A Reversible Protonic Ceramic Cell with Symmetrically Designed Pr2NiO4+δ-Based Electrodes: Fabrication and Electrochemical Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Characterization of Materials
2.3. Fabrication of the PCC
2.4. Characterization of the PCC
3. Results and Discussion
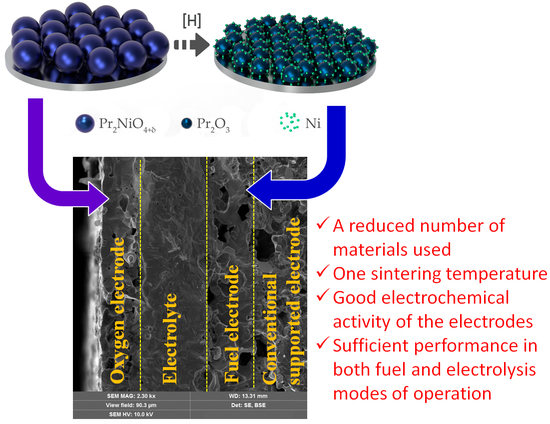
3.1. Pr1.9Ba0.1NiO4+δ Functionality
3.2. Microstructural Features
3.3. Volt-Ampere Dependences and Related Properties
3.4. Analysis of Impedance Data
- (1)
- (2)
- The slight temperature behavior of the total resistance of the PCC is a characteristic feature of the cells, corresponding to the condition of Rp < RO. Therefore, their output parameters (as shown in Figure 4d) also change slightly with temperature variation.
3.5. Effect of Air and Hydrogen Humidification
- (1)
- The absolute value of R1 is equal to 0.02 Ω cm2, but its contribution as part of Rp decreases from 30 to 17%;
- (2)
- The contribution of R1’ in Rp does not exceed 4.5% or 0.004 Ω cm2 in absolute units;
- (3)
- The contribution of R2 increases from 70 to 80%, remaining the dominant parameter in the electrode performance.
3.6. Electrolytic Properties of the BCZD Membrane
- (1)
- The BCZD electrolyte forms the basis for the design of novel electrochemical cells with improved output parameters due to its higher ionic conductivity compared with those for the most-studied Y-containing cerate-zirconates;
- (2)
- Despite the negative electrochemical response of the electrodes to gas humidification, the average ionic transference and ionic conductivity values take the opposite direction, resulting in improved PCC efficiency.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A



| p’H2O, atm | RO, Ω cm2 | Rp, Ω cm2 | Pmax, mW cm−2 | jH2, ml min−1 cm−2 |
|---|---|---|---|---|
| 0.03 | 0.47 | 0.12 | 293 | 5.1 |
| 0.50 | 0.45 | 0.21 | 284 | 5.6 |


References
- Medvedev, D.; Murashkina, A.; Pikalova, E.; Demin, A.; Podias, A.; Tsiakaras, P. BaCeO3: Materials development, properties and application. Prog. Mater. Sci. 2014, 60, 72–129. [Google Scholar] [CrossRef]
- Bi, L.; Boulfrada, S.; Traversa, E. Steam electrolysis by solid oxide electrolysis cells (SOECs) with proton-conducting oxides. Chem. Soc. Rev. 2014, 43, 8255–8270. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; An, H.; Yoon, K.J.; Kim, B.-K.; Lee, H.-W.; Son, J.-W.; Kim, H.; Shin, D.; Ji, H.-L. Electrochemical analysis of high-performance protonic ceramic fuel cells based on a columnar-structured thin electrolyte. Appl. Energy 2019, 233–234, 29–36. [Google Scholar] [CrossRef]
- Mohato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Gómez, S.Y.; Hotza, D. Current developments in reversible solid oxide fuel cells. Renew. Sust. Energy Rev. 2016, 61, 155–174. [Google Scholar] [CrossRef]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.-I.; Haile, S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef]
