Recent Advances in Fluorescent Probes for Lipid Droplets
Abstract
:1. Introduction
2. Common Methods and Probes in LDs Fluorescence Bioimaging
2.1. Fluorescence Immunostaining
2.2. Diazo Dyes
2.3. Nile Red
2.4. BODIPY 493/503
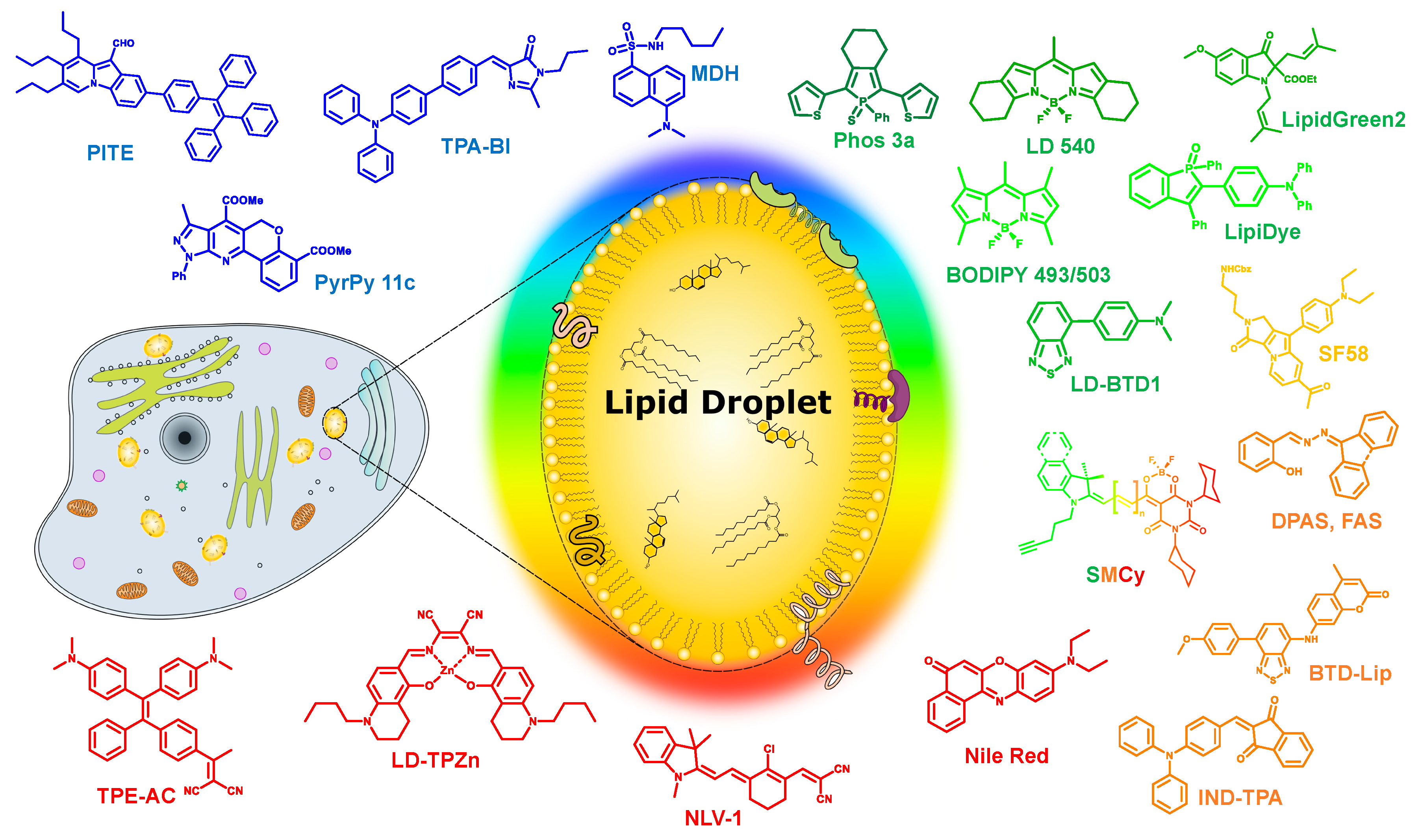
3. Recent Developments in LDs’ Selective Fluorescent Probes
3.1. Blue Emitting LD Probes (λem < 500 nm)
3.2. Green Emitting LD Probes (λem = 500–550 nm)
3.3. Orange Emitting LD Probes (550 nm < λem < 600 nm)
3.4. Red to Near-Infrared Emitting LD Probes (λem > 600 nm)
3.5. Families of LD Probes with Tunable Emission Color
4. Application of Fluorescent LD Probes
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, S.; Parton, R.G. Opinion: Lipid Droplets: A Unified View of a Dynamic Organelle. Nat. Rev. Mol. Cell Biol. 2006, 7, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Farese, R.V.; Walther, T.C. Lipid Droplets Finally Get a Little R-E-S-P-E-C-T. Cell 2009, 139, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Thiam, A.R.; Farese, R.V., Jr.; Walther, T.C. The Biophysics and Cell Biology of Lipid Droplets. Nat. Rev. Mol. Cell Biol. 2013, 14, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Blom, T.; Somerharju, P.; Ikonen, E. Synthesis and Biosynthetic Trafficking of Membrane Lipids. CSH Perspect. Biol. 2011, 3, a004713. [Google Scholar] [CrossRef] [PubMed]
- Olzmann, J.A.; Richter, C.M.; Kopito, R.R. Spatial Regulation of UBXD8 and P97/VCP Controls ATGL-Mediated Lipid Droplet Turnover. Proc. Natl. Acad. Sci. USA 2013, 110, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Onal, G.; Kutlu, O.; Gozuacik, D.; Dokmeci Emre, S. Lipid Droplets in Health and Disease. Lipids Health Dis. 2017, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Ohsaki, Y.; Suzuki, M.; Cheng, J. Chapter 13—Imaging Lipid Droplets by Electron Microscopy. In Methods in Cell Biology; Yang, H., Li, P., Eds.; Lipid Droplets; Academic Press: Cambridge, MA, USA, 2013; Volume 116, pp. 227–251. [Google Scholar]
