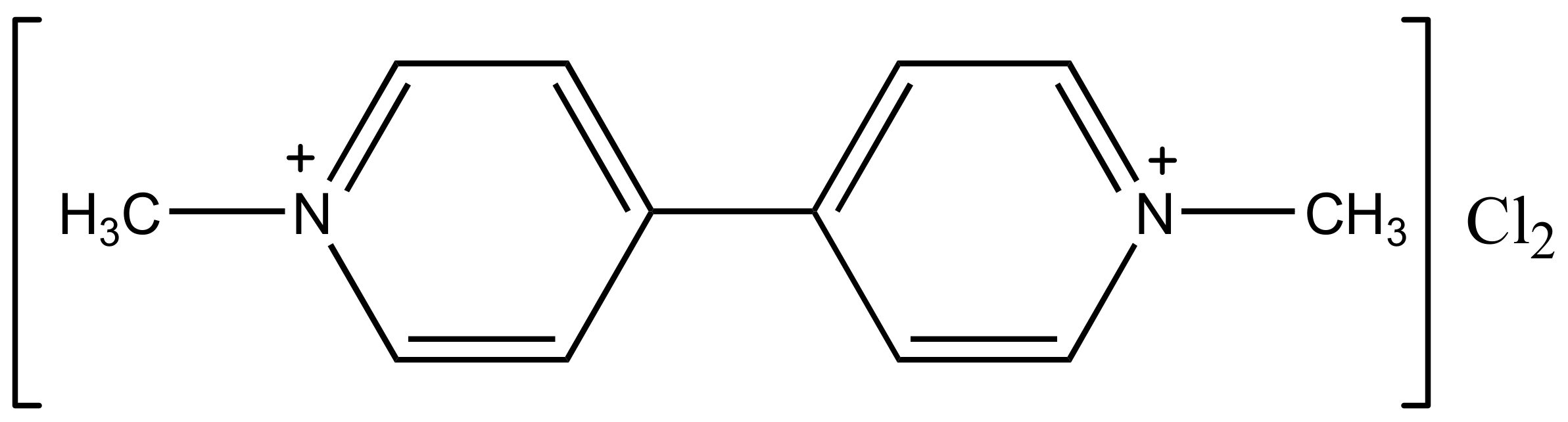
Photoelectrocatalytic Degradation of Paraquat by Pt Loaded TiO2 Nanotubes on Ti Anodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Ti Plates
2.2. Preparation of Thermally Oxidized Photoanode (Ti/TiO2)
2.3. Preparation of Nanotube Structured TiO2 Photoanodes by Anodic Oxidation in Hydrogen Fluoride Solution (Ti/TiO2NTHF-X)
2.4. Preparation of Nanotube Structured TiO2 Photoanodes by Anodic Oxidation in Ethylene Glycol Solution (Ti/TiO2NTEG-X)
2.5. Pt Loading on Ti/TiO2NTEG-3h-500 Photoanodes
2.6. Photoanode Characterization
2.7. Photo-, Electro- and Photoelectro-Reactivity Set up and Procedure
2.8. Analytical Techniques
3. Results and Discussion
3.1. Characterization Results
3.2. Photoelectrocatalytic Activity Results
4. Conclusions
Supplementary Materials
 ), Ti/TiO2NTHF-1h-500 (
), Ti/TiO2NTHF-1h-500 (  ), Ti/TiO2NTEG-1h-500 (
), Ti/TiO2NTEG-1h-500 (  ), Ti/TiO2NTEG-3h-500 (
), Ti/TiO2NTEG-3h-500 (  ) and Ti/TiO2NTEG-6h-500 (
) and Ti/TiO2NTEG-6h-500 (  ) for paraquat degradation. Potential: 1V, Figure S15. UV-Vis absorbance values of the samples taken from the reaction medium at fixed times during PEC degradation of paraquat (37.4 μM) at 1 V by using the Ti/TiO2NTEG-3h-500 photoanode.