- Bae, K.; Jang, D.Y.; Choi, H.J.; Kim, D.; Hong, J.; Kim, B.-K.; Lee, J.-H.; Son, J.-W.; Shim, J.H. Demonstrating the potential of yttrium-doped barium zirconate electrolyte for high-performance fuel cells. Nat. Commun. 2017, 8, 14553. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; O’Hayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef]
- Shim, J.H. Ceramics breakthrough. Nat. Energy 2018, 3, 168–169. [Google Scholar] [CrossRef]
- Ding, D.; Li, X.; Lai, S.Y.; Gerdes, K.; Liu, M. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 2014, 7, 552–575. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Wang, W.; Liu, M.; Tadé, M.O.; Shao, Z. Progress and prospects in symmetrical solid oxide fuel cells with two identical electrodes. Adv. Energy Mater. 2015, 5, 1500188. [Google Scholar] [CrossRef]
- Choi, S.; Sengodan, S.; Park, S.; Ju, Y.-W.; Kim, J.; Hyodo, J.; Jeong, H.Y.; Ishihara, T.; Shin, J.; Kim, G. A robust symmetrical electrode with layered perovskite structure for direct hydrocarbon solid oxide fuel cells: PrBa0.8Ca0.2Mn2O5+δ. J. Mater. Chem. A 2016, 4, 1747–1753. [Google Scholar] [CrossRef]
- Shen, J.; Chen, Y.; Yang, G.; Zhou, W.; Tadé, M.O.; Shao, Z. Impregnated LaCo0.3Fe0.67Pd0.03O3−δ as a promising electrocatalyst for “symmetrical” intermediate-temperature solid oxide fuel cells. J. Power Sources 2016, 306, 92–99. [Google Scholar] [CrossRef]
- Tao, H.; Xie, J.; Wu, Y.; Wang, S. Evaluation of PrNi0.4Fe0.6O3−δ as a symmetrical SOFC electrode material. Int. J. Hydrogen Energy 2018, 43, 15423–15432. [Google Scholar] [CrossRef]
- Niu, B.; Jin, F.; Zhang, L.; Shen, P.; He, T. Performance of double perovskite symmetrical electrode materials Sr2TiFe1−xMoxO6−δ (x = 0.1, 0.2) for solid oxide fuel cells. Electrochim. Acta 2018, 263, 217–227. [Google Scholar] [CrossRef]
- Lan, R.; Cowin, P.I.; Sengodan, S.; Tao, S. A perovskite oxide with high conductivities in both air and reducing atmosphere for use as electrode for solid oxide fuel cells. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Gao, Z.; Ding, X.; Ding, D.; Ding, L.; Zhang, S.; Yuan, G. Infiltrated Pr2NiO4 as promising bi-electrode for symmetrical solid oxide fuel cells. Int. J. Hydrogen Energy 2018, 43, 8953–8961. [Google Scholar] [CrossRef]
- Nicollet, C.; Flura, A.; Vibhu, V.; Rougier, A.; Bassat, J.-M.; Grenier, J.-C. An innovative efficient oxygen electrode for SOFC: Pr6O11 infiltrated into Gd-doped ceria backbone. Int. J. Hydrogen Energy 2016, 41, 15538–15544. [Google Scholar] [CrossRef]
- Lyagaeva, J.; Medvedev, D.; Pikalova, E.; Plaksin, S.; Brouzgou, A.; Demin, A.; Tsiakaras, P. A detailed analysis of thermal and chemical compatibility of cathode materials suitable for BaCe0.8Y0.2O3−δ and BaZr0.8Y0.2O3−δ proton electrolytes for solid oxide fuel cell application. Int. J. Hydrogen Energy 2017, 42, 1715–1723. [Google Scholar] [CrossRef]
- Pikalova, E.; Kolchugin, A.; Bogdanovich, N.; Medvedev, D.; Lyagaeva, J.; Vedmid’, L.; Anayev, M.; Plaksin, S.; Farlenkov, A. Suitability of Pr2−xCaxNiO4+δ as cathode materials for electrochemical devices based on oxygen ion and proton conducting solid state electrolytes. Int. J. Hydrogen Energy 2018. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y.; Fang, S.; Zhu, Z.; Yan, L.; Liu, W. Evaluation of BaZr0.1Ce0.7Y0.2O3−δ-based proton-conducting solid oxide fuel cells fabricated by a one-step co-firing process. Electrochim. Acta 2011, 56, 1447–1454. [Google Scholar] [CrossRef]
