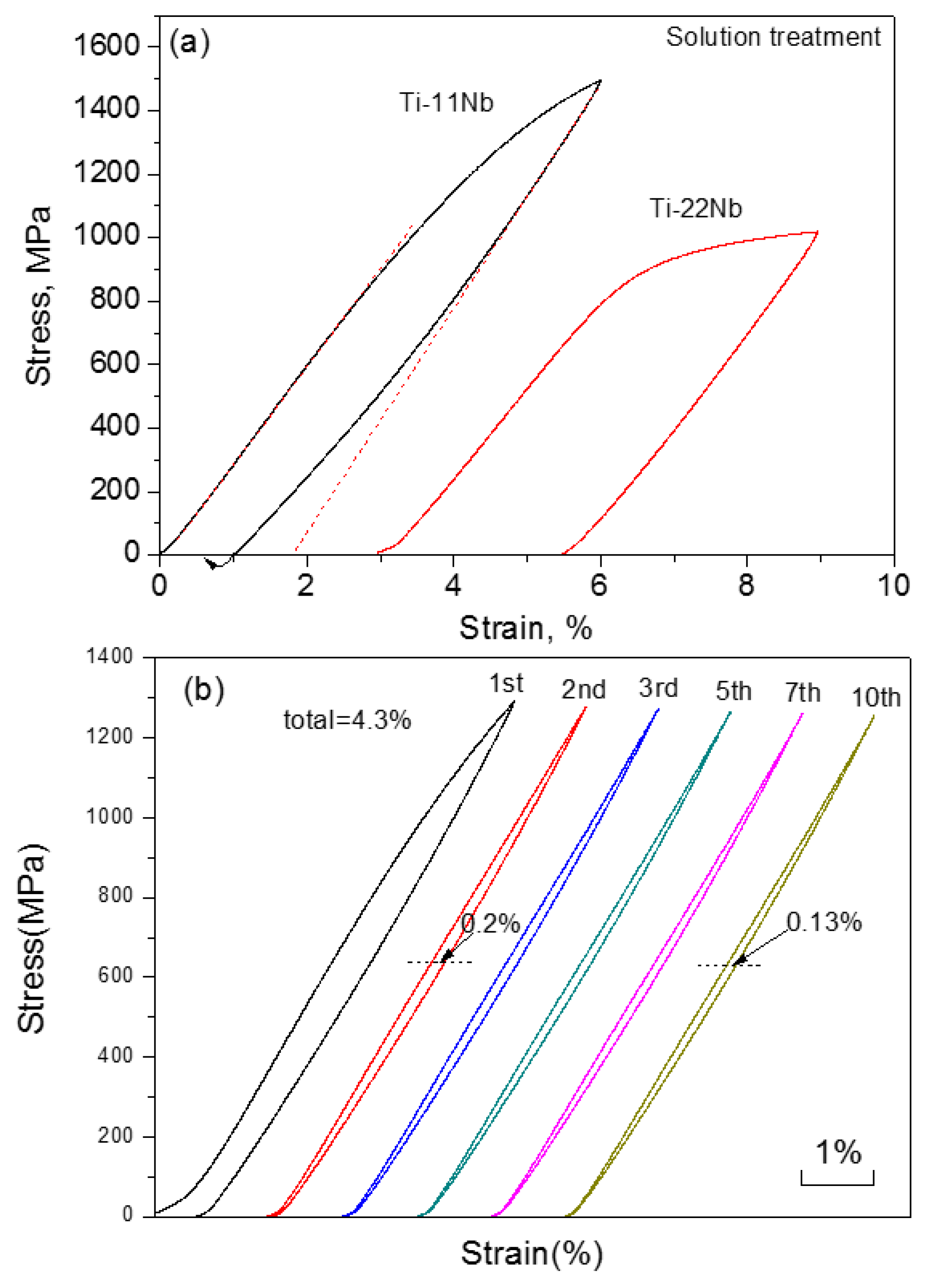




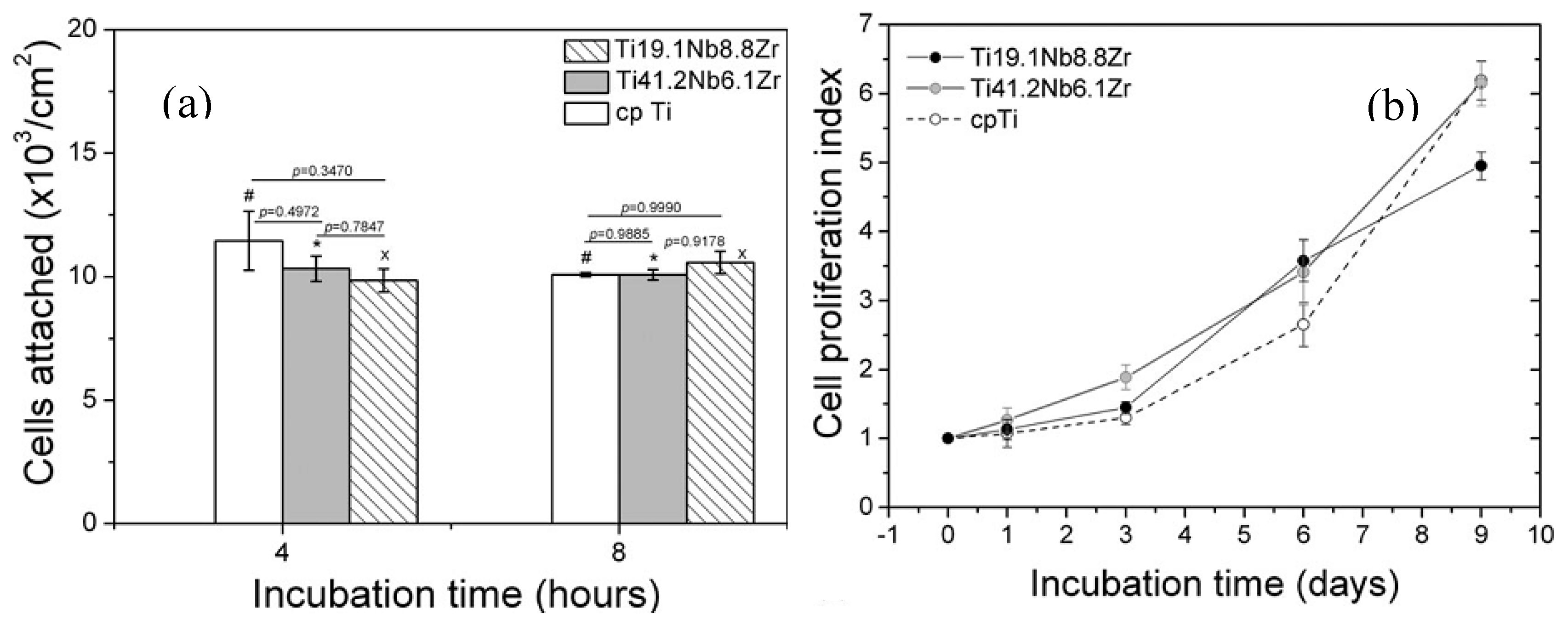







| Hard-Tissue | Human Bone | Human Teeth | |||
|---|---|---|---|---|---|
| Properties | Cortical Bone | Cancellous Bones | Enamel | Dentin | |
| density | 1.99 g/cm3 [28] | 0.05–1 g/cm3 [29] | 3 g/cm3 | 2–2.1 g/cm3 | |
| porosity | 5–30% [22,24,25,26,27] | 30–90% [22,29] | 7% | 59–70% | |
| Pore size | 10–500 μm [22,23,24,25,26,27] | 50–300 μm [24,25,26,27] | 1.7–2.5 μm [33] | ||
| Tension Strength | 79–151 MPa(longitudinal) [28] 51–56 MPa (transverse) | 34–61 MPa [34] | |||
| Compression strength | 131–224 MPa(longitudinal) 106–133 MPa(transverse) [28] | 2–5 MPa [31] | |||
| Elastic modulus | 17–20 GPa(longitudinal) [30,31] 6–13 GPa(transverse) | 0.76–4 GPa [31] | 3–25 GPa [35] | ||
| Recoverable strain | 2–2.5% [32] | ||||
| Fabrication Method | Porosity/% | Pore Size/μm | Pore Shape | Pore Connectivity | Pore Distribution |
|---|---|---|---|---|---|
| CS | 20–50% | 10–100 | irregular | good | homogeneous |
| HIP/CF-HIP | 10–80% | 100–3000 | rounded | bad | homogeneous or gradient |
| SHS | 30–70% | 300–500 | directional | very good | homogeneous |