) for paraquat degradation. Potential: 1V, Figure S15. UV-Vis absorbance values of the samples taken from the reaction medium at fixed times during PEC degradation of paraquat (37.4 μM) at 1 V by using the Ti/TiO2NTEG-3h-500 photoanode.Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marien, C.B.D.; Cottineau, T.; Robert, D.; Drogui, P. TiO2 Nanotube arrays: Influence of tube length on the photocatalytic degradation of Paraquat. Appl. Catal. B Environ. 2016, 194, 1–6. [Google Scholar] [CrossRef]
- Kowal, S.; Balsaa, P.; Werres, F.; Schmidt, T.C. Determination of the polar pesticide degradation product N, N-dimethylsulfamide in aqueous matrices by UPLC–MS/MS. Anal. Bioanal. Chem. 2009, 395, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, F.; Behpour, M.; Ghoreishi, S.M.; Khalilian, H. Photocatalytic degradation of paraquat herbicide in the presence TiO2 nanostructure thin films under visible and sun light irradiation using continuous flow photoreactor. Sol. Energy 2015, 120, 287–295. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Photoelectrocatalytic technologies for environmental applications. J. Photochem. Photobiol. A Chem. 2012, 238, 41–52. [Google Scholar] [CrossRef]
- Suhadolnik, L.; Pohar, A.; Likozar, B.; Ceh, M. Mechanism and kinetics of phenol photocatalytic, electrocatalytic and photoelectrocatalytic degradation in a TiO2-nanotube fixed-bed microreactor. Chem. Eng. J. 2016, 303, 292–301. [Google Scholar] [CrossRef]
- Serpone, N.; Pelizzetti, E. Photocatalysis: Fundamentals and Applications; Wiley: New York, NY, USA, 1989; ISBN 9780471967545-20160527. [Google Scholar]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Augugliaro, V.; Camera-Roda, G.; Loddo, V.; Palmisano, G.; Palmisano, L.; Soria, J.; Yurdakal, S. Heterogeneous photocatalysis and photoelectrocatalysis: From unselective abatement of noxious species to selective production of high-value chemicals. J. Phys. Chem. Lett. 2015, 6, 1968–1981. [Google Scholar] [CrossRef] [PubMed]
