Phase Transformation and Morphology Evolution of Ti50Cu25Ni20Sn5 during Mechanical Milling
Abstract
:1. Introduction
2. Materials and Methods
3. Experimental Results and Discussion
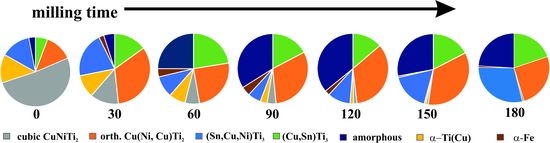
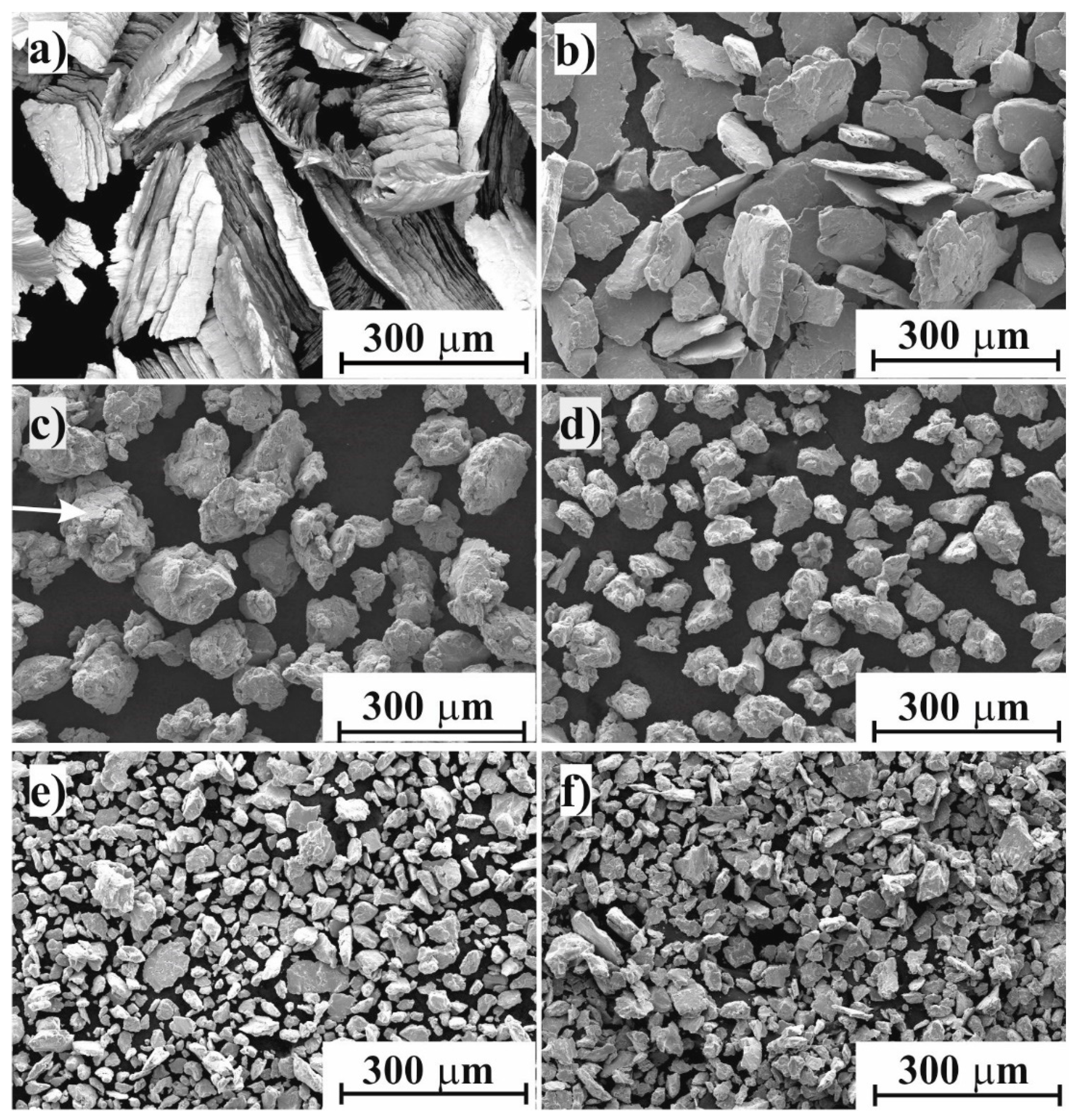
- 30 to 120 min—amorphization period, during which the milled powder was mainly composed of nanocrystalline phases. Accumulation of various crystal defects played a dominating role, and an increase in the storage energy of milled sample by crystal defects was evidenced by strong cold welding. The amorphization was enhanced through the destruction of nanocrystalline phases.