- Horn, P.J.; Ledbetter, N.R.; James, C.N.; Hoffman, W.D.; Case, C.R.; Verbeck, G.F.; Chapman, K.D. Visualization of Lipid Droplet Composition by Direct Organelle Mass Spectrometry. J. Biol. Chem. 2011, 286, 3298–3306. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Surmacki, J.; Kopeć, M.; Olejnik, A.K.; Lubecka-Pietruszewska, K.; Fabianowska-Majewska, K. The Role of Lipid Droplets and Adipocytes in Cancer. Raman Imaging of Cell Cultures: MCF10A, MCF7, and MDA-MB-231 Compared to Adipocytes in Cancerous Human Breast Tissue. Analyst 2015, 140, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.; Yoon, J.; Heo, J.; Choi, C.; Park, Y. Three-Dimensional Label-Free Imaging and Quantification of Lipid Droplets in Live Hepatocytes. Sci. Rep. 2016, 6, 36815. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York City, NY, USA, 2006. [Google Scholar]
- Lavis, L.D. Teaching Old Dyes New Tricks: Biological Probes Built from Fluoresceins and Rhodamines. Annu. Rev. Biochem. 2017, 86, 825–843. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, J.; Du, J.; Peng, X. Fluorescent Probes for Sensing and Imaging within Specific Cellular Organelles. Acc. Chem. Res. 2016, 49, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Kuerschner, L.; Moessinger, C.; Thiele, C. Imaging of Lipid Biosynthesis: How a Neutral Lipid Enters Lipid Droplets. Traffic 2007, 9, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Listenberger, L.L.; Brown, D.A. Fluorescent Detection of Lipid Droplets and Associated Proteins. Curr. Protoc. Cell Biol. 2007, 35, 24. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Hagiwara, H.; Fujimoto, T. Peculiar Distribution of Fodrin in Fat-Storing Cells. Exp. Cell Res. 1997, 234, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Schaart, G.; Hesselink, M.K. Optimisation of Oil Red O Staining Permits Combination with Immunofluorescence and Automated Quantification of Lipids. Histochem. Cell. Biol. 2001, 116, 63–68. [Google Scholar] [PubMed]
- Ohsaki, Y.; Shinohara, Y.; Suzuki, M.; Fujimoto, T. A Pitfall in Using BODIPY Dyes to Label Lipid Droplets for Fluorescence Microscopy. Histochem. Cell. Biol. 2010, 133, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Fujimoto, T. Deformation of Lipid Droplets in Fixed Samples. Histochem. Cell. Biol. 2002, 118, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile Red: A Selective Fluorescent Stain for Intracellular Lipid Droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Thermo Fisher Scientific. BODIPY 493/503. Available online: https://www.thermofisher.com/order/catalog/product/D3922 (accessed on 26 July 2018).
