Effect of Recrystallization Annealing on Corrosion Behavior of Ta-4%W Alloy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, Z.J.; Xie, H.; Wang, B.J.; Wei, X.W.; Zhao, D.W. A novel Tantalum coating on porous SiC used for bone filling material. Mater. Lett. 2016, 179, 166–169. [Google Scholar] [CrossRef]
- Hosseini, E.; Kazeminezhad, M. Dislocation structure and strength evolution of heavily deformed tantalum. Int. J. Refract. Metals Hard Mater. 2009, 27, 605–610. [Google Scholar] [CrossRef]
- Yang, H.L.; Li, J.; Zhou, Z.C.; Ruan, J.M. Structural preparation and biocompatibility evaluation of highly porous Tantalum scaffolds. Mater. Lett. 2013, 100, 152–155. [Google Scholar] [CrossRef]
- Bischof, M.; Mayer, S.; Leitner, H.; Clemens, H.; Staron, P.; Geiger, E.; Voiticek, A.; Knabl, W. On the development of grain growth resistant tantalum alloys. Int. J. Refract. Metals Hard Mater. 2006, 24, 437–444. [Google Scholar] [CrossRef]
- Bermúdez, M.D.; Carrión, F.J.; Martínez-Nicolás, G.; López, R. Erosion–corrosion of stainless steels, titanium, tantalum and zirconium. Wear 2005, 258, 693–700. [Google Scholar] [CrossRef]
- Seidl, W.M.; Bartosik, M.; Kolozsvári, S.; Bolvardi, H.; Mayrhofer, P.H. Improved mechanical properties, thermal stabilities, and oxidation resistance of arc evaporated Ti-Al-N coatings through alloying with Ta. Surf. Coat. Technol. 2018, 344, 244–249. [Google Scholar] [CrossRef]
