The Effects of Using Aluminum Oxide Nanoparticles as Heat Transfer Fillers on Morphology and Thermal Performances of Form-Stable Phase Change Fibrous Membranes Based on Capric–Palmitic–Stearic Acid Ternary Eutectic/Polyacrylonitrile Composite
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of Electrospun PAN/Al2O3 Supporting Membranes
2.3. Fabrication of CA–PA–SA/PAN/Al2O3 Form-Stable PCCFMs
2.4. Characterizations
2.4.1. Scanning Electron Microscopy
2.4.2. Differential Scanning Calorimeter
2.4.3. Measurement of Melting and Freezing Times
3. Results and Discussion
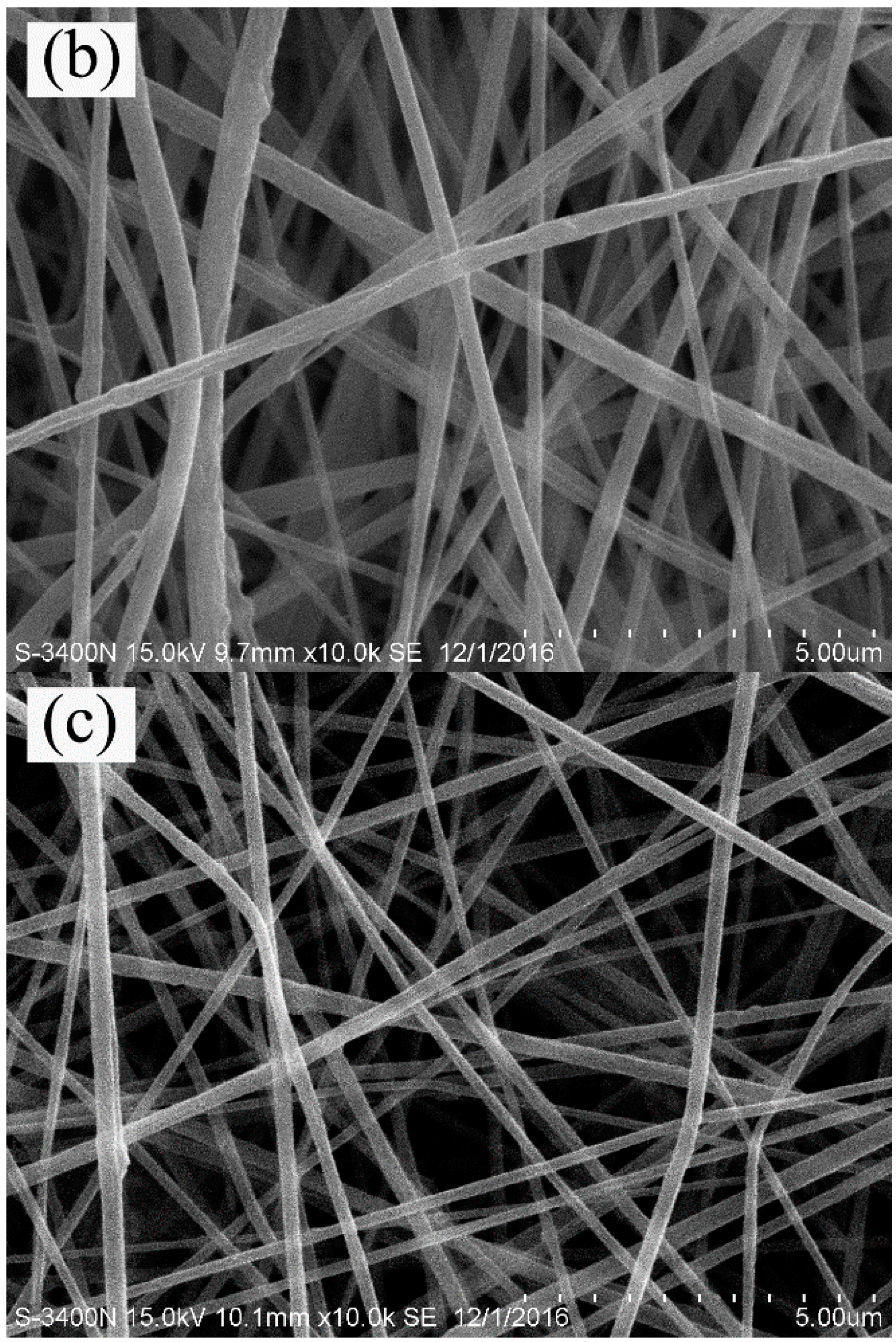
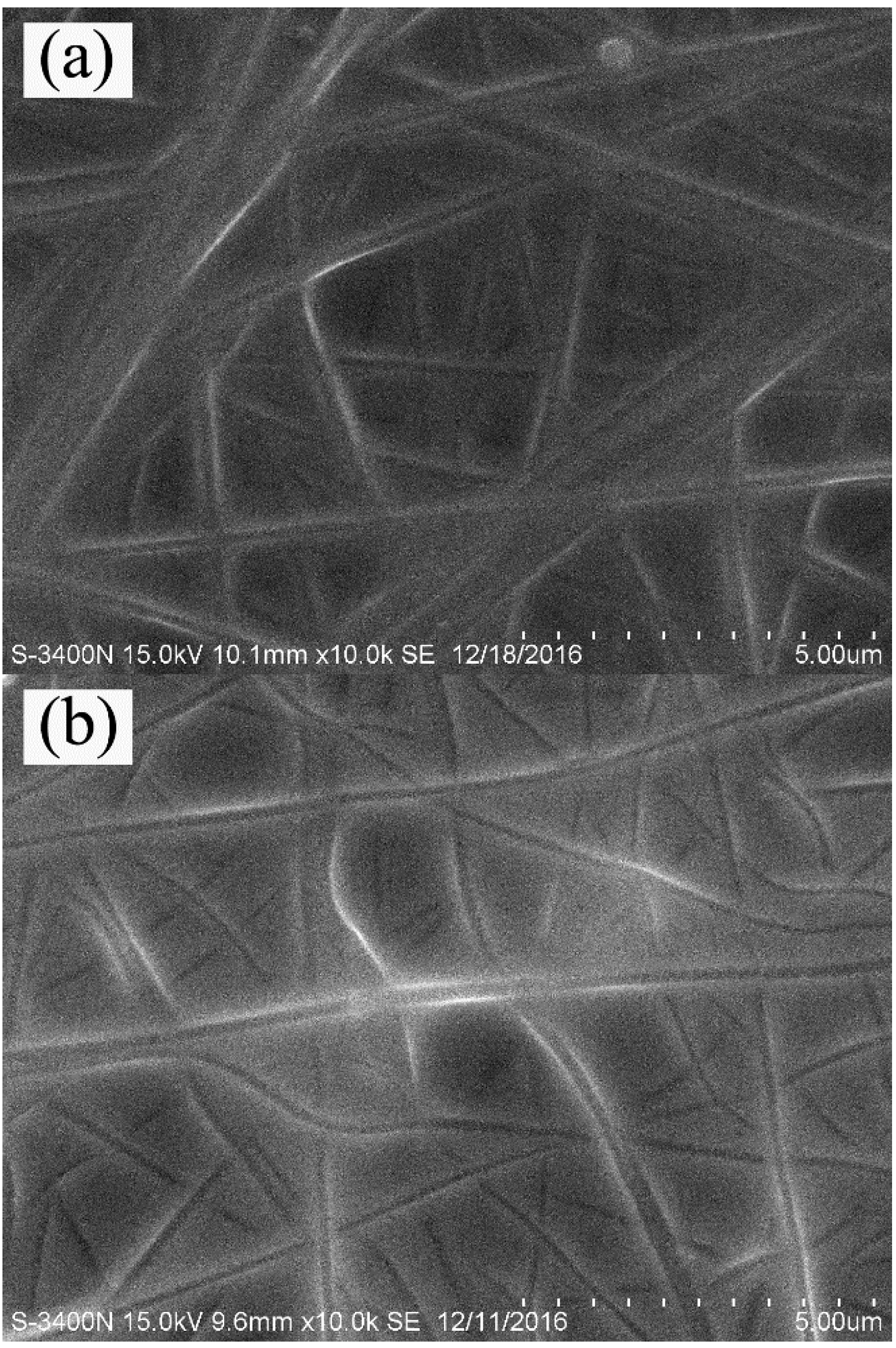
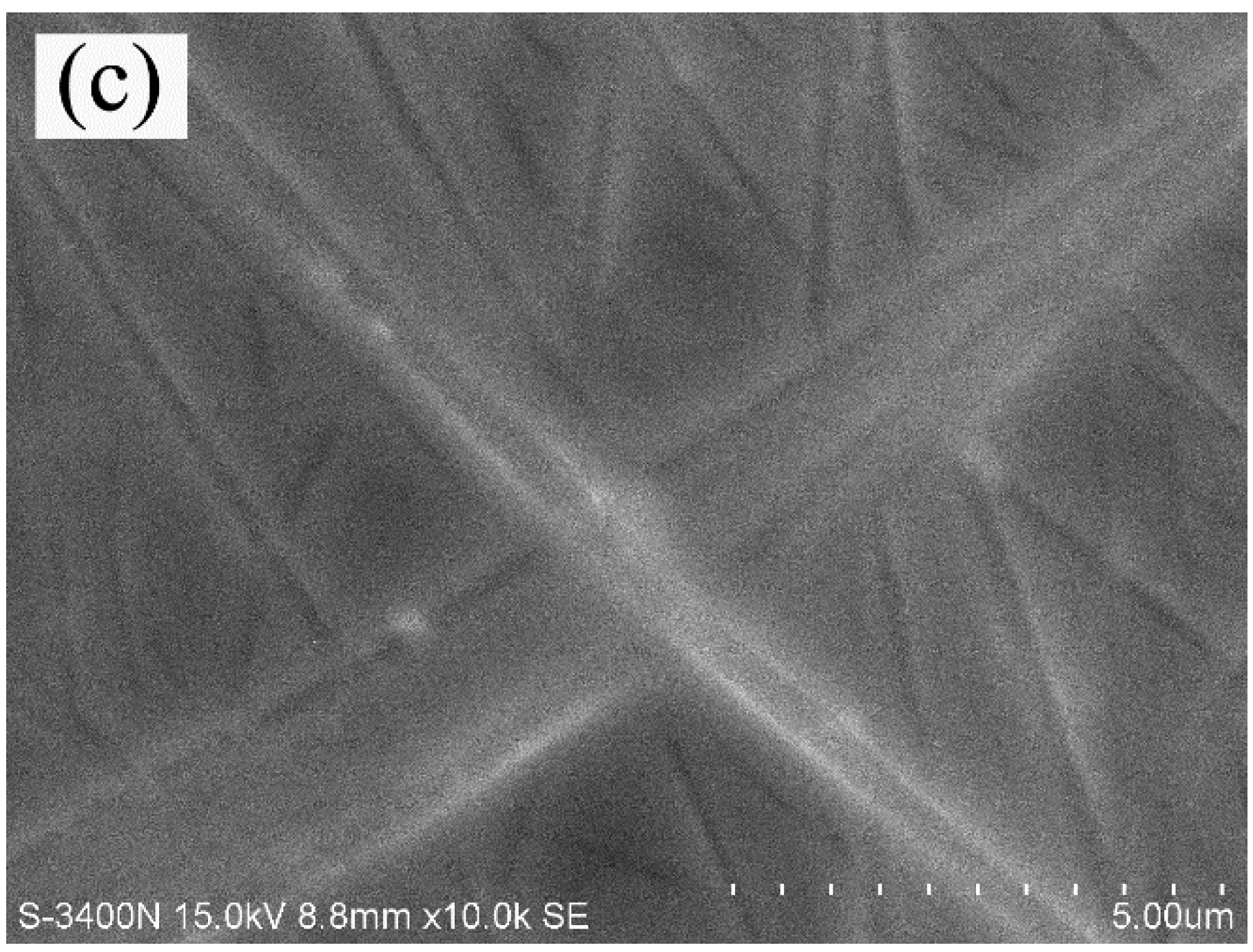
3.1. Morphological Structure
3.2. Thermal Energy Storage Properties
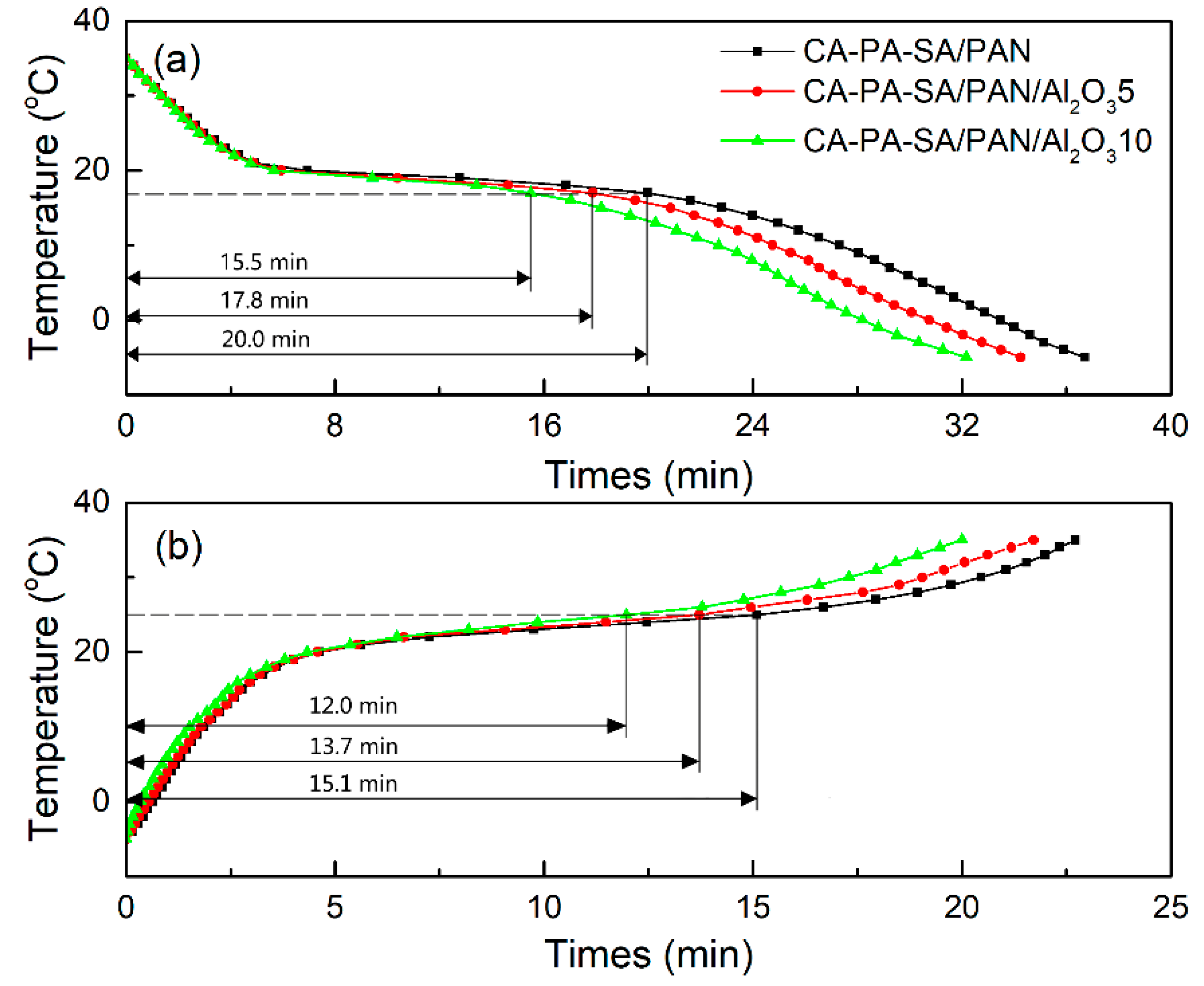
3.3. Thermal Energy Storage and Release Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Serale, G.; Goia, F.; Perino, M. Numerical model and simulation of a solar thermal collector with slurry phase change material (PCM) as the heat transfer fluid. Sol. Energy 2016, 134, 429–444. [Google Scholar] [CrossRef]
- Zhang, S.L.; Wu, W.; Wang, S.F. Integration highly concentrated photovoltaic module exhaust heat recovery system with adsorption air-conditioning module via phase change materials. Energy 2017, 118, 1187–1197. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, M.Z.; Liu, Q.T.; Wan, J.M.; Hu, J.X. Preparation and thermal properties of molecular-bridged expanded graphite/polyethylene glycol composite phase change materials for building energy conservation. Materials 