- Collot, M.; Fam, T.K.; Ashokkumar, P.; Faklaris, O.; Galli, T.; Danglot, L.; Klymchenko, A.S. Ultrabright and Fluorogenic Probes for Multicolor Imaging and Tracking of Lipid Droplets in Cells and Tissues. J. Am. Chem. Soc. 2018, 140, 5401–5411. [Google Scholar] [CrossRef] [PubMed]
- Gocze, P.M.; Freeman, D.A. Factors Underlying the Variability of Lipid Droplet Fluorescence in MA-10 Leydig Tumor Cells. Cytometry 1994, 17, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Koreivienė, J. Microalgae Lipid Staining with Fluorescent BODIPY Dye. In SpringerLink; Methods in Molecular Biology; Humana Press: New York City, NY, USA, 2017; pp. 1–7. [Google Scholar]
- Yang, H.-J.; Hsu, C.-L.; Yang, J.-Y.; Yang, W.Y. Monodansylpentane as a Blue-Fluorescent Lipid-Droplet Marker for Multi-Color Live-Cell Imaging. PLoS ONE 2012, 7, e32693. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Ruiz, M.; Vargas, V.; Jara, P.; Tirapegui, C.; Carrasco, C.; Nuñez, M.; Lezana, N.; Galdámez, A.; Vilches-Herrera, M. Blue-Fluorescent Probes for Lipid Droplets Based on Dihydrochromeno-Fused Pyrazolo- and Pyrrolopyridines. Eur. J. Org. Chem. 2018, 34, 4795–4801. [Google Scholar] [CrossRef]
- Sk, B.; Thakre, P.K.; Tomar, R.S.; Patra, A. A Pyridoindole-Based Multifunctional Bioprobe: PH-Induced Fluorescence Switching and Specific Targeting of Lipid Droplets. Chem. Asian J. 2017, 12, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Zhao, E.; Hong, Y.; Lam, J.W.Y.; Tang, B.Z. A Highly Selective AIE Fluorogen for Lipid Droplet Imaging in Live Cells and Green Algae. J. Mater. Chem. B 2014, 2, 2013–2019. [Google Scholar] [CrossRef]
- Jiang, M.; Gu, X.; Lam, J.W.Y.; Zhang, Y.; Kwok, R.T.K.; Wong, K.S.; Tang, B.Z. Two-Photon AIE Bio-Probe with Large Stokes Shift for Specific Imaging of Lipid Droplets. Chem. Sci. 2017, 8, 5440–5446. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; So, J.-H.; Jeon, J.H.; Choi, E.B.; Lee, Y.-R.; Chang, Y.-T.; Kim, C.-H.; Bae, M.A.; Ahn, J.H. Synthesis of a New Fluorescent Small Molecule Probe and Its Use for in Vivolipid Imaging. Chem. Commun. 2011, 47, 7500–7502. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.-S.; Jeon, J.H.; Pagire, H.S.; Lee, J.H.; Chung, H.-C.; Park, M.J.; So, J.-H.; Ryu, J.-H.; Kim, C.-H.; Ahn, J.H.; et al. Synthesis of LipidGreen2 and Its Application in Lipid and Fatty Liver Imaging. Mol. BioSyst. 2013, 9, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Spandl, J.; White, D.J.; Peychl, J.; Thiele, C. Live Cell Multicolor Imaging of Lipid Droplets with a New Dye, LD540. Traffic 2009, 10, 1579–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Umar, S.; Kar, P.; Singh, K.; Sachdev, M.; Goel, A. A New Type of Biocompatible Fluorescent Probe AFN for Fixed and Live Cell Imaging of Intracellular Lipid Droplets. Analyst 2015, 141, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Zhang, R.; Kwong, J.P.C.; Lam, J.W.Y.; Chen, C.; Wang, J.; Chen, Y.; Feng, X.; Kwok, R.T.K.; Sung, H.H.