Fabrication of Porous Al2O3 Ceramics with Submicron-Sized Pores Using a Water-Based Gelcasting Method
Abstract
:1. Introduction
2. Experimental
2.1. Material Preparation
2.2. Material Characterization
3. Results and Discussion
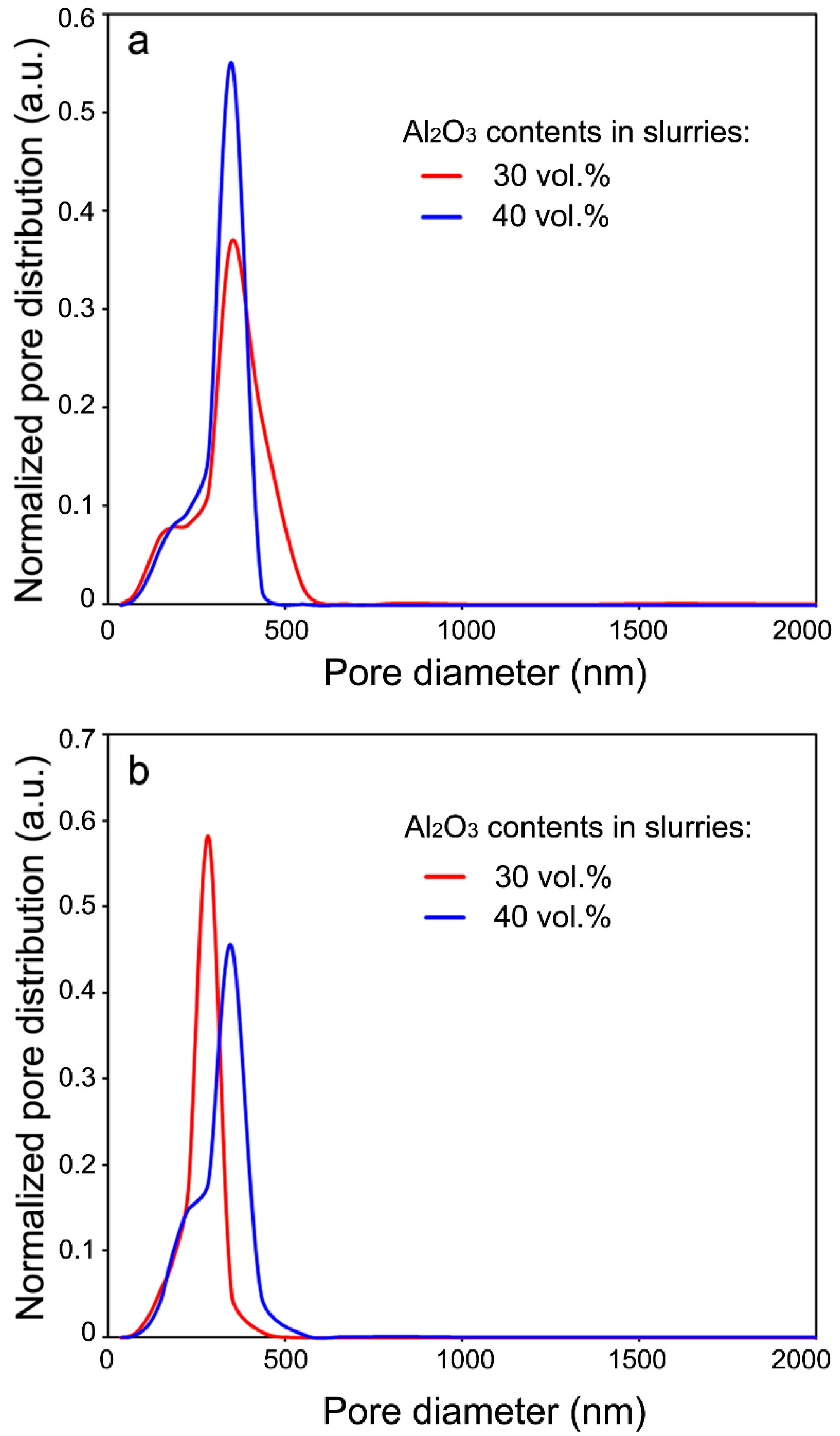
3.1. Microstructural Characteristics
3.2. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hammel, E.C.; Ighodaro, O.L.-R.; Okoli, O.I. Processing and properties of advanced porous ceramics: An application based review. Ceram. Int. 2014, 40, 15351–15370. [Google Scholar]
- Li, D.; Li, M. Porous Y2SiO5 Ceramic with low thermal conductivity. J. Mater. Sci. Technol. 2012, 28, 799–802. [Google Scholar] [CrossRef]
- Sobsey, M.D.; Stauber, C.E.; Casanova, L.M.; Brown, J.M.; Elliott, M.A. Point of use household drinking water filtration: A practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ. Sci. Technol. 2008, 42, 4261–4267. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, S. Hot gas filtration—A review. Fuel 2013, 104, 83–94. [Google Scholar] [CrossRef]