- Chen, Y.I.; Chen, S.M. Annealing effects on nanostructure and mechanical properties of nanolaminated Ta–Zr coatings. Surf. Coat. Technol. 2013, 215, 209–217. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Attarzadeh, F.R.; Vafaeian, S.; Haghshenas, M.; Keshavarz, M.K. Electrochemical behavior assessment of tantalum in aqueous KOH solutions. Int. J. Refract. Metals Hard Mater. 2017, 64, 168–175. [Google Scholar] [CrossRef]
- Silva, R.A.; Walls, M.; Rondot, B.; Da Cunha Belo, M.; Guidoin, R. Electrochemical and microstructural studies of tantalum and its oxide films for biomedical applications in endovascular surgery. J. Mater. Sci. Mater. Med. 2002, 13, 495–500. [Google Scholar] [CrossRef]
- Robin, A.; Rosa, J.L. Corrosion behavior of niobium, tantalum and their alloys in hot hydrochloric and phosphoric acid solutions. Int. J. Refract. Metals Hard Mater. 2000, 18, 13–21. [Google Scholar] [CrossRef]
- Ghorbani, H.; Abdollah-Zadeh, A.; Bagheri, F.; Poladi, A. Improving the bio-corrosion behavior of AISI316L stainless steel through deposition of Ta-based thin films using PACVD. Appl. Surf. Sci. 2018, 456, 398–402. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Jiang, C.; Li, Y.; Fu, Y. In vitro corrosion behavior and cytocompatibility of pure Fe implanted with Ta. Surf. Coat. Technol. 2017, 320, 201–205. [Google Scholar] [CrossRef]
- Wei, D.B.; Chen, X.H.; Zhang, P.Z.; Ding, F.; Li, F.K.; Yao, Z.J. Plasma surface tantalum alloying on titanium and its corrosion behavior in sulfuric acid and hydrochloric acid. Appl. Surf. Sci. 2018, 441, 448–457. [Google Scholar] [CrossRef]