-Y.; et al. Specific Two-Photon Imaging of Live Cellular and Deep-Tissue Lipid Droplets by Lipophilic AIEgens at Ultralow Concentration. Chem. Mater. 2018, 30, 4778–4787. [Google Scholar] [CrossRef]
- Appelqvist, H.; Stranius, K.; Börjesson, K.; Nilsson, K.P.R.; Dyrager, C. Specific Imaging of Intracellular Lipid Droplets Using a Benzothiadiazole Derivative with Solvatochromic Properties. Bioconjug. Chem. 2017, 28, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, E.; Wang, C.; Fukazawa, A.; Taki, M.; Sato, Y.; Sasaki, T.; Ueda, M.; Sasaki, N.; Higashiyama, T.; Yamaguchi, S. Environment-Sensitive Fluorescent Probe: A Benzophosphole Oxide with an Electron-Donating Substituent. Angew. Chem. Int. Edit. 2015, 127, 4622–4626. [Google Scholar] [CrossRef]
- High Sensitive Lipid Droplets Imaging Fluorescent Dye | LipiDye | Funakoshi Co., Ltd.: Tokyo, Japan. Available online: https://www.funakoshi.co.jp/exports_contents/80682 (accessed on 30 July 2018).
- Niko, Y.; Didier, P.; Mely, Y.; Konishi, G.; Klymchenko, A.S. Bright and Photostable Push-Pull Pyrene Dye Visualizes Lipid Order Variation between Plasma and Intracellular Membranes. Sci. Rep. 2016, 6, 18870. [Google Scholar] [CrossRef] [PubMed]
- Öberg, E.; Appelqvist, H.; Nilsson, K.P.R. Non-Fused Phospholes as Fluorescent Probes for Imaging of Lipid Droplets in Living Cells. Front. Chem. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- De Moliner, F.; King, A.; Dias, G.G.; de Lima, G.F.; de Simone, C.A.; da Silva Júnior, E.N.; Vendrell, M. Quinone-Derived π-Extended Phenazines as New Fluorogenic Probes for Live-Cell Imaging of Lipid Droplets. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lee, S.; Park, S.B. A Seoul-Fluor-Based Bioprobe for Lipid Droplets and Its Application in Image-Based High Throughput Screening. Chem. Commun. 2012, 48, 2331–2333. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Na, S.; Lee, S.; Jeon, N.L.; Park, S.B. Optimization of Seoul-Fluor-Based Lipid Droplet Bioprobes and Their Application in Microalgae for Bio-Fuel Study. Mol. Biosyst. 2013, 9, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gui, C.; Zhao, E.; Wang, J.; Li, X.; Qin, A.; Zhao, Z.; Yu, Z.; Tang, B.Z. Specific Fluorescence Probes for Lipid Droplets Based on Simple AIEgens. ACS Appl. Mater. Interfaces 2016, 8, 10193–10200. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.A.R.; Correa, J.R.; de Andrade, L.P.; Assumpção, J.A.F.; de Souza Cintra, G.A.; Freitas-Junior, L.H.; da Silva, W.A.; de Oliveira, H.C.B.; Neto, B.A.D. From Live Cells to Caenorhabditis Elegans: Selective Staining and Quantification of Lipid Structures Using a Fluorescent Hybrid Benzothiadiazole Derivative. ACS Omega 2018, 3, 3874–3881. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Su, H.; Li, S.; Lin, Y.; Ling, X.; Qin, A.; Tang, B.Z. An Easily Accessible Aggregation-Induced Emission Probe for Lipid Droplet-Specific Imaging and Movement Tracking. Chem. Commun. 2017, 53, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ling, X.; Lin, Y.; Qin, A.; Gao, M.; Tang, B.Z. In Situ Generation of Photoactivatable Aggregation-Induced Emission Probes for Organelle-Specific Imaging. Chem. Sci. 2018, 9, 5730–5735. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, Y.; Yin, H.-Y.; Xu, G.; Zhang, J.-L. Precise Labeling and Tracking of Lipid Droplets in Adipocytes Using a Luminescent ZnSalen Complex. Chem. Asian J. 2017, 12, 2533–2538. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Jana, N.R. Quantum Dot-Based Designed Nanoprobe for Imaging Lipid Droplet. J. Phys. Chem. C 2017, 121, 23727–23735. [Google Scholar] [CrossRef]
- Gao, M.; Su, H.; Lin, Y.; Ling, X.; Li, S.; Qin, A.; Tang, B.Z. Photoactivatable Aggregation-Induced Emission Probes for Lipid Droplets-Specific Live Cell Imaging. Chem. Sci. 2017, 8, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Gu, X.; Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Li, F.; Tang, B.Z. A Near-Infrared AIEgen for Specific Imaging of Lipid Droplets. Chem. Commun. 2016, 52, 5957–5960. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Su, H.; Kwok, R.T.K.; Shan, G.; Leung, A.C.S.; Lee, M.M.S.; Sung, H.H.Y.; Williams, I.D.; Lam, J.W.Y.; Tang, B.Z. Facile Synthesis of Red/NIR AIE Luminogens with Simple Structures, Bright Emissions, and High Photostabilities, and Their Applications for Specific Imaging of Lipid Droplets and Image-Guided Photodynamic Therapy. Adv. Funct. Mater. 2017, 27, 1704039. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, T.; Liu, H.; Chen, Y.; Kwok, R.T.K.; Ma, C.; Zhang, P.; Sung, H.H.Y.; Williams, I.D.; Lam, J.W.Y.; et al. Bright Near-Infrared Aggregation-Induced Emission Luminogens with Strong Two-Photon Absorption, Excellent Organelle Specificity, and Efficient Photodynamic Therapy Potential. ACS Nano 2018, 12, 8145–8159. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Yin, J.; Ma, Y.; Li, G.; Wang, Q.; Lin, W. A Novel NIR Probe for Detection of Viscosity in Cellular Lipid Droplets, Zebra Fishes and Living Mice. Sensor. Actuators B-Chem. 2018, 271, 321–328. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific, LipidTOX Neutral Lipid Stain. Available online: https://www.thermofisher.com/order/catalog/product/H34475 (accessed on 27 July 2018).