- Özcan, L.; Yurdakal, S.; Augugliaro, V.; Loddo, V.; Palmas, S.; Palmisano, G.; Palmisano, L. Photoelectrocatalytic selective oxidation of 4-methoxybenzyl alcohol in water by TiO2 supported on titanium anodes. Appl. Catal. B Environ. 2013, 132, 535–542. [Google Scholar] [CrossRef]
- Rajeshwar, K. Photoelectrochemistry and the environment. J. Appl. Electrochem. 1995, 25, 1067–1082. [Google Scholar] [CrossRef]
- Palmisano, G.; Loddo, V.; El Nazer, H.H.; Yurdakal, S.; Augugliaro, V.; Ciriminna, R.; Pagliaro, M. Graphite-supported TiO2 for 4-nitrophenol degradation in a photoelectrocatalytic reactor. Chem. Eng. J. 2009, 155, 339–346. [Google Scholar] [CrossRef]
- Liu, D.; Yang, T.; Chen, J.; Chou, K.C.; Hou, X. Pt-Co alloys-loaded cubic SiC electrode with improved photoelectrocatalysis property. Materials 2017, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Ensaldo-Rentería, M.K.; Ramírez-Robledo, G.; Sandoval-González, A.; Pineda-Arellano, C.A.; Álvarez-Gallegos, A.A.; Zamudio-Lara, Á.; Silva-Martínez, S. Photoelectrocatalytic oxidation of acid green 50 dye in aqueous solution using Ti/TiO2-NT electrode. J. Environ. Chem. Eng. 2018, 6, 1182–1188. [Google Scholar] [CrossRef]
- Chai, S.; Zhao, G.; Li, P.; Lei, Y.; Zhang, Y.N.; Li, D. Novel sieve-like SnO2/TiO2 nanotubes with integrated photoelectrocatalysis: Fabrication and application for efficient toxicity elimination of nitrophenol wastewater. J. Phys. Chem. C 2011, 115, 18261–18269. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, M.; Zhou, J.; Li, W.; Wang, H.; Xu, Z.; Lu, L.; Pei, L.; Shi, Z.; Yan, S.; et al. Surface states as electron transfer pathway enhanced charge separation in TiO2 nanotube water splitting photoanodes. Appl. Catal. B Environ. 2018, 234, 100–108. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; Misra, M.; Mahajan, V.K.; Raja, K.S. A novel method for the synthesis of titania nanotubes using sonoelectrochemical method and its application for photoelectrochemical splitting of water. J. Catal. 2007, 246, 362–369. [Google Scholar] [CrossRef]