- Sundaram, S.; Colombo, P.; Katoh, Y. Selected emerging opportunities for ceramics in energy, environment, and transportation. Int. J. Appl. Ceram. Technol. 2013, 10, 731–739. [Google Scholar] [CrossRef]
- Zhou, M.; Shu, D.; Li, K.; Zhang, W.Y.; Ni, H.J.; Sun, B.D.; Wang, J. Deep filtration of molten aluminum using ceramic foam filters and ceramic particles with active coatings. Metall. Mater. Trans. A 2003, 34, 1183–1191. [Google Scholar] [CrossRef]
- Olson, R.A.; Martins, L.C.B. Cellular ceramics in metal filtration. Adv. Eng. Mater. 2005, 7, 187–192. [Google Scholar] [CrossRef]
- Deville, S.; Saiz, E.; Tomsia, A.P. Freeze casting of hydroxyapatite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 5480–5489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moene, R.; Makkee, M.; Moulijn, J.A. High surface area silicon carbide as catalyst support characterization and stability. Appl. Catal. A Gen. 1998, 167, 321–330. [Google Scholar] [CrossRef]
- Linul, E.; Movahedi, N.; Marsavina, L. On the lateral compressive behavior of empty and ex-situ Aluminum foam-filled tubes at high temperature. Materials 2018, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Linul, E.; Movahedi, N.; Marsavina, L. The temperature and anisotropy effect on compressive behavior of cylindrical closed-cell aluminum-alloy foams. J. Alloy. Compd. 2018, 740, 1172–1179. [Google Scholar] [CrossRef]