- Dai, H.; Da’as, E.H.; Shafi, S.P.; Wang, H.; Bi, L. Tailoring cathode composite boosts the performance of proton-conducting SOFCs fabricated by a one-step co-firing method. J. Eur. Ceram. Soc. 2018, 38, 2903–2908. [Google Scholar] [CrossRef]
- Lyagaeva, J.; Danilov, N.; Vdovin, G.; Bu, J.; Medvedev, D.; Demin, A.; Tsiakaras, P. A new Dy-doped BaCeO3–BaZrO3 proton-conducting material as a promising electrolyte for reversible solid oxide fuel cells. J. Mater. Chem. A 2016, 4, 15390–15399. [Google Scholar] [CrossRef]
- Lyagaeva, Y.G.; Danilov, N.A.; Gorshkov, M.Y.; Vdovin, G.K.; Antonov, B.D.; Demin, A.K.; Medvedev, D.A. Functionality of lanthanum, neodymium, and praseodymium nickelates as promising electrode systems for proton-conducting electrolytes. Russ. J. Appl. Chem 2018, 91, 583–590. [Google Scholar] [CrossRef]
- Equipment. Shared Access Center. Available online: http://www.ihte.uran.ru/?page_id=3154 (accessed on 11 December 2018).
- DRTTOOLS. Available online: https://sites.google.com/site/drttools/ (accessed on 11 December 2018).
- Pikalova, E.; Kolchugin, A.; Filonova, E.; Bogdanovich, N.; Pikalov, S.; Ananyev, M.; Molchanova, N.; Farlenkov, A. Validation of calcium-doped neodymium nickelates as SOFC air electrode materials. Solid State Ionics 2018, 319, 130–140. [Google Scholar] [CrossRef]
- Akbari-Fakhrabadi, A.; Toledo, E.G.; Canales, J.I.; Meruane, V.; Chan, S.H.; Gracia-Pinilla, M.A. Effect of Sr2+ and Ba2+ doping on structural stability and mechanical properties of La2NiO4+δ. Ceram. Int. 2018, 44, 10551–10557. [Google Scholar] [CrossRef]
- Kravchenko, E.; Khalyavin, D.; Zakharchuk, K.; Grins, J.; Svensson, G.; Pankov, V.; Yaremchenko, A. High-temperature characterization of oxygen-deficient K2NiF4-type Nd2−xSrxNiO4−δ (x = 1.0–1.6) for potential SOFC/SOEC applications. J. Mater. Chem. A 2015, 3, 23852–23863. [Google Scholar] [CrossRef]
- Hua, B.; Li, M.; Sun, Y.-F.; Li, J.-H.; Luo, J.-L. Enhancing perovskite electrocatalysis of solid oxide cells through controlled exsolution of nanoparticles. ChemSusChem 2017, 10, 3333–3341. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Medvedev, D.A.; Khasanov, A.F. Structure, stability, and thermomechanical properties of Ca-substituted Pr2NiO4+δ. Phys. Solid State 2017, 59, 694–702. [Google Scholar] [CrossRef]
- Ceretti, M.; Wahyudi, O.; André, G.; Meven, M.; Villesuzanne, A.; Paulus, W. (Nd/Pr)2NiO4+δ: Reaction intermediates and redox behavior explored by in situ neutron powder diffraction during electrochemical oxygen intercalation. Chem. Mater. 2018, 57, 4657–4666. [Google Scholar] [CrossRef] [PubMed]
- Løken, A.; Ricote, S.; Wachowski, S. Thermal and chemical expansion in proton ceramic electrolytes and compatible electrodes. Crystals 2018, 8, 365. [Google Scholar] [CrossRef]