- Bettoni, M.; Rol, C.; Sebastiani, G.V. Photoelectrochemistry on TiO2/Ti anodes as a tool to increase the knowledge about some photo-oxidation mechanisms in CH3CN. J. Phys. Org. Chem. 2008, 21, 219–224. [Google Scholar] [CrossRef]
- Özcan, L.; Yalçın, P.; Alagöz, O.; Yurdakal, S. Selective photoelectrocatalytic oxidation of 5-(hydroxymethyl)-2-furaldehyde in water by using Pt loaded nanotube structure of TiO2 on Ti photoanodes. Catal. Today 2017, 281, 205–213. [Google Scholar] [CrossRef]
- Jia, Y.; Ye, L.; Kang, X.; You, H.; Wang, S.; Yao, J. Photoelectrocatalytic reduction of perchlorate in aqueous solutions over Ag doped TiO2 nanotube arrays. J. Photochem. Photobiol. A Chem. 2016, 328, 225–232. [Google Scholar] [CrossRef]
- Assefpour-Dezfuly, M.; Vlachos, C.; Andrews, E.H. Oxide morphology and adhesive bonding on titanium surfaces. J. Mater. Sci. 1984, 19, 3626–3639. [Google Scholar] [CrossRef]
- Zwilling, V.; Darque-Ceretti, E.; Boutry-Forveille, A.; David, D.; Perrin, M.Y.; Aucouturier, M. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf. Interface Anal. 1999, 27, 629–637. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.C.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Macak, J.M.; Sirotna, K.; Schmuki, P. Self-organized porous titanium oxide prepared in Na2SO4/NaF electrolytes. Electrochim. Acta 2005, 50, 3679–3684. [Google Scholar] [CrossRef]
- Yasuda, K.; Schmuki, P. Control of morphology and composition of self-organized zirconium titanate nanotubes formed in (NH4)2SO4/NH4F electrolytes. Electrochim. Acta 2007, 52, 4053–4061. [Google Scholar] [CrossRef]
- Paulose, M.; Prakasam, H.E.; Varghese, O.K.; Peng, L.; Popat, K.C.; Mor, G.K.; Desai, T.A.; Grimes, C.A. TiO2 nanotube arrays of 1000 μm length by anodization of titanium foil: Phenol red diffusion. J. Phys. Chem. C 2007, 111, 14992–14997. [Google Scholar] [CrossRef]