- Robin, A. Corrosion behavior of niobium, tantalum and their alloys in boiling sulfuric acid solutions. Int. J. Refract. Metals Hard Mater. 1997, 15, 317–323. [Google Scholar] [CrossRef]
- Souza, A.D.; Robin, A. Influence of concentration and temperature on the corrosion behavior of titanium, titanium-20 and 40% tantalum alloys and tantalum in sulfuric acid solutions. Mater. Chem. Phys. 2007, 103, 351–360. [Google Scholar] [CrossRef]
- Friedrich, C.; Kritzer, P.; Boukis, N.; Frans, G.; Dinjus, E. The corrosion of tantalum in oxidizing sub- and supercritical aqueous solutions of HCl, H2SO4 and H3PO4. J. Mater. Sci. 1999, 34, 3137–3141. [Google Scholar] [CrossRef]
- Piotrowski, O.; Madore, C.; Landolt, D. Electropolishing of tantalum in sulfuric acid-methanol electrolytes. Electrochim. Acta 1999, 44, 3389–3399. [Google Scholar] [CrossRef]
- Carmo, D.R.D.; Picone, C.A. Corrosion resistance of niobium, tantalum and titanium in sea water and sulfuric acid. Electrochem. Soc. 2013, 224, 1722. [Google Scholar]
- Zhang, J.; Ma, G.Q.; Godfrey, A.; Shu, D.Y.; Chen, Q.; Wu, G.L. Orientation dependence of the deformation microstructure of Ta-4%W after cold-rolling. IOP Conf. Ser. Mater. Sci. Eng. 2017, 219, 012051. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.Z.; Dong, C.F.; Xiao, K.; Li, X.G. Effect of cold deformation on the corrosion behaviour of UNS S31803 duplex stainless steel in simulated concrete pore solution. Corros. Sci. 2017, 124, 178–192. [Google Scholar] [CrossRef]
- Brandon, D.G. The structure of high-angle grain boundaries. Acta Metall. 1966, 14, 1479–1484. [Google Scholar] [CrossRef]