- Kharton, V.V.; Kovalevsky, A.V.; Avdeev, M.; Tsipis, E.V.; Patrakeev, M.V.; Yaremchenko, A.A.; Naumovich, E.N.; Frade, J.R. Chemically induced expansion of La2NiO4+δ-based materials. Chem. Mater. 2007, 19, 2027–2033. [Google Scholar] [CrossRef]
- Kochetova, N.; Animitsa, I.; Medvedev, D.; Demin, A.; Tsiakaras, P. Recent activity in the development of proton-conducting oxides for high-temperature applications. RSC Adv. 2016, 6, 73222–73268. [Google Scholar] [CrossRef]
- Jeong, S.; Kobayashi, T.; Kuroda, K.; Kwon, H.; Zhu, C.; Habazaki, H.; Aoki, Y. Evaluation of thin film fuel cells with Zr-rich BaZrxCe0.8−xY0.2O3−δ electrolytes (x ≥ 0.4) fabricated by a single-step reactive sintering method. RSC Adv. 2018, 8, 26309–26317. [Google Scholar] [CrossRef]
- Danilov, N.A.; Lyagaeva, J.G.; Medvedev, D.A.; Demin, A.K.; Tsiakaras, P. Transport properties of highly dense proton-conducting BaCe0.8−xZrxDy0.2O3−δ materials in low- and high-temperature ranges. Electrochim. Acta 2018, 284, 551–559. [Google Scholar] [CrossRef]
- Liu, M.; Hu, H. Effect of interfacial resistance on determination of transport properties of mixed-conducting electrolytes. J. Electrochem. Soc. 1996, 143, L109–L112. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton-conducting oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Malavasi, L.; Fisher, C.A.J.; Islam, M.S. Oxide-ion and proton conducting electrolyte materials for clean energy applications: Structural and mechanistic features. Chem. Soc. Rev. 2010, 39, 4370–4387. [Google Scholar] [CrossRef]
- Gregori, G.; Merkle, R.; Maier, J. Ion conduction and redistribution at grain boundaries in oxide systems. Prog. Mater. Sci. 2017, 89, 252–305. [Google Scholar] [CrossRef]
- Nechache, A.; Cassir, M.; Ringuedé, A. Solid oxide electrolysis cell analysis by means of electrochemical impedance spectroscopy: A review. J. Power Sources 2014, 258, 164–181. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, S.; Yang, Y.; Xia, C. Barium carbonate as a synergistic catalyst for the H2O/CO2 reduction reaction at Ni–yttria stabilized zirconia cathodes for solid oxide electrolysis cells. J. Mater. Chem. A 2018, 6, 2721–2729. [Google Scholar] [CrossRef]
- Sun, S.; Cheng, Z. Electrochemical behaviors for Ag, LSCF and BSCF as oxygen electrodes for proton conducting IT-SOFC. J. Electrochem. Soc. 2017, 164, F3104–F3113. [Google Scholar] [CrossRef]
- Shi, N.; Su, F.; Huan, D.; Xie, Y.; Lin, J.; Tan, W.; Peng, R.; Xia, C.; Chen, C.; Lu, Y. Performance and DRT analysis of P-SOFCs fabricated using new phase inversion combined tape casting technology. J. Mater. Chem. A 2017, 5, 19664–19671. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Z.; Zhang, T.; Kang, J.; Ou, X.; Feng, P.; Wang, S.; Zhou, F.; Ling, Y. Charge transfer modeling and polarization DRT analysis of proton ceramics fuel cells based on mixed conductive electrolyte with the modified anode-electrolyte interface. ACS Appl. Mater. Interfaces 2018, 10, 35047–35059. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, B.A. Fourier transform distribution function of relaxation times; application and limitations. Electrochim. Acta 2015, 154, 35–46. [Google Scholar] [CrossRef]