- Chang, S.; Li, L.; Lu, L.; Fuh, J.Y.H. Selective laser sintering of porous silica enabled by carbon additive. Materials 2017, 10, 1313. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Haniu, H.; Kamanaka, T.; Takizawa, T.; Sobajima, A.; Yoshida, K.; Aoki, K.; Okamoto, M.; Kato, H.; Saito, N. Physico-chemical, in vitro, and in vivo evaluation of a 3D unidirectional porous hydroxyapatite scaffold for bone regeneration. Materials 2017, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-P.; Lin, D.-J.; Yeh, M.-L. Phenolic modified ceramic coating on biodegradable Mg alloy: The improved corrosion resistance and osteoblast-like cell activity. Materials 2017, 10, 696. [Google Scholar] [CrossRef] [PubMed]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Liu, R.; Xu, T.; Wang, C.-A. A review of fabrication strategies and applications of porous ceramics prepared by freeze-casting method. Ceram. Int. 2016, 42, 2907–2925. [Google Scholar] [CrossRef]
- Sofie, S.W.; Dogan, F. Freeze casting of aqueous alumina slurries with glycerol. J. Am. Ceram. Soc. 2001, 84, 1459–1464. [Google Scholar] [CrossRef]
- Fukasawa, T.; Deng, Z.-Y.; Ando, M.; Ohji, T.; Goto, Y. Pore structure of porous ceramics synthesized from water-based slurry by freeze-dry process. J. Mater. Sci. 2001, 36, 2523–2527. [Google Scholar] [CrossRef]
- Araki, K.; Halloran, J.W. Porous ceramic bodies with interconnected pore channels by a novel freeze casting technique. J. Am. Ceram. Soc. 2005, 88, 1108–1114. [Google Scholar] [CrossRef]
- Koh, Y.H.; Sun, J.J.; Kim, H.E. Freeze casting of porous Ni–YSZ cermets. Mater. Lett. 2007, 61, 1283–1287. [Google Scholar] [CrossRef]
- Chen, R.; Wang, C.-A.; Huang, Y.; Ma, L.; Lin, W. Ceramics with special porous structures fabricated by freeze-gelcasting: Using tert-butyl alcohol as a template. J. Am. Ceram. Soc. 2007, 90, 3478–3484. [Google Scholar] [CrossRef]
- Deville, S.; Saiz, E.; Nalla, R.K.; Tomsia, A.P. Freezing as a path to build complex composites. Science 2006, 311, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fidelis, C.L.; Serva, A.L.T.; Wilhelm, M.; Rezwan, K. Water-based