- Yoriya, S.; Grimes, C.A. Self-assembled TiO2 nanotube arrays by anodization of titanium in diethylene glycol: Approach to extended pore widening. Langmuir 2010, 26, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.N.; Altomare, M.; Yoo, J.; Schmuki, P. Efficient photocatalytic H2 evolution: Controlled dewetting-dealloying to fabricate site-selective high-activity nanoporous Au particles on highly ordered TiO2 nanotube arrays. Adv. Mater. 2015, 27, 3208–3215. [Google Scholar] [CrossRef]
- Xie, K.P.; Sun, L.; Wang, C.L.; Lai, Y.K.; Wang, M.Y.; Chen, H.B.; Lin, C.J. Photoelectrocatalytic properties of Ag nanoparticles loaded TiO2 nanotube arrays prepared by pulse current deposition. Electrochim. Acta 2010, 55, 7211–7218. [Google Scholar] [CrossRef]
- Qin, Y.H.; Yang, H.H.; Lv, R.L.; Wang, W.G.; Wang, C.W. TiO2 nanotube arrays supported Pd nanoparticles for ethanol electrooxidation in alkaline media. Electrochim. Acta 2013, 106, 372–377. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, N.Q.; Lin, S.W. Influence of Pt deposition on water-splitting hydrogen generation by highly-ordered TiO2 nanotube arrays. Int. J. Hydrogen Energy 2014, 39, 13474–13480. [Google Scholar] [CrossRef]
- Ge, M.Z.; Cao, C.Y.; Huang, J.Y.; Li, S.H.; Zhang, S.N.; Deng, S.; Li, O.S.; Zhang, K.Q.; Lai, Y.K. Synthesis, modification, and photo/photoelectro catalytic degradation applications of TiO2 nanotube arrays: A review. Nanotechnol. Rev. 2016, 5, 75–112. [Google Scholar] [CrossRef]
- Moctezuma, E.; Leyva, E.; Monreal, E.; Villegas, N.; Infante, D. Photocatalytic degradation of the herbicide “paraquat”. Chemosphere 1999, 39, 511–517. [Google Scholar] [CrossRef]
- Kanchanatip, E.; Grisdanurak, N.; Thongruang, R.; Neramittagapong, A. Degradation of paraquat under visible light over fullerene modified V-TiO2. React. Kinet. Mech. Cat. 2011, 103, 227–237. [Google Scholar] [CrossRef]