- Fujimoto, T.; Ohsaki, Y.; Cheng, J.; Suzuki, M.; Shinohara, Y. Lipid Droplets: A Classic Organelle with New Outfits. Histochem. Cell. Biol. 2008, 130, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, N.; Choudhary, V.; Mari, M.; Toulmay, A.; Reggiori, F.; Schneiter, R. Lipid Droplets Are Functionally Connected to the Endoplasmic Reticulum in Saccharomyces Cerevisiae. J. Cell. Sci. 2011, 124, 2424–2437. [Google Scholar] [CrossRef] [PubMed]
- Layerenza, J.P.; González, P.; García de Bravo, M.M.; Polo, M.P.; Sisti, M.S.; Ves-Losada, A. Nuclear Lipid Droplets: A Novel Nuclear Domain. BBA-Mol. Cell Biol. Lipids 2013, 1831, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Nevo-Yassaf, I.; Lovelle, M.; Nahmias, Y.; Hirschberg, K.; Sklan, E.H. Live Cell Imaging and Analysis of Lipid Droplets Biogenesis in Hepatatis C Virus Infected Cells. Methods 2017, 127, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tuohetahuntila, M.; Molenaar, M.R.; Spee, B.; Brouwers, J.F.; Wubbolts, R.; Houweling, M.; Yan, C.; Du, H.; VanderVen, B.C.; Vaandrager, A.B.; et al. Lysosome-Mediated Degradation of a Distinct Pool of Lipid Droplets during Hepatic Stellate Cell Activation. J. Biol. Chem. 2017, 292, 12436–12448. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X.; Li, L.; Qiu, H.; Zhang, Z.; Wang, Y.; Sun, G. Application of the Fluorescent Dye BODIPY in the Study of Lipid Dynamics of the Rice Blast Fungus Magnaporthe Oryzae. Molecules 2018, 23, 1594. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.; Braverman, J.; Asfaha, K.; Gronert, K.; Stanley, S. Lipid Droplet Formation in Mycobacterium Tuberculosis Infected Macrophages Requires IFN-γ/HIF-1α Signaling and Supports Host Defense. PLOS Pathog. 2018, 14, e1006874. [Google Scholar] [CrossRef] [PubMed]
- Masedunskas, A.; Chen, Y.; Stussman, R.; Weigert, R.; Mather, I.H.; Nusrat, A. Kinetics of Milk Lipid Droplet Transport, Growth, and Secretion Revealed by Intravital Imaging: Lipid Droplet Release Is Intermittently Stimulated by Oxytocin. MBoC 2017, 28, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, J.G.; Moens, S.J.B.; Tiessens, F.; Bakker, G.J.; Dallinga-Thie, G.M.; Groen, A.K.; Nieuwdorp, M.; Stroes, E.S.G.; Kroon, J. Nile Red Quantifier: A Novel and Quantitative Tool to Study Lipid Accumulation in Patient-Derived Circulating Monocytes Using Confocal Microscopy. J. Lipid. Res. 2017, 58, 2210–2219. [Google Scholar] [CrossRef] [PubMed]
- Held, P. Lipid Accumulation in HepG2 Cells Exposed to Free Fatty Acids. Available online: https://www.biotek.com/resources/application-notes/lipid-accumulation-in-hepg2-cells-exposed-to-free-fatty-acids/ (accessed on 14 August 2017).
- Helmchen, F.; Denk, W. Deep Tissue Two-Photon Microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Hu, F.; Shen, Y.; Chen, Z.; Yu, Y.; Lin, C.-C.; Wang, M.C.; Min, W. Live-Cell Imaging of Alkyne-Tagged Small Biomolecules by Stimulated Raman Scattering. Nat. Methods 2014, 11, 410–412. [Google Scholar] [CrossRef] [PubMed]