- Over 120 min—recrystallization period, when reduction of the storage energy of milled sample by atomic migration/recrystallization dominated and then the amorphous phase transformed back into nanocrystalline phase.
4. Conclusions
- Mechanical milling of Ti50Cu25Ni20Sn5 alloy by high-energy planetary ball milling up to 180 min led to the formation of nanocrystalline and amorphous composite. Amorphous fraction increased during milling due to intense shearing and plastic deformation induced by mechanical effect of balls.
- After a short milling time (120 min), an amorphous structure with nanocrystalline phases of sizes in the 1.1–13.1 nm range was produced.
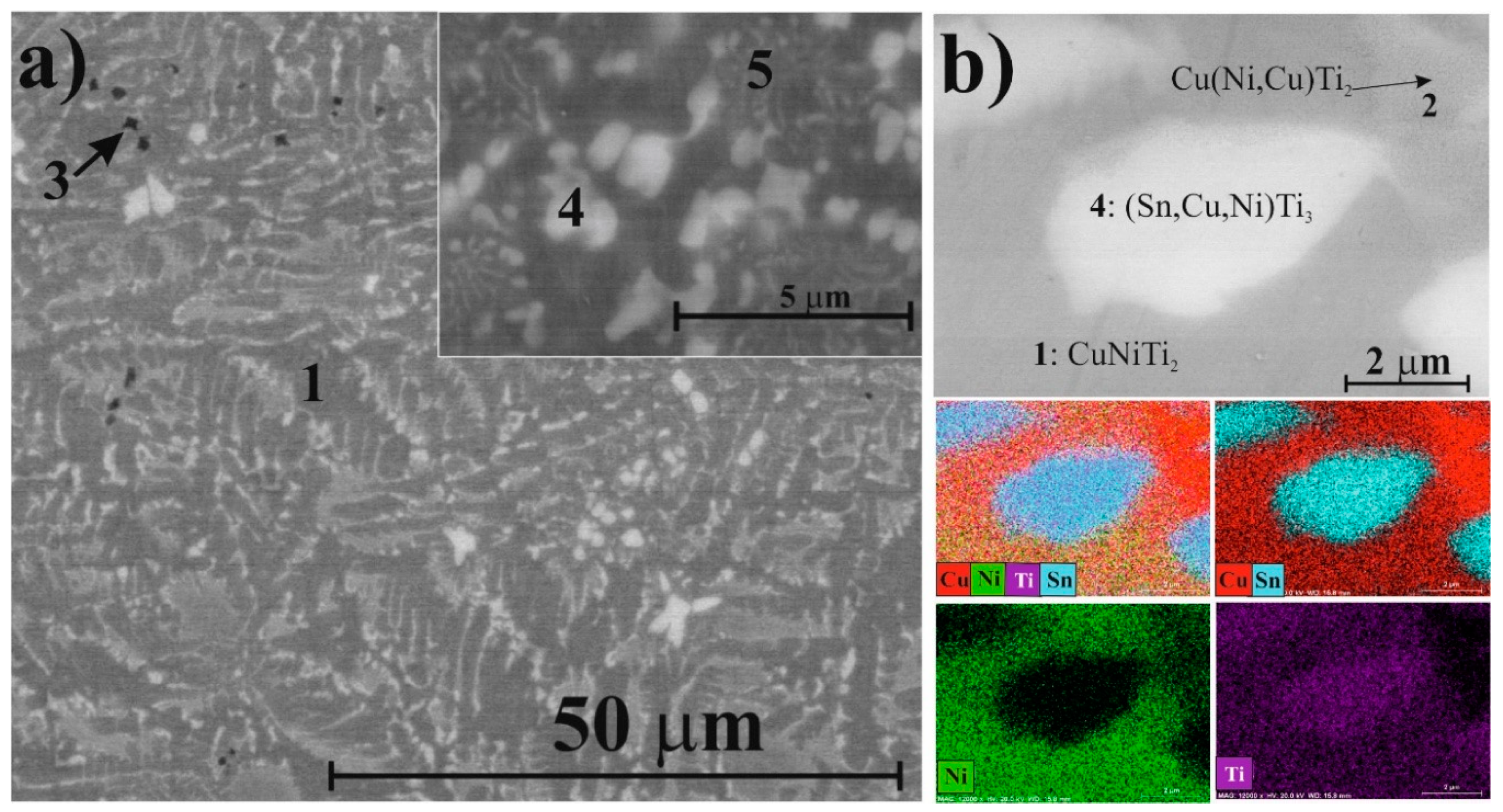
- The maximal amorphous fraction (36 wt %) was obtained after 120 min of milling. Two short range ordered phases could be distinguished based on X-ray diffraction, indicating atomic segregation in the transforming solid phases.
- Cyclic phase transformation took place during the milling process; Amorphization of a nanocrystalline alloy phase and recrystallization of an amorphous alloy phase occurred due to the high milling energy applied.
- The crystallite size continuously decreased; the interval of crystallite size was between 1 and 10 nm after 180 min of milling.
- Cubic Cu(Ni,Cu)Ti2 structure was transformed into the orthorhombic structure owing to the shear/stress, dislocations, and Cu substitution during the milling process.
- Fe contamination from milling media increased with the milling time up to 90 min of milling, but longer milling time alloyed Fe into the milled material.
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Pang, S.; Li, H.; Hu, Q.; Chen, B.; Zhang, T. Formation and properties of Ti-based Ti-Zr-Cu-Fe-Sn-Si bulk metallic glasses with different (Ti + Zr)/Cu ratios for biomedical application. Intermetallics 2016, 72, 36–43. [Google Scholar] [CrossRef]