- Shankar, K.; Mor, G.K.; Prakasam, H.E.; Yoriya, S.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Highly-ordered TiO2 nanotube arrays up to 220 µm in length: Use in water photoelectrolysis and dye-sensitized solar cells. Nanotechnology 2007, 18, 065707. [Google Scholar] [CrossRef]
- Yurdakal, S.; Tek, B.S.; Alagöz, O.; Augugliaro, V.; Loddo, V.; Palmisano, G.; Palmisano, L. Photocatalytic selective oxidation of 5-(hydroxymethyl)-2-furaldehyde to 2,5-furandicarbaldehyde in water by using anatase, rutile, and brookite TiO2 nanoparticles. ACS Sustain. Chem. Eng. 2013, 1, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Schiavello, M. Heterogeneous Photocatalysis; Wiley: Chichester, UK, 1997; ISBN 0-471-96754-8. [Google Scholar]
- Loddo, V.; Yurdakal, S.; Palmisano, G.; Imoberdorf, G.E.; Irazoqui, H.A.; Alfano, O.M.; Augugliaro, V.; Berber, H.; Palmisano, L. Selective photocatalytic oxidation of 4-methoxybenzyl alcohol to p-anisaldehyde in organic-free water in a continuous annular fixed bed reactor. Int. J. Chem. React. Eng. 2007, 5, A57. [Google Scholar] [CrossRef]






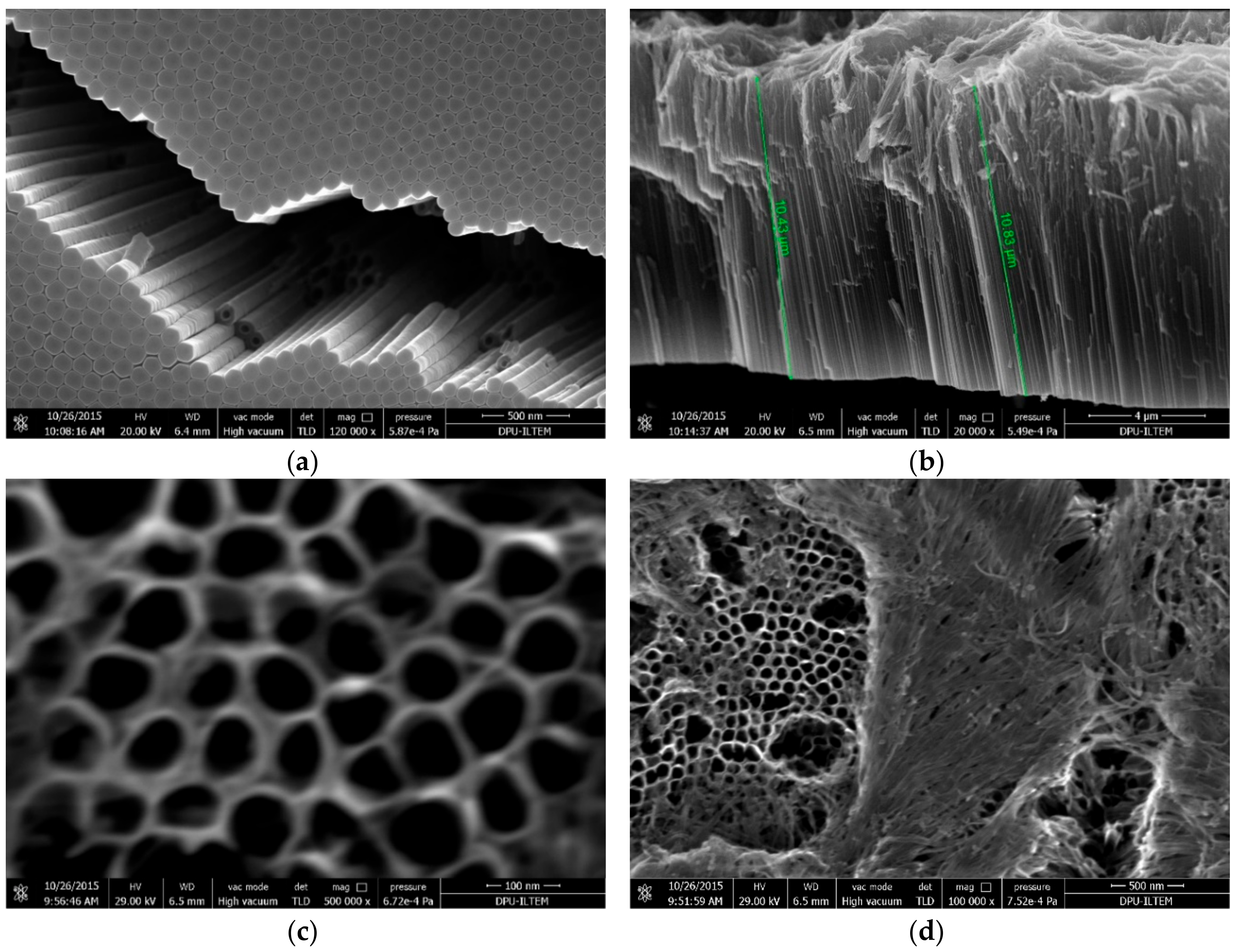

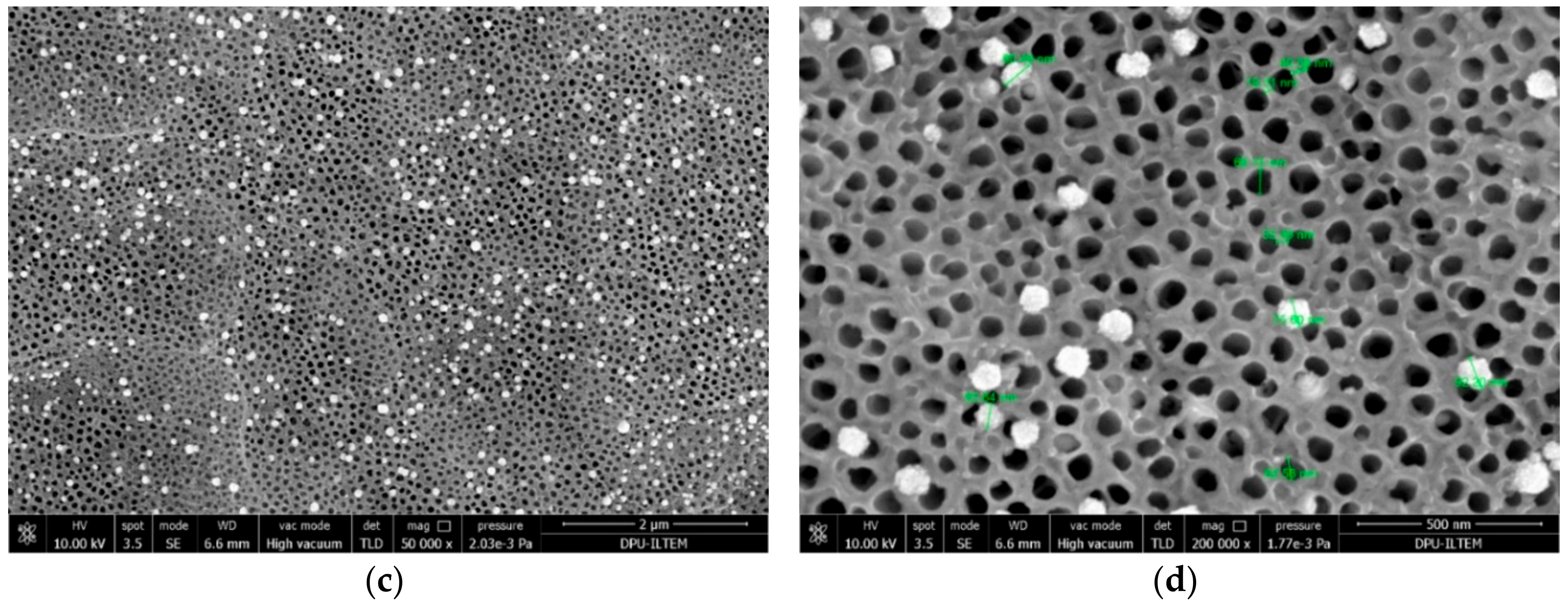




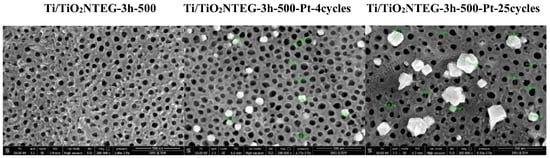
| Electrode | Crystal Phase | Peak Area of Anatase (101) | Primary Particle Size (nm) of Anatase | Primary Particle Size (nm) of Rutile | Primary Particle Size (nm) of Pt |
|---|---|---|---|---|---|
| Ti/TiO2NTEG-3h-450 | A | 423 | 37 | ||
| Ti/TiO2NTEG-3h-500-Pt-25cycles | A + R | 450 | 33 | 28 | |
| Ti/TiO2NTEG-3h-550 | A + R | 477 | 35 | 43 | |
| Ti/TiO2NTEG-1h-500 | A + R | 194 | 35 | 22 | |
| Ti/TiO2NTEG-6h-500 | A + R | 558 | 38 | 37 | |
| Ti/TiO2NTHF-6h-500 | A + R | 48 | 32 | 23 |
| Electrode | Wall Thickness (nm) | Internal Diameter (nm) | Tube Length (μm) | Pt Nanoparticle Diameter (nm) |
|---|---|---|---|---|
| Ti/TiO2NTEG-1h-500 | 35 | 43 | 1.7 | |
| Ti/TiO2NTEG-2h-500 | 30 | 47 | ||
| Ti/TiO2NTEG-3h-500 | 35 | 60 | ||
| Ti/TiO2NTEG-4h-500 | 35 | 47 | 9.8 | |
| Ti/TiO2NTEG-6h-500 | 20 | 80 | 11 | |
| Ti/TiO2NTHF-6h-500 | 14 | 90 | ||
| Ti/TiO2NTHF-6h-650 | 30 | 75 | 1.0 | |