freeze casting: Adjusting hydrophobic polymethylsiloxane for obtaining hierarchically ordered porous SiOC. J. Am. Ceram. Soc. 2017, 100, 1907–1918. [Google Scholar] [CrossRef]
- Fukushima, M.; Yoshizawa, Y.-I. Fabrication and morphology control of highly porous mullite thermal insulators prepared by gelation freezing route. J. Eur. Ceram. Soc. 2016, 36, 2947–2953. [Google Scholar] [CrossRef]
- Tang, Y.; Qiu, S.; Wu, C.; Miao, Q.; Zhao, K. Freeze cast fabrication of porous ceramics using tert-butyl alcohol–water crystals as template. J. Eur. Ceram. Soc. 2016, 36, 1513–1518. [Google Scholar] [CrossRef]
- Xu, T.; Wang, C.-A. Effect of two-step sintering on micro-honeycomb BaTiO3 ceramics prepared by freeze-casting process. J. Eur. Ceram. Soc. 2016, 36, 2647–2652. [Google Scholar] [CrossRef]
- Liu, X.; Xue, W.; Shi, C.; Sun, J. Fully interconnected porous Al2O3 scaffolds prepared by a fast cooling freeze casting method. Ceram. Int. 2015, 41, 11922–11926. [Google Scholar] [CrossRef]
- Franks, G.V.; Tallon, C.; Studart, A.R.; Sesso, M.L.; Leo, S. Colloidal processing: Enabling complex shaped ceramics with unique multiscale structures. J. Am. Ceram. Soc. 2017, 100, 458–490. [Google Scholar] [CrossRef]