2018, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, R.; Principi, P.; Copertaro, B. A refrigerated container envelope with a PCM (phase change material) layer: Experimental and theoretical investigation in a representative town in Central Italy. Energy Convers. Manag. 2016, 122, 131–141. [Google Scholar] [CrossRef]
- Ziapour, B.M.; Hashtroudi, A. Performance study of an enhanced solar greenhouse combined with the phase change material using genetic algorithm optimization method. Appl. Therm. Eng. 2017, 110, 253–264. [Google Scholar] [CrossRef]
- Jiang, G.W.; Huang, J.H.; Fu, Y.S.; Cao, M.; Liu, M.C. Thermal optimization of composite phase change material/expanded graphite for Li-ion battery thermal management. Appl. Therm. Eng. 2016, 108, 1119–1125. [Google Scholar] [CrossRef]
- Iqbal, K.; Sun, D.M. Development of thermal stable multifilament yarn containing micro-encapsulated phase change materials. Fiber. Polym. 2015, 16, 1156–1162. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A. Thermal cycle test of binary mixtures of some fatty acids as phase change materials for building applications. Energy Build. 2015, 99, 196–203. [Google Scholar] [CrossRef]
- Li, X.Y.; Chen, H.S.; Liu, L.; Lu, Z.Y.; Sanjayan, J.G.; Duan, W.H. Development of granular expanded perlite/paraffin phase change material composites and prevention of leakage. Sol. Energy 2016, 137, 179–188. [Google Scholar] [CrossRef]