- Hughes, D.A.; Hansen, N.; Bammann, D.J. Geometrically necessary boundaries, incidental dislocation boundaries and geometrically necessary dislocations. Scr. Mater. 2003, 48, 147–153. [Google Scholar] [CrossRef]
- Rofagha, R.; Erb, U.; Ostrander, D.; Palumbo, G.; Aust, K.T. The effects of grain size and phosphorus on the corrosion of nanocrystalline Ni-P alloys. Nanostruct. Mater. 1993, 2, 1–10. [Google Scholar] [CrossRef]
- Liu, Q.; Hansen, N. Geometrically necessary boundaries and incidental dislocation boundaries formed during cold deformation. Scr. Metall. Mater. 1995, 32, 1289–1295. [Google Scholar] [CrossRef]
- Liu, Q.; Jensen, D.; Hansen, N. Effect of grain orientation on deformation structure in cold-rolled polycrystalline aluminium. Acta Mater. 1998, 46, 5819–5838. [Google Scholar] [CrossRef]
- Hughes, D.A.; Hansen, N. Microstructural evolution in nickel during rolling from intermediate to large strains. Metall. Trans. A 1993, 24, 2022–2037. [Google Scholar] [CrossRef]
- Hughes, D.A.; Hansen, N. Microstructure and strength of nickel at large strains. Acta Mater. 2000, 48, 2985–3004. [Google Scholar] [CrossRef]
- Li, B.L.; Godfrey, A.; Meng, Q.C.; Liu, Q.; Hansen, N. Microstructural evolution of IF-steel during cold rolling. Acta Mater. 2004, 52, 1069–1081. [Google Scholar] [CrossRef]