- Leo, S.; Tallon, C.; Stone, N.; Franks, G.V. Near-net-shaping methods for ceramic elements of (body) armor systems. J. Am. Ceram. Soc. 2014, 97, 3013–3033. [Google Scholar] [CrossRef]
- Hu, Y.; Du, G.; Chen, N. A novel approach for Al2O3/epoxy composites with high strength and thermal conductivity, Compos. Sci. Technol. 2016, 124, 36–43. [Google Scholar]
- Barsoum, M.W. Fundamentals of Ceramics; McGraw Hill: New York, NY, USA, 1997. [Google Scholar]
- Sun, Z.; Fan, J.; Hu, P.; Ding, F.; Yang, J.; Yuan, F. A novel low-temperature strategy for synthesis of alumina ceramics with uniform and interconnected pores by silica coating. J. Mater. Sci. 2017, 52, 1603–1616. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Li, Y.; Sang, S.; Li, S. Effects of pore structure on thermal conductivity and strength of alumina porous ceramics using carbon black as pore-forming agent. Ceram. Int. 2016, 42, 8221–8228. [Google Scholar] [CrossRef]

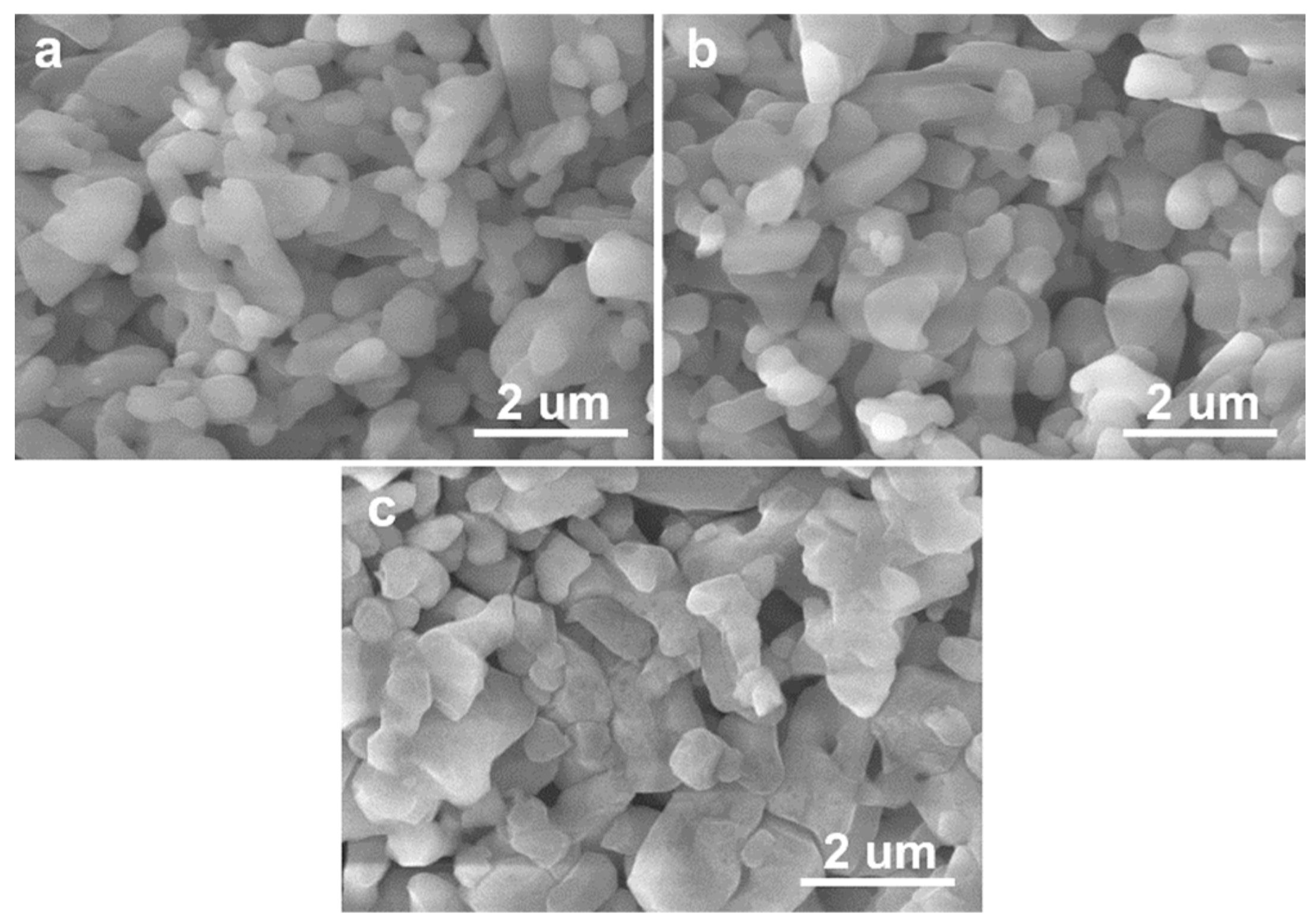
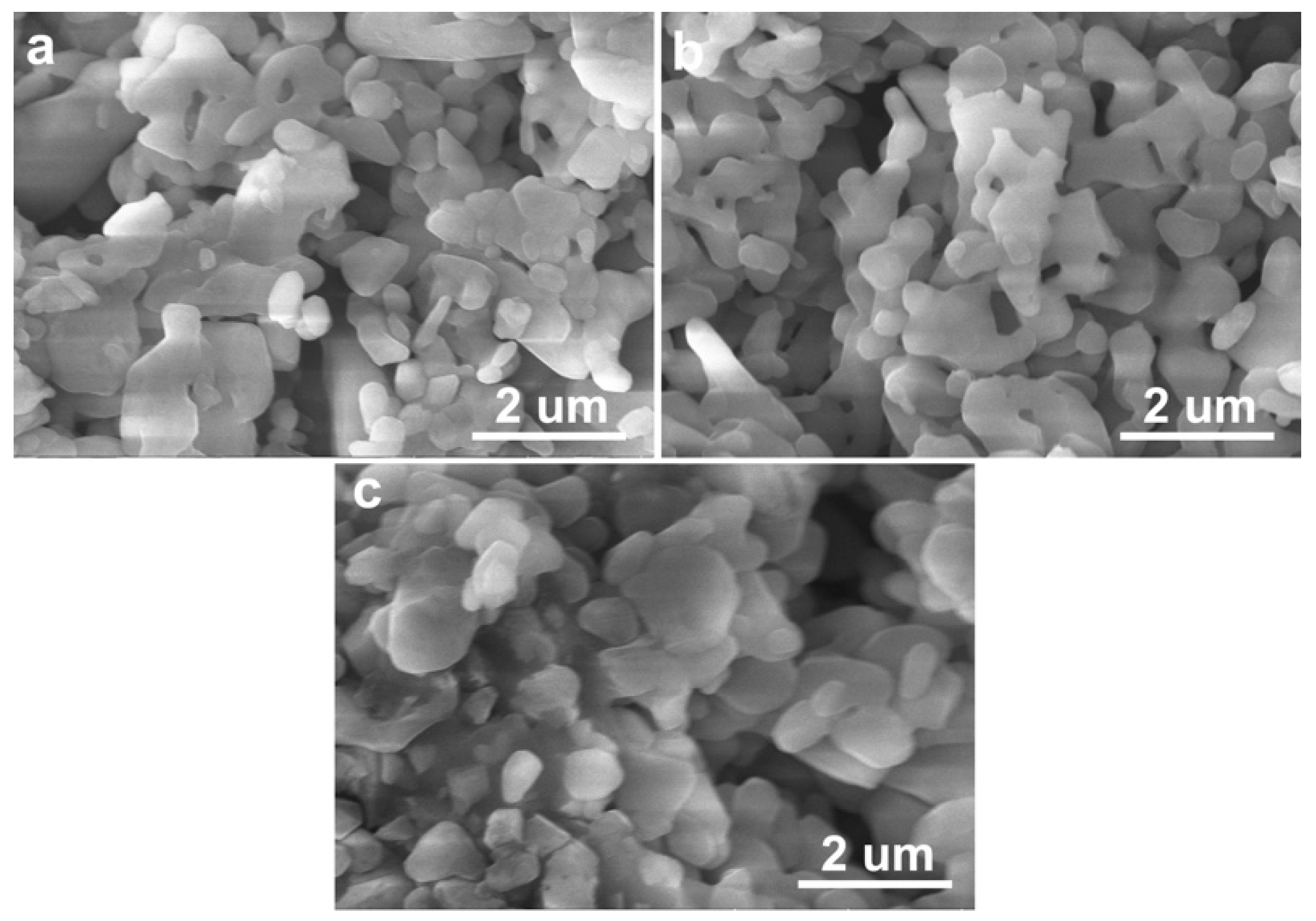
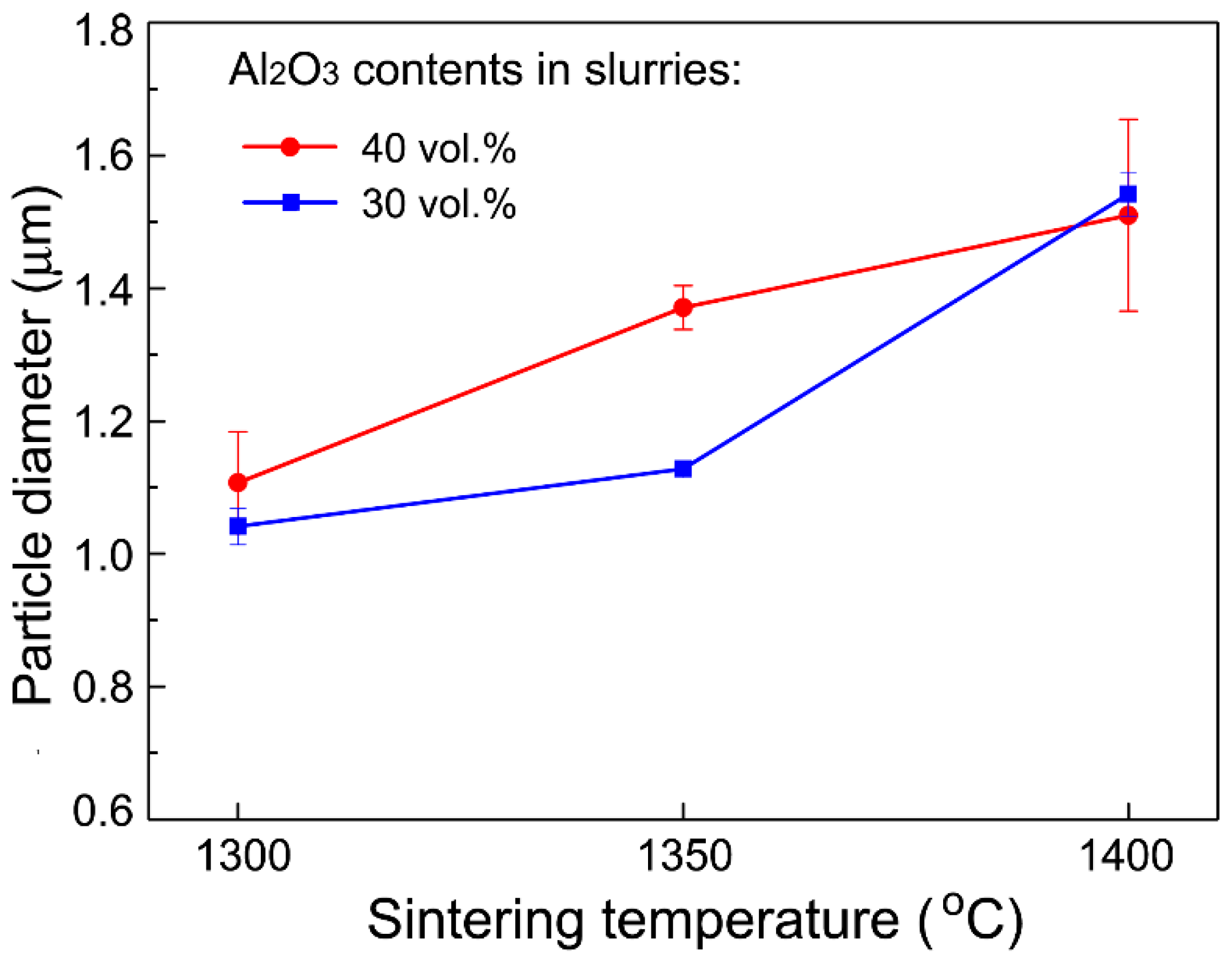