- Tang, F.; Su, D.; Tang, Y.J.; Fang, G.Y. Synthesis and thermal properties of fatty acid eutectics and diatomite composites as shape-stabilized phase change materials with enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2015, 141, 218–224. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Z.H.; Zeng, X.R.; Gao, X.N.; Zhang, Z.G. Poly(methyl methacrylate) copolymer nanocapsules containing phase-change material (n-dodecanol) prepared via miniemulsion polymerization. J. Appl. Polym. Sci. 2015, 132, 42334. [Google Scholar] [CrossRef]
- Sobolciak, P.; Abdelrazeq, H.; Ouederni, M.; Karkri, M.; Al-Maadeed, M.A.; Krupa, I. The stabilizing effect of expanded graphite on the artificial aging of shape stabilized phase change materials. Polym. Test. 2015, 46, 65–71. [Google Scholar] [CrossRef]
- Li, X.Q.; Wei, H.T.; Lin, X.S.; Xie, X.Z. Preparation of stearic acid/modified expanded vermiculite composite phase change material with simultaneously enhanced thermal conductivity and latent heat. Sol. Energy Mater. Sol. Cells 2016, 155, 9–13. [Google Scholar] [CrossRef]
- Tian, B.Q.; Yang, W.B.; Luo, L.J.; Wang, J.; Zhang, K.; Fan, J.H.; Wu, J.Y.; Xing, T. Synergistic enhancement of thermal conductivity for expanded graphite and carbon fiber in paraffin/EVA form-stable phase change materials. Sol. Energy 2016, 127, 48–55. [Google Scholar] [CrossRef]
- Ke, H.Z.; Li, Y.G. A series of electrospun fatty acid ester/polyacrylonitrile phase change composite nanofibers as novel form-stable phase change materials for storage and retrieval of thermal energy. Text. Res. J. 2017, 87, 2314–2322. [Google Scholar] [CrossRef]