| Space holder assisted sintering | 10–80% | 10–3000 | Depend on space holder | Depend on the space holder | homogeneous or gradient |
| AM | 0–90% | >150 | Depend on designing | very good | homogeneous or gradient |
| Melting point | 1750–1800 °C | Elastic Modulus | 35–120 GPa |
| Density | 4.8–5.5 g/cm3 | Poisson’s Ratio | 0.33 |
| Yield Strength | 250–790 MPa | Tension strength | 590–1074 MPa (rolling and aging) |
| Biocompatibility | Excellent, compared Ti | Elongation | 15–90% |
| Corrosion resistance | Excellent, better than Ti | Recoverable strain | Maximum 6% |
| Wear resistance | Good, better than Ti | Fatigue life | 3 × 104–1 × 107 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, B.; Zhu, M.; Chung, C.Y. Biomedical Porous Shape Memory Alloys for Hard-Tissue Replacement Materials. Materials 2018, 11, 1716. https://doi.org/10.3390/ma11091716
Yuan B, Zhu M, Chung CY. Biomedical Porous Shape Memory Alloys for Hard-Tissue Replacement Materials. Materials. 2018; 11(9):1716. https://doi.org/10.3390/ma11091716
Chicago/Turabian StyleYuan, Bin, Min Zhu, and Chi Yuen Chung. 2018. "Biomedical Porous Shape Memory Alloys for Hard-Tissue Replacement Materials" Materials 11, no. 9: 1716. https://doi.org/10.3390/ma11091716