- Gong, P.; Deng, L.; Jin, J.; Wang, S.; Wang, X.; Yao, K. Review on the Research and Development of Ti-Based Bulk Metallic Glasses. Metals 2016, 6, 264. [Google Scholar] [CrossRef]
- Bera, S.; Sarac, B.; Balakin, S.; Ramasamy, P.; Stoica, M.; Calin, M.; Eckert, J. Micro-patterning by thermoplastic forming of Ni-free Ti-based bulk metallic glasses. Mater. Des. 2017, 120, 204–211. [Google Scholar] [CrossRef]
- Inoue, A. Bulk amorphous and nanocrystalline alloys with high functional properties. Mater. Sci. Eng. A 2001, 304–306, 1–10. [Google Scholar] [CrossRef]
- Roy, D.; Mitra, R.; Chudoba, T.; Witczak, Z.; Lojkowski, W.; Fecht, H.-J.; Manna, I. Structure and mechanical properties of Al65Cu20Ti15-based amorphous/nanocrystalline alloys prepared by high-pressure sintering. Mater. Sci. Eng. A 2008, 497, 93–100. [Google Scholar] [CrossRef]
- Li, M.M.; Inoue, A.; Han, Y.; Kong, F.L.; Zhu, S.L.; Shalaan, E.; Al-Marzouki, F. Influence of Ag replacement on supercooled liquid region and icosahedral phase precipitation of Zr65Al7.5Ni10Cu17.5−xAgx (x = 0–17.5 at%) glassy alloys. J. Alloy. Compd. 2018, 735, 1712–1721. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, L.X.; Wang, Y.H.; Ni, S.; Guo, S.F.; Song, M. Combined effect of isothermal annealing and pre-compression on mechanical properties of Cu36Zr48Al8Ag8 bulk metallic glass. Trans. Nonferr. Met. Soc. China 2016, 26, 1620–1628. [Google Scholar] [CrossRef]
- Gong, P.; Wang, S.; Liu, Z.; Chen, W.; Lia, N.; Wang, X.; Yaoe, K.F. Lightweight Ti-based bulk metallic glasses with superior thermoplastic. Intermetallics 2018, 98, 54–59. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, J.T.; Mun, S.C.; Kim, Y.S.; Park, H.J.; Na, Y.S.; Lim, K.R.; Park, J.M.; Kim, K.B. Influence of spherical particles and interfacial stress distribution on viscous flow behavior of Ti-Cu-Ni-Zr-Sn bulk metallic glass composites. Intermetallics 2017, 91, 90–94. [Google Scholar] [CrossRef]