| Ti/TiO2NTEG-3h-500-Pt-4cycles | 35 | 60 | 80 | |
| Ti/TiO2NTEG-3h-500-Pt-25cycles | 40 | 60 | 150 |
| Photoanode | Calcination Temperature (°C) | Conversion for 3 h (%) |
|---|---|---|
| Ti/TiO2-500 | 500 | 17 |
| Ti/TiO2NTHF-1h-400 | 400 | 28 |
| Ti/TiO2NTHF-1h-500 | 500 | 30 |
| Ti/TiO2NTHF-1h-600 | 600 | 23 |
| Ti/TiO2NTHF-1h-650 | 650 | 26 |
| Ti/TiO2NTHF-1h-750 | 750 | 0 |
| Photoanode | Method | Anodic Oxidation Time | Conversion for 1 h (%) | Conversion for 3 h (%) |
|---|---|---|---|---|
| Ti/TiO2NTEG-1h-500 | PEC | 1 | 43 | 86 |
| Ti/TiO2NTEG-2h-500 | PEC | 2 | 52 | 93 |
| Ti/TiO2NTEG-3h-500 | PEC | 3 | 60 | 98 |
| Ti/TiO2NTEG-4h-500 | PEC | 4 | 62 | 95 |
| Ti/TiO2NTEG-6h-500 | PEC | 6 | 50 | 90 |
| Ti/TiO2NTEG-6h-500 | PC | 6 | 7 | 26 |
| Ti/TiO2NTEG-6h-500 | EC | 6 | 0 | 1 |
| Ti/TiO2NTHF-1h-500 | PEC | 1 | 8 | 30 |
| Ti/TiO2NTHF-6h-500 | PEC | 6 | 9 | 48 |
| Photoanode | Cycle Count | Conversion for 1 h (%) |
|---|---|---|
| Ti/TiO2NTEG-3h-500 | - | 60 |
| Ti/TiO2NTEG-3h-500-Pt-1cycle | 1 | 49 |
| Ti/TiO2NTEG-3h-500-Pt-3cycles | 3 | 60 |
| Ti/TiO2NTEG-3h-500-Pt-4cycles | 4 | 75 |
| Ti/TiO2NTEG-3h-500-Pt-5cycles | 5 | 68 |
| Ti/TiO2NTEG-3h-500-Pt-6cycles | 6 | 60 |
| Ti/TiO2NTEG-3h-500-Pt-7cycles | 7 | 52 |
| Ti/TiO2NTEG-3h-500-Pt-10cycles | 10 | 46 |
| Ti/TiO2NTHF-3h-500-Pt-25cycles | 25 | 42 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özcan, L.; Mutlu, T.; Yurdakal, S. Photoelectrocatalytic Degradation of Paraquat by Pt Loaded TiO2 Nanotubes on Ti Anodes. Materials 2018, 11, 1715. https://doi.org/10.3390/ma11091715
Özcan L, Mutlu T, Yurdakal S. Photoelectrocatalytic Degradation of Paraquat by Pt Loaded TiO2 Nanotubes on Ti Anodes. Materials. 2018; 11(9):1715. https://doi.org/10.3390/ma11091715
Chicago/Turabian StyleÖzcan, Levent, Turan Mutlu, and Sedat Yurdakal. 2018. "Photoelectrocatalytic Degradation of Paraquat by Pt Loaded TiO2 Nanotubes on Ti Anodes" Materials 11, no. 9: 1715. https://doi.org/10.3390/ma11091715