| Dye | λex/λem, nm | ε, M−1 cm−1 | QY | Excitation Mode | Application | Reference |
|---|---|---|---|---|---|---|
| Commonly used | ||||||
| Nile Red | 553/635 (MeOH) | 44,000 | n.r. | 1P | Cells | [21] |
| BODIPY 493/503 | 493/503 (MeOH) | 90,000 | n.r. | 1P | Cells | [22] |
| Blue | ||||||
| PITE | 313 a/487 (MeCN) | n.r. | 0.48 (MeCN) | 1P | Mammalian and bacterial cells | [28] |
| MDH | 405/570 (MeOH) | n.r. | n.r. | 1P, 2P (δ2PA n.r.) | Cells | [26] |
| TPA-BI | 447/619 (MeCN) | 34,000 | 0.22 (water 70%) | 1P, 2P (δ2PA = 213 GM at 840 nm) | 1PE and 2PE imaging in cells | [31] |
| TPE-AmAl | 400/470 (THF) | n.r. | 0.22 (solid state) | 1P | Cells and algae | [30] |
| PyrPy 10d | 356/449 (CHCl3) | 12,400 | 0.322 (CHCl3) | 1P | Cells | [27] |
| PyrPy 11c | 344/447 (CHCl3) | 13,200 | 0.029 (CHCl3) | 1P | Cells | [27] |
| Green | ||||||
| BODIPY 505/515 | 505/515 (MeOH) | 94,000 | n.r. | 1P | Cells | [22] |
| LipidGreen | 485/515 (PBS) | n.r. | n.r. | 1P | See footnote 1 | [32] |
| LipidGreen2 | 456/534 (PBS) | n.r. | n.r. | 1P | See footnote 2 | [33] |
| LD540 | 540/545.5 (oil) | n.r. | n.r. | 1P | Multicolor imaging in cells | [34] |
| LD-BTD1 | 420/511 (hexane) | 7100 | 0.66 (hexane) | 1P | Cells | [37] |
| AF8 | 380/479 (DMSO) | n.r. | 0.31 (DMSO) | 1P | Cells | [35] |
| AFN | 428/592 (DMSO) | n.r. | 0.17 (DMSO) | 1P | Cells | [35] |
| AF10 | 356/477 (DMSO) | n.r. | 0.18 (DMSO) | 1P | Cells | [35] |
| NAP-Ph | 409/523 (water) | n.r. | 0.018 (water) | 1P, 2P (δ2PA = 100 GM at 860 nm) | 1PE and 2PE imaging in cells and tissues | [36] |
| NAP-Br | 409/525 (water) | n.r. | 0.014 (water) | 1P, 2P (δ2PA = ~50 GM at 860 nm) | 1PE and 2PE imaging in cells and tissues | [36] |
| NAP-CF3 | 425/560 (water) | n.r. | 0.016 (water) | 1P, 2P (δ2PA = ~50 GM at 860 nm) | 1PE and 2PE imaging in cells and tissues | [36] |
| NAP-Py | 413/541 (water) | n.r. | 0.015 (water) | 1P, 2P (δ2PA = 45 GM at 860 nm) | 1PE and 2PE imaging in cells and tissues | [36] |
| LipiDye | 415 (toluene) | 18,700 | 0.94 (toluene) | 1P | Cells | [38,39] |
| PA | 434/521 (toluene) | 25,000 | 0.95 (toluene) | 1P, 2P (δ2PA =35 GM at 820 nm) | Lipid order in cells | [40] |
| Phos 2a | 439/554 (DMSO) | n.r. | n.r. | 1P | Cells | [41] |
| Phos 3a | 429/512 (DMSO) | n.r. | n.r. | 1P | Cells | [41] |
| Phos 2b | 437/554 (DMSO) | n.r. | n.r. | 1P | Not applicable for cells | [41] |
| Phos 3b | 374/550 (DMSO) | n.r. | n.r. | 1P | Not applicable for cells | [41] |
| P1 | 428/500 (Dioxane) | n.r. | 0.22 | 1P | Cells | [42] |
| Orange | ||||||
| SF44 | 455/626 (MeCN) | n.r. | 0.09 (MeCN) | 1P | Cells, HTS for LD modulator | [43] |
| SF58 | 440/623 (MeCN) | n.r. | 0.09 (MeCN) | 1P | Cells and algae | [44] |
| FAS | 322/600 (water) b | n.r. | 0.021 (solid state) | 1P | Cells | [45] |
| DPAS | 301/550 (water) b | n.r. | 0.03 (solid state | 1P | Cells | [45] |
| BTD-Lip | 455/624 (MeCN) | 7586 | 0.2 (MeCN) | 1P | Cells and worms | [46] |