- Manaf, A.; Buckley, A.; Davies, H.A. New nanocrystalline high-remanence Nd-Fe-B alloys by rapid solidification. J. Magn. Magn. Mater. 1993, 128, 302–306. [Google Scholar] [CrossRef]
- Guo, W.; Yamada, R.; Saida, J. Unusual plasticization for structural relaxed bulk metallic glass. Mater. Sci. Eng. A 2017, 699, 81–87. [Google Scholar] [CrossRef]
- Shahreza, P.R.; Seifoddini, A.; Hasani, S. Non-isothermal kinetic analysis of nano-crystallization process in (Fe41Co7Cr15Mo14Y2C15)94B6 amorphous alloy. Thermochim. Acta 2017, 652, 119–125. [Google Scholar] [CrossRef]
- Manna, I.; Chattopadhyay, P.P.; Banhart, F.; Fecht, H.J. development of amorphous and nanocrystalline Al65Cu35−xZrx alloys by mechanical alloying. Mater. Sci. Eng. A 2004, 379, 360–365. [Google Scholar] [CrossRef]
- Oanh, N.T.H.; Choi, P.P.; Kim, J.S.; Kwon, D.H.; Kwon, Y.S. Thermal Stability of Amorphous Ti-Cu-Ni-Sn Prepared by Mechanical Alloying. Mater. Sci. Forum 2007, 534–536, 233–236. [Google Scholar] [CrossRef]
- Chicardi, E.; García-Garrido, C.; Sayagués, M.J.; Torres, Y.; Amigó, V.; Aguilar, C. Development of a novel fcc structure for an amorphous-nanocrystalline Ti-33Nb-4Mn (at.%) ternary alloy. Mater. Charact. 2018, 135, 46–56. [Google Scholar] [CrossRef]
- Koch, C.C.; Whittenberger, J.D. Mechanical milling/alloying of intermetallics. Intermetallics 1996, 4, 339–355. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Cho, Y.S.; Koch, C.C. Mechanical milling of ordered intermetallic compounds: The role of defects in amorphization. J. Alloy. Compd. 1993, 194, 287–294. [Google Scholar] [CrossRef]
- Kishimura, H.; Matsumoto, H. Fabrication of Ti-Cu-Ni-Al amorphous alloys by mechanical alloying and mechanical milling. J. Alloy. Compd. 2011, 509, 4386–4389. [Google Scholar] [CrossRef]
- Zhang, T.; Inoue, A. Thermal and mechanical properties of Ti-Ni-Cu-Sn amorphous alloys with a wide supercooled liquid region before crystallization. Mater. Trans. JIM 1998, 39, 1001–1006. [Google Scholar] [CrossRef]