- Cai, Y.B.; Gao, C.T.; Xu, X.L.; Fu, Z.; Fei, X.Z.; Zhao, Y.; Chen, Q.; Liu, X.Z.; Wei, Q.F.; He, G.F.; et al. Electrospun ultrafine composite fibers consisting of lauric acid and polyamide 6 as form-stable phase change materials for storage and retrieval of solar thermal energy. Sol. Energy Mater. Sol. Cells 2012, 103, 53–61. [Google Scholar] [CrossRef]
- Ke, H.Z.; Li, D.W.; Wang, X.L.; Wang, H.; Cai, Y.B.; Xu, Y.; Huang, F.L.; Wei, Q.F. Thermal and mechanical properties of nanofibers-based form-stable PCMs consisting of glycerol monostearate and polyethylene terephthalate. J. Therm. Anal. Calorim. 2013, 114, 101–111. [Google Scholar] [CrossRef]
- Fang, Y.T.; Kang, H.Y.; Wang, W.L.; Liu, H.; Gao, X.N. Study on polyethylene glycol/epoxy resin composite as a form-stable phase change material. Energy Convers. Manag. 2010, 51, 2757–2761. [Google Scholar] [CrossRef]
- Ke, H.Z.; Ghulam, M.U.H.; Li, Y.G.; Wang, J.; Peng, B.; Cai, Y.B.; Wei, Q.F. Ag-coated polyurethane fibers membranes absorbed with quinary fatty acid eutectics solid-liquid phase change materials for storage and retrieval of thermal energy. Renew. Energy 2016, 99, 1–9. [Google Scholar] [CrossRef]
- Cai, Y.B.; Song, X.F.; Liu, M.M.; Li, F.; Xie, M.S.; Cai, D.L.; Wei, Q.F. Flexible cellulose acetate nano-felts absorbed with capric-myristic-stearic acid ternary eutectic mixture as form-stable phase-change materials for thermal energy storage/retrieval. J. Therm. Anal. Calorim. 2017, 128, 661–673. [Google Scholar] [CrossRef]
- Fashandi, M.; Leung, S.N. Preparation and characterization of 100% bio-based polylactic acid/palmitic acid microcapsules for thermal energy storage. Mater. Renew. Sustain. Energy 2017, 6, 1–14. [Google Scholar]