| IND-TPA | 478/594 (THF) | n.r. | 0.069 (THF) | 1P, 2P (δ2PA = 119 GM at 920 nm) | 1PE and 2PE imaging in cells | [47] |
| BZT 3a | 309/576 (water) | n.r. | n.r. | 1P, 2P at 780 nm | 1PE and 2PE imaging in cells | [49] |
| BZT 4a | -/570 (solid) | n.r. | 0.4 (solid state) | 1P, 2P at 780 nm | 1PE and 2PE imaging in cells | [49] |
| Red to NIR | ||||||
| LD-TPZn | 599/630 (DMSO) | 10,600 | 0.44 (DMSO) | 1P, 2P (δ2PA = 110 GM at 880 nm) | 1PE and 2PE imaging in cells | [50] |
| LQD | 495/600 (colloidal) | n.r. | n.r. | 1P | Cells | [51] |
| PhotoAFN 2a | 409/624 (THF) | n.r. | 0.006 (THF) | 1P | Cells | [52] |
| PhotoAFN 2b | 402/617 (THF) | n.r. | 0.005 (THF) | 1P | Cells | [52] |
| PhotoAFN 2c | 400/610 (THF) | n.r. | 0.008 (THF) | 1P | Cells | [52] |
| TPE-AC | 455/724 (THF) | n.r. | 0.05 (solid state) | 1P | Cells | [53] |
| TPMN | 441/635 (MeCN) | n.r. | 0.0021 (MeCN) | 1P | Cells and zebrafish | [54] |
| TTMN | 483/664 (MeCN) | n.r. | 0.0032 (MeCN) | 1P | Cells and zebrafish | [54] |
| MeTTMN | 441/635 (MeCN) | n.r. | 0.0021 (MeCN) | 1P | Cells and zebrafish | [54] |
| MeOTTMN | 499/-(MeCN) | n.r. | n.r. | 1P | Cells | [54] |
| DCMa | 478/665 (water) | n.r. | 0.296 (solid state) | 1P, 2P (δ2PA = 394 GM at 940 nm) | 1PE and 2PE imaging in cells | [54] |
| DCIs | 510/709 (water) | n.r. | 0.135 (solid state) | 1P, 2P (δ2PA = 548 GM at 980 nm) | 1PE and 2PE imaging in cells | [55] |
| DCFu | 538/755 (water) | n.r. | 0.017 (solid state) | 1P, 2P (δ2PA = 887 GM at 1020 nm) | 1PE and 2PE imaging in cells | [55] |
| NLV-1 | 680/719 (glycerol) | n.r. | 0.204 (glycerol) | 1P | See footnote 3 | [56] |
| Multicolor | ||||||
| LipidTox™ | Analysis of steatosis | [57] | ||||
| green | 495/505 | n.r. | n.r. | 1P | [57] | |
| red | 577/609 | n.r. | n.r. | 1P | [57] | |
| deep red | 637/655 | n.r. | n.r. | 1P | [57] | |
| SMCy family | See footnote 4 | [23] | ||||
| SMCy3 | 512/541 (oil) | 82,900 | 0.21 (oil) | 1P, 2P (δ2PA = 178 GM at 690 nm, DMSO) | [23] | |
| SMCy3.5 | 530/559 (oil) | 100,000 | 0.4 (oil) | 1P, 2P (δ2PA = 2400 GM at 760 nm, DMSO) | [23] | |
| SMCy5 | 618/648 (oil) | 256,000 | 0.6 (oil) | 1P, 2P (δ2PA = 6250 GM at 740 nm, DMSO) | [23] | |
| SMCy5.5 | 638/662 (oil) | 169,000 | 0.74 (oil) | 1P, 2P (δ2PA = 13330 GM at 770 nm and 10400 GM at 820 nm, DMSO) | [23] | |
| SMCy7 | 692/744 (oil) | 44,000 | 0.42 (oil) | 1P | [23] | |
| SMCy7.5 | 716/753 (oil) | 103,000 | 0.19 (oil) | 1P | [23] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fam, T.K.; Klymchenko, A.S.; Collot, M. Recent Advances in Fluorescent Probes for Lipid Droplets. Materials 2018, 11, 1768. https://doi.org/10.3390/ma11091768
Fam TK, Klymchenko AS, Collot M. Recent Advances in Fluorescent Probes for Lipid Droplets. Materials. 2018; 11(9):1768. https://doi.org/10.3390/ma11091768
Chicago/Turabian StyleFam, Tkhe Kyong, Andrey S. Klymchenko, and Mayeul Collot. 2018. "Recent Advances in Fluorescent Probes for Lipid Droplets" Materials 11, no. 9: 1768. https://doi.org/10.3390/ma11091768