- McCusker, L.B.; von Dreele, R.B.; Cox, D.E.; LoueÈr, D.; Scardi, P. Rietveld refinement guidelines. J. Appl. Cryst. 1999, 32, 36–50. [Google Scholar] [CrossRef] [Green Version]
- Bish, D.L.; Howard, S.A. Quantitative Phase Analysis Using the Rietveld Method. J. Appl. Cryst. 1988, 21, 86–91. [Google Scholar] [CrossRef]
- Caër, G.L.; Delcroix, P.; Colin, S.B.; Ziller, T. High-Energy Ball-Milling of Alloys and Compounds. Hyperfine Interact. 2002, 141, 63–72. [Google Scholar] [CrossRef]
- Nazari, K.A.; Nouri, A.; Hilditch, T. The addition of a surfactant at regular time intervals in the mechanical alloying process. J. Alloy. Compd. 2014, 615, 47–55. [Google Scholar] [CrossRef]
- Hao, L.; Lu, Y.; Sato, H.; Yoshida, H. Fabrication of Ni coatings by Mechanical Coating Technique. J. Eng. Technol. JET 2012, 1, 131–134. [Google Scholar]
- Kim, Y.C.; Kim, W.T.; Kim, D.H. A development of Ti-based bulk metallic glass. Mater. Sci. Eng. A 2004, 375, 127–135. [Google Scholar] [CrossRef]
- Bendersky, L.A.; Roytburd, A.; Boettinger, W.J. Transformation of BCC and B2 High Temperature Phases to HCP and Orthorhombic Structures in the Ti-Al-Nb System. Part I: Microstructural Predictions Based on a Subgroup Relation between Phases. J. Res. Natl. Inst. Stand. Technol. 1993, 98, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Sveda, M.; Sycheva, A.; Miko, T.; Kristaly, F.; Racz, A.; Ferenczi, T.; Janovszky, D. Effect of Ni and Zr on the microstructural evolution of Ti-based alloys during ball-milling. J. Non-Cryst. Solids 2017, 473, 41–46. [Google Scholar] [CrossRef]
- Abrosimova, G.E.; Aronin, A.S. Evolution of the Amorphous Phase Structure in Metal-Metal Type Metallic Glasses. J. Surf. Investig. 2015, 9, 887–893. [Google Scholar] [CrossRef]
- Abrosimova, G.; Aronin, A. Amorphous and Nanocrystalline Metallic Alloys, Progress in Metallic Alloys. Available online: https://www.intechopen.com/books/progress-in-metallic-alloys/amorphous-and-nanocrystalline-metallic-alloys (accessed on 19 October 2016).