- Ivers-Tiffée, E.; Weber, A. Evaluation of electrochemical impedance spectra by the distribution of relaxation times. J. Ceram. Soc. Jpn. 2017, 125, 193–201. [Google Scholar] [CrossRef]
- Yang, S.; Wen, Y.; Zhang, J.; Lu, Y.; Ye, X.; Wen, Z. Electrochemical performance and stability of cobalt-free Ln1.2Sr0.8NiO4 (Ln=La and Pr) air electrodes for proton-conducting reversible solid oxide cells. Electrochim. Acta 2018, 267, 269–277. [Google Scholar] [CrossRef]
- Li, W.; Guan, B.; Ma, L.; Hu, S.; Zhang, N.; Liu, X. High performing triple-conductive Pr2NiO4+δ anode for proton-conducting steam solid oxide electrolysis cell. J. Mater. Chem. A 2018, 6, 18057–18066. [Google Scholar] [CrossRef]
- Li, G.; Jin, H.; Cui, Y.; Gui, L.; He, B.; Zhao, L. Application of a novel (Pr0.9La0.1)2(Ni0.74Cu0.21Nb0.05)O4+δ-infiltrated BaZr0.1Ce0.7Y0.2O3-δ cathode for high performance protonic ceramic fuel cells. J. Power Sources 2017, 341, 192–198. [Google Scholar] [CrossRef]
- Dailly, J.; Marrony, M.; Taillades, G.; Taillades-Jacquin, M.; Grimaud, A.; Mauvy, F.; Louradour, E.; Salmi, J. Evaluation of proton conducting BCY10-based anode supported cells by co-pressing method: Up-scaling, performances and durability. J. Power Sources 2014, 255, 302–307. [Google Scholar] [CrossRef]
- Wang, W.; Medvedev, D.; Shao, Z. Gas Humidification impact on the properties and performance of perovskite-type functional materials in proton-conducting solid oxide cells. Adv. Funct. Mater. 2018, 28, 1802592. [Google Scholar] [CrossRef]
- Heras-Juaristi, G.; Pérez-Coll, D.; Mather, G.C. Temperature dependence of partial conductivities of the BaZr0.7Ce0.2Y0.1O3−δ proton conductor. J. Power Sources 2017, 364, 52–60. [Google Scholar] [CrossRef]
- Lyagaeva, J.; Danilov, N.; Korona, D.; Farlenkov, A.; Medvedev, D.; Demin, A.; Animitsa, I.; Tsiakaras, P. Improved ceramic and electrical properties of CaZrO3-based proton-conducting materials prepared by a new convenient combustion synthesis method. Ceram. Int. 2017, 43, 7184–7192. [Google Scholar] [CrossRef]
- Norby, T. EMF method determination of conductivity contributions from protons and other foreign ions in oxides. Solid State Ionics 1988, 28–30, 1586–1591. [Google Scholar] [CrossRef]
- Sutija, D. Transport number determination by the concentration-cell/open-circuit voltage method for oxides with mixed electronic, ionic and protonic conductivity. Solid State Ionics 1995, 77, 167–174. [Google Scholar] [CrossRef]
- Gong, Z.; Sun, W.; Cao, J.; Shan, D.; Wu, Y.; Liu, W. Ce0.8Sm0.2O1.9 decorated with electron-blocking acceptor-doped BaCeO3 as electrolyte for low-temperature solid oxide fuel cells. Electrochim. Acta 2017, 228, 226–232. [Google Scholar] [CrossRef]
- Huan, D.; Shi, N.; Zhang, L.; Tan, W.; Xie, Y.; Wang, W.; Xia, C.; Lu, Y. New, Efficient, and reliable air electrode material for proton-conducting reversible solid oxide cells. ACS Appl. Mater. Interfaces 2018, 10, 1761–1770. [Google Scholar] [CrossRef]