- Kamaya, M. Assessment of local deformation using EBSD: Quantification of accuracy of measurement and definition of local gradient. Ultramicroscopy 2011, 111, 1189–1199. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kobayashi, R.; Watanabe, T. Control of grain boundary connectivity based on fractal analysis for improvement of intergranular corrosion resistance in SUS316L austenitic stainless steel. Acta Mater. 2016, 102, 397–405. [Google Scholar] [CrossRef]
- Shimada, M.; Kokawa, H.; Wang, Z.J.; Sato, Y.S.; Karibe, I. Optimization of grain boundary character distribution for intergranular corrosion resistant 304 stainless steel by twin-induced grain boundary engineering. Acta Mater. 2002, 50, 2331–2341. [Google Scholar] [CrossRef]
- Godfrey, A.; Hughes, D.A. Determination of boundary area and spacing in prismatic structures with applications to dislocation boundaries. Mater. Charact. 2002, 48, 89–99. [Google Scholar] [CrossRef]
- Tan, L.; Ren, X.; Sridharan, K.; Allen, T.R. Corrosion behavior of Ni-base alloys for advanced high temperature water-cooled nuclear plants. Corros. Sci. 2008, 50, 3056–3062. [Google Scholar] [CrossRef]
- Song, G.L.; Xu, Z.Q. The surface, microstructure and corrosion of magnesium alloy AZ31 sheet. Electrochim. Acta 2010, 55, 4148–4161. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, Y.; Meng, G.; Cui, Z.; Wang, F. Corrosion of hot extrusion AZ91 magnesium alloy: I-relation between the microstructure and corrosion behavior. Corros. Sci. 2011, 53, 1960–1968. [Google Scholar] [CrossRef]
- Rault, V.; Vignal, V.; Krawiec, H.; Dufour, F. Quantitative assessment of local misorientations and pitting corrosion behaviour of pearlitic steel using electron backscattered diffraction and microcapillary techniques. Corros. Sci. 2015, 100, 667–671. [Google Scholar] [CrossRef]