- Yamamoto, T.; Takahashi, T.; Kimura, H.; Inoue, A. Effect of ball-milling and shot-peening on Zr55Al10Ni5Cu30 alloys. J. Alloy. Compd. 2007, 430, 97–101. [Google Scholar] [CrossRef]
- Scudino, S.; Sakaliyska, M.; Surreddi, K.B.; Eckert, J. Mechanical alloying and milling of Al-Mg alloys. J. Alloy. Compd. 2009, 483, 2–7. [Google Scholar] [CrossRef]



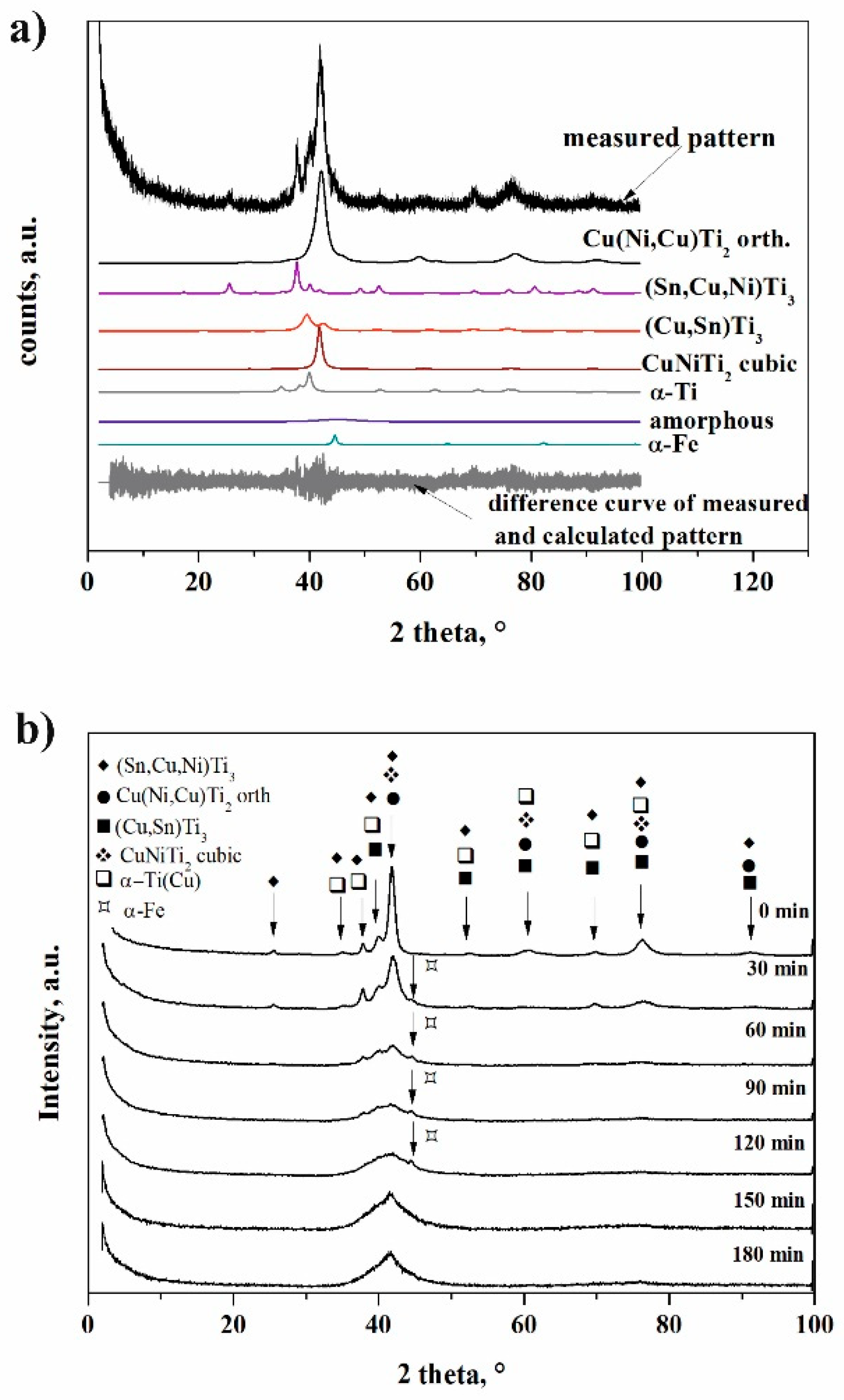


| Phase | Milling Time, min | 0 | 30 | 60 | 90 | 120 | 150 | 180 |
|---|---|---|---|---|---|---|---|---|
| cubic CuNiTi2 space group Pm-3m | a, nm | 0.3050 | 0.3054 | 0.3089 | 0.3082 | 0.3100 | 0.3074 | 0.3069 |
| crystallite size, nm | 16.2–25.5 ± 2.8 | 9.6–6.1 ± 1.3 | 3.1–4.8 ± 0.6 | 1.7–2.6 ± 0.5 | 1.5–2.3 ± 0.7 | 2.6–4.0 ± 0.3 | 2.7–4.3 ± 0.4 | |
| wt % Rietveld | 53.0 | 13.0 | 6.6 | 4.1 | 1.6 | 1.0 | 0.8 | |
| cell volume, nm3 | 28.49 | 28.49 | 28.62 | 29.28 | 29.79 | 29.05 | 28.92 | |
| R Bragg | 3.60 | 2.26 | 2.31 | 2.82 | 74.63 | 29.58 | 8.95 | |
| orthorhombic Cu(Ni,Cu)Ti2 space group Pmmb | a, nm | 0.2990 | 0.2950 | 0.2950 | 0.2950 | 0.2907 | 0.2850 | 0.2850 |
| b, nm | 0.4330 | 0.4300 | 0.4300 | 0.4300 | 0.4300 | 0.4300 | 0.4300 | |
| c, nm | 0.4460 | 0.4450 | 0.4450 | 0.4450 | 0.4450 | 0.4450 | 0.4450 | |
| crystallite size, nm | 10.5–16.4 ± 1.6 | 3.8–6.0 ± 0.6 | 2.0–3.1 ± 0.4 | 1.0–1.6 ± 0.4 | 1.1–1.8 ± 0.3 | ~1.3 ± 0.2 | ~1.0 | |
| wt % Rietveld | 13.3 | 33.4 | 24.8 | 31.3 | 34.5 | 35.0 | 25.7 | |
| cell volume, nm3 | 57.76 | 56.45 | 56.45 | 56.45 | 55.61 | 54.53 | 54.53 | |
| R Bragg | 4.70 | 3.02 | 3.83 | 4.14 | 67.07 | 27.87 | 8.28 | |
| hexagonal α-Ti(Cu) space group P63/mmc | a, nm | 0.2970 | 0.2967 | 0.2967 | 0.2967 | 0.2967 | 0.2967 | - |