- Zhang, P.; Meng, Z.N.; Zhu, H.; Wang, Y.L.; Peng, S.P. Melting heat transfer characteristics of a composite phase change material fabricated by paraffin and metal foam. Appl. Energy 2017, 185, 1971–1983. [Google Scholar] [CrossRef]
- Karaipekli, A.; Biçer, A.; Sarı, A.; Tyagi, V.V. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]
- Yang, J.; Qi, G.Q.; Liu, Y.; Bao, R.Y.; Liu, Z.Y.; Yang, W.; Xie, B.H.; Yang, M.B. Hybrid graphene aerogels/phase change material composites: Thermal conductivity, shape-stabilization and light-to-thermal energy storage. Carbon 2016, 100, 693–702. [Google Scholar] [CrossRef]
- Su, D.; Jia, Y.T.; Alva, G.; Tang, F.; Fang, G.Y. Preparation and thermal properties of n-octadecane/stearic acideutectic mixtures with hexagonal boron nitride as phase change materials for thermal energy storage. Energy Build. 2016, 131, 35–41. [Google Scholar] [CrossRef]
- Esfe, M.H.; Karimipour, A.; Yan, W.M.; Akbari, M.; Safaei, M.R.; Dahari, M. Experimental study on thermal conductivity of ethylene glycol based nanofluids containing Al2O3 nanoparticles. Int. J. Heat Mass Transf. 2015, 88, 728–734. [Google Scholar] [CrossRef]
- Ke, H.Z. Phase diagrams, eutectic mass ratios and thermal energy storage properties of multiple fatty acid eutectics as novel solid-liquid phase change materials for storage and retrieval of thermal energy. Appl. Therm. Eng. 2017, 113, 1319–1331. [Google Scholar] [CrossRef]