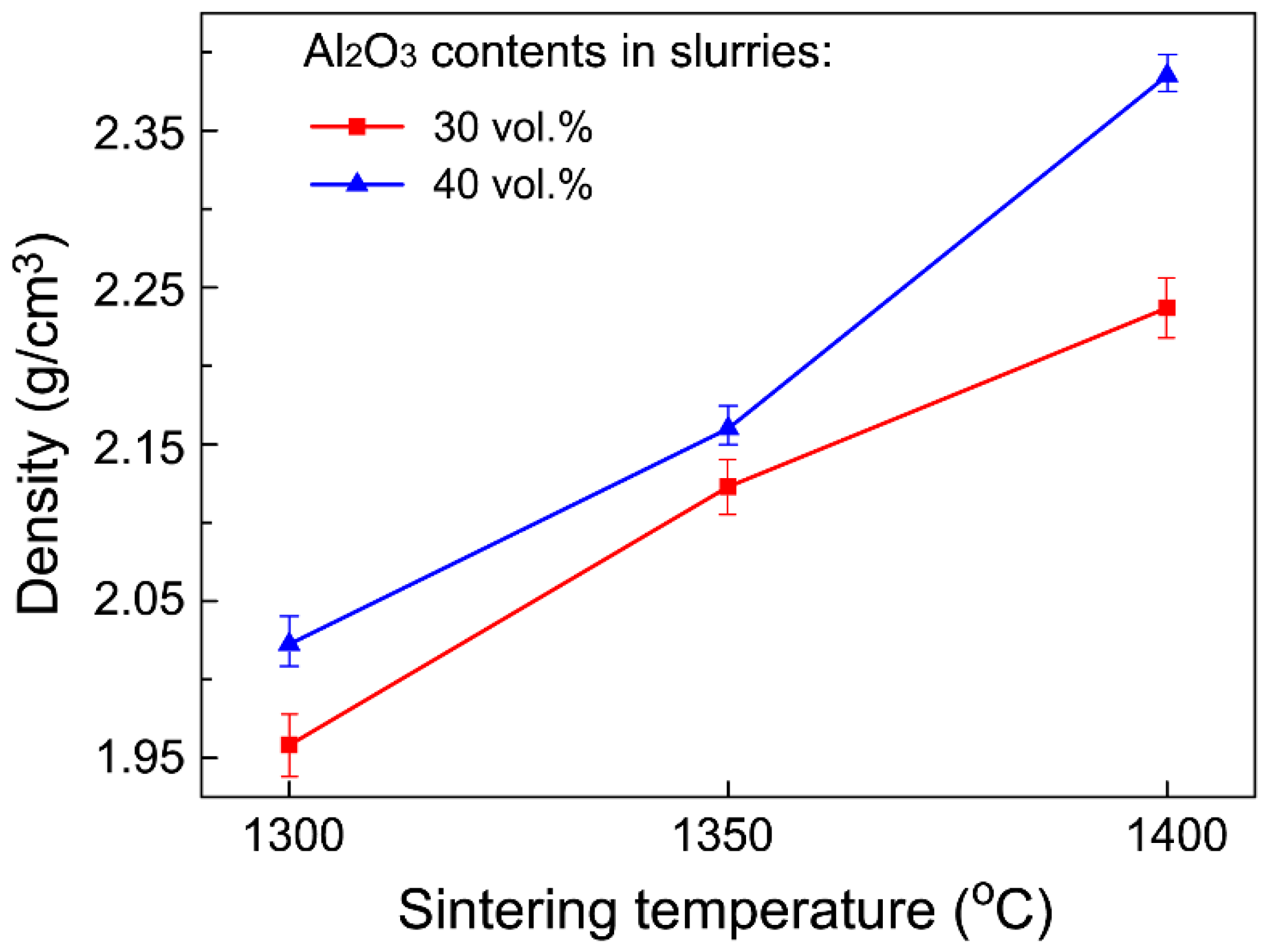

| Sintering Temperature (°C) | Open Porosity (%) | Closed Porosity (%) | Median Pore Diameter (nm) |
|---|---|---|---|
| 1300 | 47.2 | 1.6 | 371.9 |
| 1350 | 42.5 | 2.2 | 330.6 |
| 1400 | 38.3 | 3.2 | 299.8 |
| Sintering Temperature (°C) | Open Porosity (%) | Closed Porosity (%) | Median Pore Diameter (nm) |
|---|---|---|---|
| 1300 | 46.5 | 0.8 | 363.1 |
| 1350 | 41.7 | 1.9 | 358.5 |
| 1400 | 37.0 | 0.9 | 355.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Chen, N.; Qin, X. Fabrication of Porous Al2O3 Ceramics with Submicron-Sized Pores Using a Water-Based Gelcasting Method. Materials 2018, 11, 1784. https://doi.org/10.3390/ma11091784
Yang Z, Chen N, Qin X. Fabrication of Porous Al2O3 Ceramics with Submicron-Sized Pores Using a Water-Based Gelcasting Method. Materials. 2018; 11(9):1784. https://doi.org/10.3390/ma11091784
Chicago/Turabian StyleYang, Zhihong, Nan Chen, and Xiaomei Qin. 2018. "Fabrication of Porous Al2O3 Ceramics with Submicron-Sized Pores Using a Water-Based Gelcasting Method" Materials 11, no. 9: 1784. https://doi.org/10.3390/ma11091784