| c, nm | 0.4700 | 0.4700 | 0.4700 | 0.4700 | 0.4700 | 0.4700 | - | |
| crystallite size, nm | 6.4–10 ± 0.8 | 6.4–10 ± 1.5 | 6.2–9.7 ± 1.8 | 8.4–13.1 ± 5.8 | 8.8–13.1 ± 8.5 | 9.6–15.1 ± 11.8 | - | |
| wt % Rietveld | 13.7 | 11 | 7.5 | 3.2 | 1.6 | 0.8 | 0 | |
| cell volume, nm3 | 35.83 | 35.83 | 35.83 | 35.83 | 35.83 | 35.83 | - | |
| R Bragg | 6.71 | 4.80 | 4.12 | 3.85 | 65.6 | 27.55 | - | |
| hexagonal (Sn,Cu,Ni)Ti3 space group P63/mmc | a, nm | 0.5894 | 0.5890 | 0.5890 | 0.5923 | 0.6303 | 0.6157 | 0.5834 |
| c, nm | 0.4759 | 0.4765 | 0.4765 | 0.4764 | 0.4741 | 0.4730 | 0.5080 | |
| crystallite size, nm | 14.9–26.2 ± 3.8 | 7.7–13.7 ± 0.7 | 7.1–11.1 ± 1.3 | 6.7–10.5 ± 1.9 | 2.3–3.6 ± 0.8 | 1.4–2.2 ± 0.5 | 1.2 ± 0.2 | |
| wt % Rietveld | 14.2 | 20.9 | 9.8 | 6.0 | 10.5 | 17.3 | 29.1 | |
| cell volume, nm3 | 143.18 | 143.16 | 143.16 | 144.72 | 163.11 | 155.26 | 149.71 | |
| R Bragg | 8.52 | 6.78 | 5.29 | 5.22 | 49.61 | 35.61 | 7.95 | |
| tetragonal (Cu,Sn)Ti3 space group P4/mmm | a, nm | 0.4150 | 0.4250 | 0.4219 | 0.4250 | 0.4250 | 0.4222 | 0.4250 |
| c, nm | 0.3580 | 0.3500 | 0.3552 | 0.3535 | 0.3582 | 0.3600 | 0.3510 | |
| crystallite size, nm | 6.4–10 ± 1.5 | 3.2–5 ± 0.7 | 2.5–3.9 ± 0.5 | 2.4–3.8 ± 0.9 | 1.9–3 ± 0.7 | 1.8–2.8 ± 0.4 | 1.3–2.1 ± 0.3 | |
| wt % Rietveld | 5.8 | 15.0 | 22.0 | 17.2 | 13.7 | 35.0 | 25.7 | |
| cell volume, nm3 | 61.64 | 63.22 | 63.22 | 63.85 | 64.70 | 64.18 | 63.41 | |
| R Bragg | 8.10 | 4.36 | 4.22 | 4.03 | 65.5 | 27.9 | 8.86 | |
| cubic α-Iron space group Im-3m | a, nm | - | 0.2872 | 0.2873 | 0.2873 | 0.2873 | 0.2876 | 0.2883 |