- Ke, H.Z.; Pang, Z.Y.; Peng, B.; Wang, J.; Cai, Y.B.; Huang, F.L.; Wei, Q.F. Thermal energy storage and retrieval properties of form-stable phase change nanofibrous mats based on ternary fatty acid eutectics/polyacrylonitrile composite by magnetron sputtering of silver. J. Therm. Anal. Calorim. 2016, 123, 1293–1307. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Q.L.; Zhang, Z.G.; Gao, X.N.; Huang, G.S. Investigation on the properties of a new type of concrete blocks incorporated with PEG/SiO2 composite phase change material. Build. Environ. 2016, 104, 172–177. [Google Scholar] [CrossRef]
- Mondal, S. Phase change materials for smart textiles-An overview. Appl. Therm. Eng. 2008, 28, 1536–1550. [Google Scholar] [CrossRef]



| Samples | Melting Process | Freezing Process | ||||||
|---|---|---|---|---|---|---|---|---|
| To (°C) | Tm (°C) | Te (°C) | ΔHm (kJ/kg) | To (°C) | Tf (°C) | Te (°C) | ΔHf (kJ/kg) | |
| CA–PA–SA | 19.72 | 25.12 | 33.08 | 145.7 | 17.64 | 15.60 | 6.29 | 144.5 |
| CA–PA–SA/PAN | 19.10 | 24.06 | 27.02 | 138.6 | 17.50 | 17.17 | 11.30 | 137.4 |
| CA–PA–SA/PAN/Al2O35 | 19.17 | 24.29 | 28.85 | 132.3 | 17.33 | 16.81 | 9.59 | 130.3 |
| CA–PA–SA/PAN/Al2O310 | 19.55 | 25.20 | 29.56 | 131.1 | 17.46 | 16.55 | 9.52 | 127.0 |
| Cycle No. | Melting Process | Freezing Process | ||||||
|---|---|---|---|---|---|---|---|---|
| To (°C) | Tm (°C) | Te (°C) | ΔHm (kJ/kg) | To (°C) | Tf (°C) | Te (°C) | ΔHf (kJ/kg) | |
| 50 cycles | 19.48 | 27.33 | 33.61 | 131.1 | 18.24 | 13.76 | 6.47 | 129.7 |
| 100 cycles | 19.47 | 26.93 | 33.39 | 130.6 | 18.25 | 13.84 | 6.51 | 129.4 |
| Form-Stable PCMs | Melting Process | Freezing Process | References | ||
|---|---|---|---|---|---|
| Tm (°C) | ΔHm (kJ/kg) | Tf (°C) | ΔHf (kJ/kg) | ||
| paraffin/EP | 25.10 | 63.30 | - | - | [9] |