- Maric, M.; Muránsky, O.; Karatchevtseva, I.; Ungár, T.; Hester, J. The effect of cold-rolling on the microstructure and corrosion behaviour of 316L alloy in FLiNaK molten salt. Corros. Sci. 2018, 142, 133–144. [Google Scholar] [CrossRef]
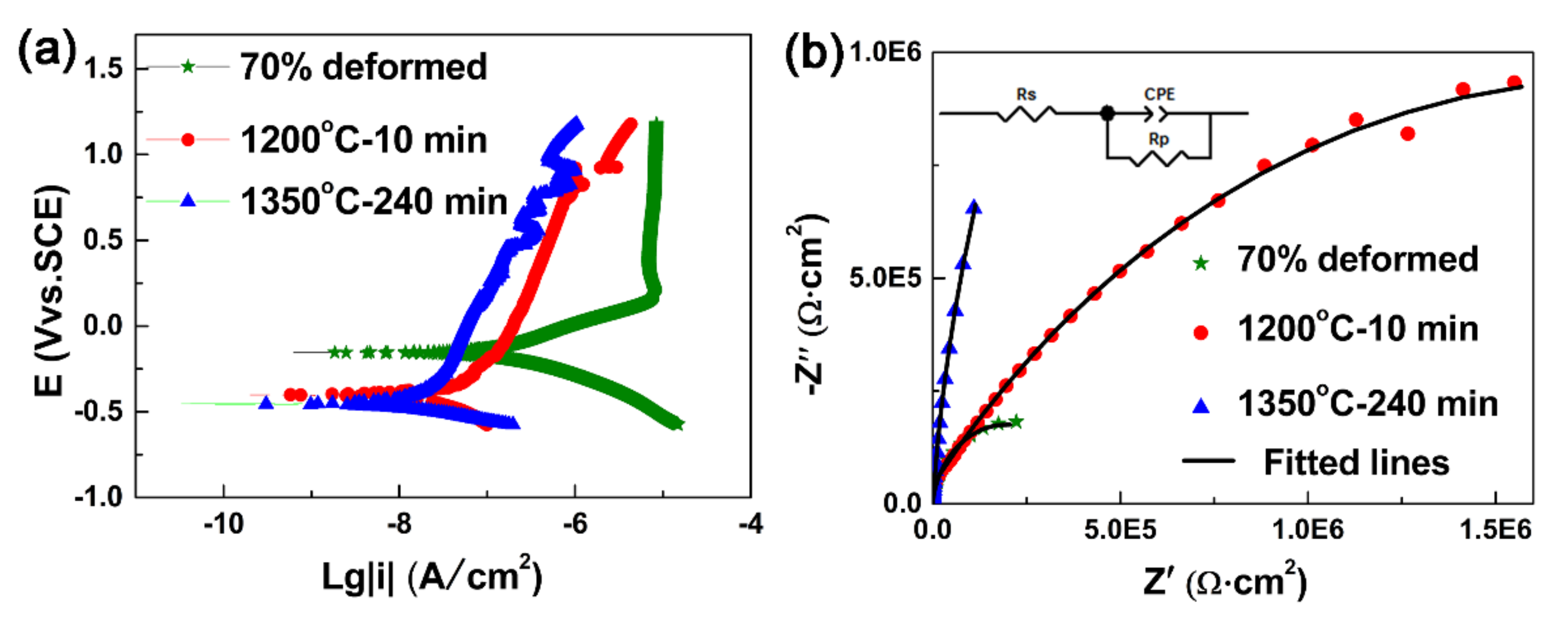

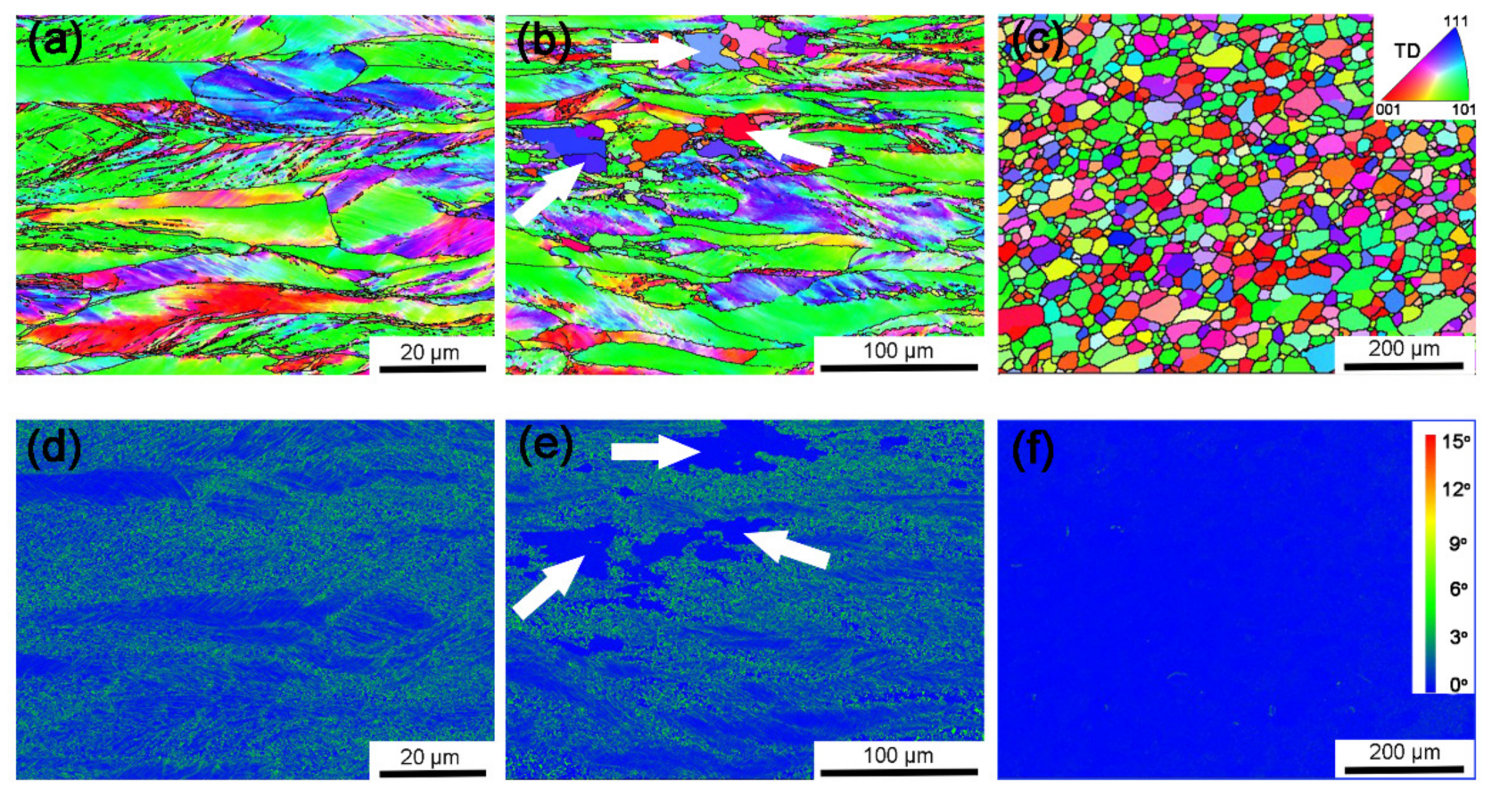
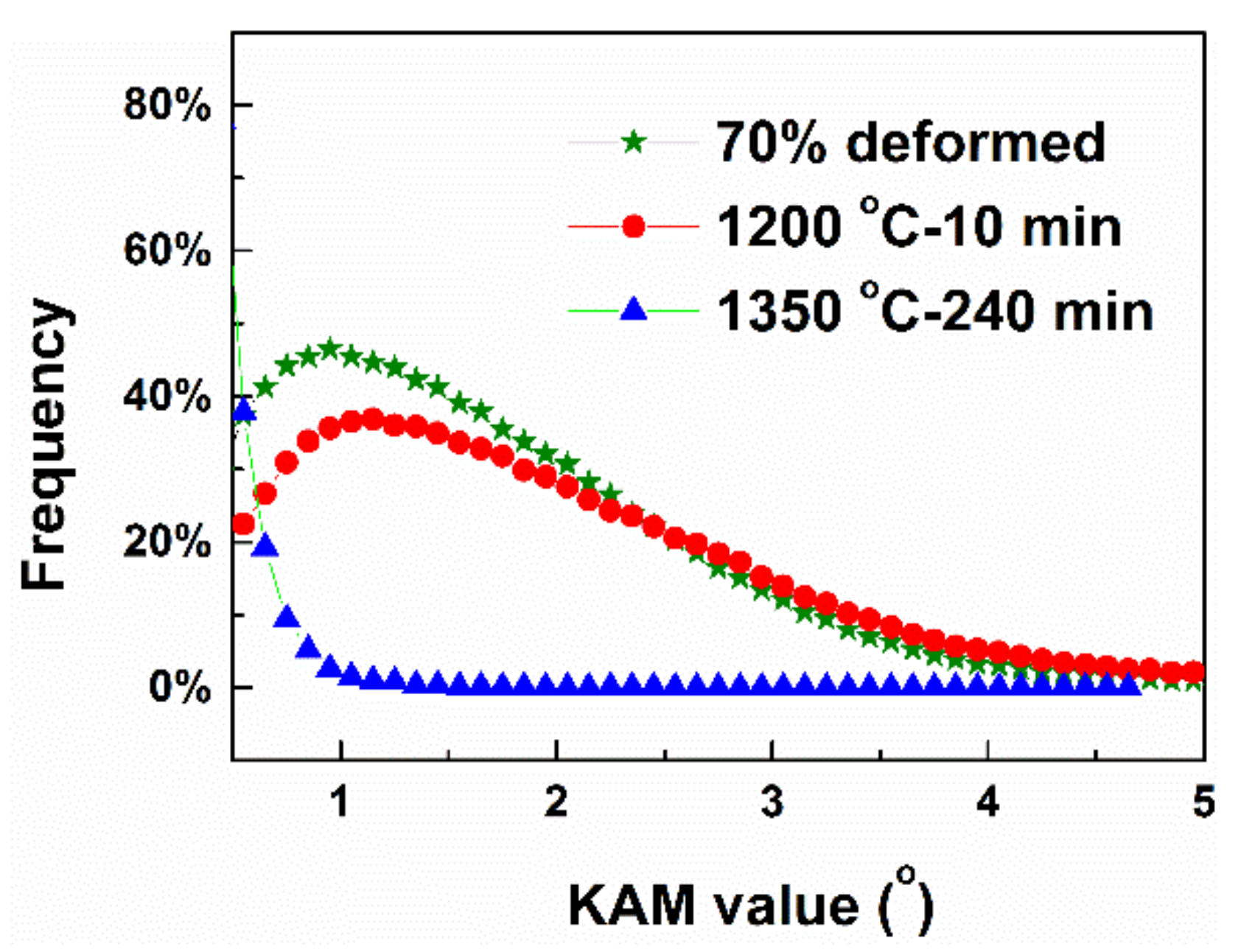


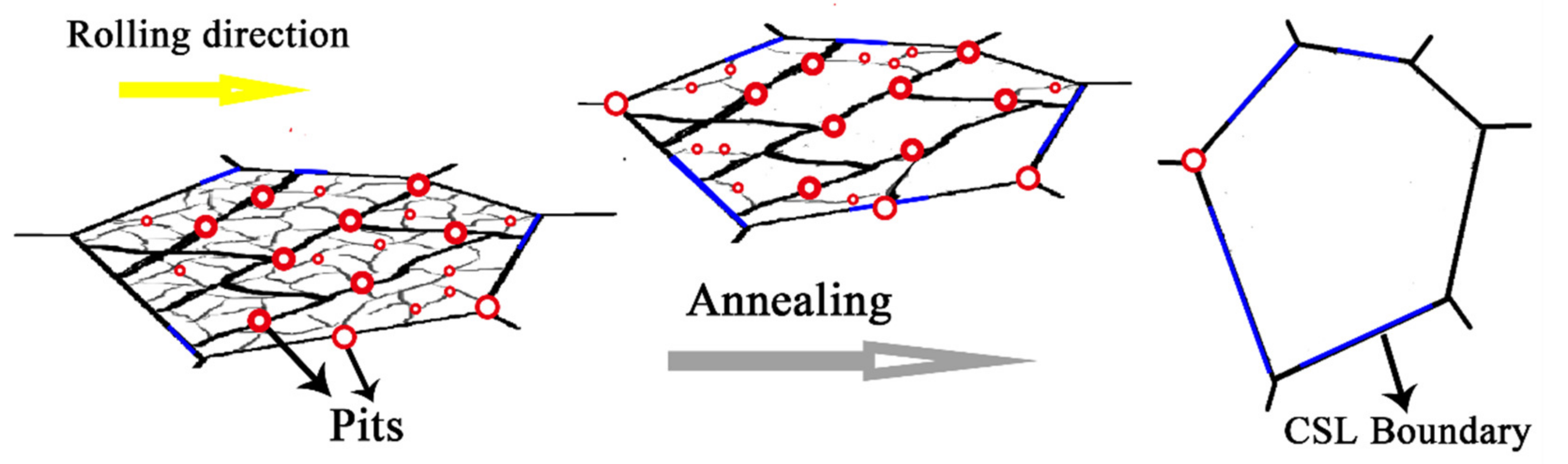
| Sample | ρG (m−2) | p(CSL)/p(θ>15°) |
|---|---|---|
| deformed | 3.74 × 1015 | 3.75% |
| 1200 °C-10 min | 3.08 × 1015 | 5.06% |
| 1350 °C-240 min | 3.01 × 1014 | 10.65% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; He, Q.; Luo, X.; Wu, G.; Chen, Q. Effect of Recrystallization Annealing on Corrosion Behavior of Ta-4%W Alloy. Materials 2019, 12, 117. https://doi.org/10.3390/ma12010117
Ma G, He Q, Luo X, Wu G, Chen Q. Effect of Recrystallization Annealing on Corrosion Behavior of Ta-4%W Alloy. Materials. 2019; 12(1):117. https://doi.org/10.3390/ma12010117
Chicago/Turabian StyleMa, Guoqiang, Qiongyao He, Xuan Luo, Guilin Wu, and Qiang Chen. 2019. "Effect of Recrystallization Annealing on Corrosion Behavior of Ta-4%W Alloy" Materials 12, no. 1: 117. https://doi.org/10.3390/ma12010117
APA StyleMa, G., He, Q., Luo, X., Wu, G., & Chen, Q. (2019). Effect of Recrystallization Annealing on Corrosion Behavior of Ta-4%W Alloy. Materials, 12(1), 117. https://doi.org/10.3390/ma12010117