| crystallite size, nm | - | 9.8–15.4 ± 2.1 | 9.5–15.0 ± 2.5 | 7.4–11.6 ± 2.2 | 9.8–15.4 ± 3.3 | 6.4–10.0 ± 7.7 | 6.4–10.0 ± 4.9 | |
| wt % Rietveld | - | 2 | 3.8 | 4.2 | 2.3 | 0.7 | 0.7 | |
| cell volume, nm3 | - | 23.70 | 23.72 | 23.71 | 23.71 | 23.78 | 23.96 | |
| R Bragg | - | 3.00 | 8.81 | 3.31 | 72.7 | 22.65 | 10.05 |
| Milling Time, min | Amorphous Fraction, wt % | Amorphous Halo | |||||
|---|---|---|---|---|---|---|---|
| First Peak | Second Peak | ||||||
| Position, nm | Size, nm | Area cps*2Th | Position, nm | Size, nm | Area cps*2Th | ||
| 0 | 3 | 0.1891 | 1.0 | 2.2 | - | - | - |
| 30 | 5 | 0.2006 | 1.0 | 2.2 | - | - | - |
| 60 | 25 | 0.1938 | 0.8 | 10.4 | 0.1309 | 0.9 | 30.2 |
| 90 | 34 | 0.1841 | 0.6 | 17.7 | 0.1318 | 0.6 | 60.6 |
| 120 | 36 | 0.1781 | 0.6 | 32.1 | 0.1336 | 0.6 | 99.1 |
| 150 | 28 | 0.1646 | 0.6 | 44.4 | 0.1330 | 0.6 | 117.8 |
| 180 | 24 | 0.1608 | 0.7 | 36.1 | 0.1321 | 0.6 | 109.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janovszky, D.; Kristaly, F.; Miko, T.; Racz, A.; Sveda, M.; Sycheva, A.; Koziel, T. Phase Transformation and Morphology Evolution of Ti50Cu25Ni20Sn5 during Mechanical Milling. Materials 2018, 11, 1769. https://doi.org/10.3390/ma11091769
Janovszky D, Kristaly F, Miko T, Racz A, Sveda M, Sycheva A, Koziel T. Phase Transformation and Morphology Evolution of Ti50Cu25Ni20Sn5 during Mechanical Milling. Materials. 2018; 11(9):1769. https://doi.org/10.3390/ma11091769
Chicago/Turabian StyleJanovszky, Dora, Ferenc Kristaly, Tamas Miko, Adam Racz, Maria Sveda, Anna Sycheva, and Tomasz Koziel. 2018. "Phase Transformation and Morphology Evolution of Ti50Cu25Ni20Sn5 during Mechanical Milling" Materials 11, no. 9: 1769. https://doi.org/10.3390/ma11091769