| CA–PA/diatomite/EG | 26.69 | 98.26 | 21.85 | 90.03 | [10] |
| SA/aEVT | 65.90 | 146.8 | - | - | [13] |
| paraffin/EVA/EG–CF | 45.63 | 167.4 | - | - | [14] |
| LA/PA6 | 44.53 | 70.44 | 40.67 | 57.14 | [16] |
| GMS/PET | 57.89 | 66.99 | 46.65 | 66.02 | [17] |
| CA–MA–SA/CA | 21.80 | 69.60 | 14.50 | 68.80 | [20] |
| PEG/GO/GNP | 65.50 | 177.8 | 1.70 | 170.1 | [24] |
| CA–PA–SA/PAN | 24.06 | 138.6 | 17.17 | 137.4 | Present work |
| CA–PA–SA/PAN/Al2O35 | 24.29 | 132.3 | 16.81 | 130.3 | Present work |
| CA–PA–SA/PAN/Al2O310 | 25.20 | 131.1 | 16.55 | 127.0 | Present work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, H.; Li, Y. The Effects of Using Aluminum Oxide Nanoparticles as Heat Transfer Fillers on Morphology and Thermal Performances of Form-Stable Phase Change Fibrous Membranes Based on Capric–Palmitic–Stearic Acid Ternary Eutectic/Polyacrylonitrile Composite. Materials 2018, 11, 1785. https://doi.org/10.3390/ma11091785
Ke H, Li Y. The Effects of Using Aluminum Oxide Nanoparticles as Heat Transfer Fillers on Morphology and Thermal Performances of Form-Stable Phase Change Fibrous Membranes Based on Capric–Palmitic–Stearic Acid Ternary Eutectic/Polyacrylonitrile Composite. Materials. 2018; 11(9):1785. https://doi.org/10.3390/ma11091785
Chicago/Turabian StyleKe, Huizhen, and Yonggui Li. 2018. "The Effects of Using Aluminum Oxide Nanoparticles as Heat Transfer Fillers on Morphology and Thermal Performances of Form-Stable Phase Change Fibrous Membranes Based on Capric–Palmitic–Stearic Acid Ternary Eutectic/Polyacrylonitrile Composite" Materials 11, no. 9: 1785. https://doi.org/10.3390/ma11091785