- Philippeau, B.; Mauvy, F.; Mazataud, C.; Fourcade, S.; Grenier, J.-C. Comparative study of electrochemical properties of mixed conducting Ln2NiO4+δ (Ln = La, Pr and Nd) and La0.6Sr0.4Fe0.8Co0.2O3−δ as SOFC cathodes associated to Ce0.9Gd0.1O2−δ, La0.8Sr0.2Ga0.8Mg0.2O3−δ and La9Sr1Si6O26.5 electrolytes. Solid State Ionics 2013, 249–250, 17–25. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizunuma, S.; Kimura, Y.; Mikami, Y.; Yamauchi, K.; Kuroha, T.; Taniguchi, N.; Tsuji, Y.; Okuyama, Y.; Amezawa, K. Energy efficiency of ionic transport through proton conducting ceramic electrolytes for energy conversion applications. J. Mater. Chem. A 2018, 6, 15771–15780. [Google Scholar] [CrossRef]
- Ji, H.-I.; Kim, H.; Lee, H.-W.; Kim, B.-K.; Son, J.-W.; Yoon, K.J.; Lee, J.-H. Open-cell voltage and electrical conductivity of a protonic ceramic electrolyte under two chemical potential gradients. Phys. Chem. Chem. Phys. 2018, 20, 14997–15001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Lei, L.-B.; Liu, D.; Zhao, F.-Y.; Ni, M.; Chen, F. Mathematical modeling of a proton-conducting solid oxide fuel cell with current leakage. J. Power Sources 2018, 400, 333–340. [Google Scholar] [CrossRef]
- Wachowski, S.; Li, Z.; Polfus, J.M.; Norby, T. Performance and stability in H2S of SrFe0.75Mo0.25O3-δ as electrode in proton ceramic fuel cells. J. Eur. Ceram. Soc. 2018, 38, 163–171. [Google Scholar] [CrossRef]
- Nasani, N.; Ramasamy, D.; Mikhalev, S.; Kovalevsky, A.V.; Fagg, D.P. Fabrication and electrochemical performance of a stable, anode supported thin BaCe0.4Zr0.4Y0.2O3−δ electrolyte protonic ceramic fuel cell. J. Power Sources 2015, 278, 582–589. [Google Scholar] [CrossRef]
- Wan, Y.; He, B.; Wang, R.; Ling, Y.; Zhao, L. Effect of Co doping on sinterability and protonic conductivity of BaZr0.1Ce0.7Y0.1Yb0.1O3−δ for protonic ceramic fuel cells. J. Power Sources 2017, 347, 14–20. [Google Scholar] [CrossRef]
- Dailly, J.; Taillades, G.; Ancelin, M.; Pers, P.; Marrony, M. High performing BaCe0.8Zr0.1Y0.1O3−δ−Sm0.5Sr0.5CoO3−δ based protonic ceramic fuel cell. J. Power Sources 2017, 361, 221–226. [Google Scholar] [CrossRef]
- Ding, H.; Xue, X. GdBa0.5Sr0.5Co2O5+δ layered perovskite as promising cathode for proton conducting solid oxide fuel cells. J Alloys Compd. 2010, 496, 683–686. [Google Scholar] [CrossRef]
- Shi, H.; Ding, Z.; Ma, G. Electrochemical performance of cobalt-free Nd0.5Ba0.5Fe1−xNixO3−δ cathode materials for intermediate temperature solid oxide fuel cells. Fuel Cells 2016, 16, 258–262. [Google Scholar] [CrossRef]
- Dailly, J.; Ancelin, M.; Marrony, M. Long term testing of BCZY-based protonic ceramic fuel cell PCFC: Micro-generation profile and reversible production of hydrogen and electricity. Solid State Ionics 2017, 306, 69–75. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.; Wee, S.; Baek, H.; Shin, D. One-dimensional structured La0.6Sr0.4Co0.2Fe0.8O3−δ-BaCe0.5Zr0.35Y0.15O3−δ composite cathode for protonic ceramic fuel cells. Solid State Ionics 2018, 320, 347–352. [Google Scholar] [CrossRef]
- Amiri, T.; Singh, K.; Sandhu, N.K.; Hanifi, A.R.; Etsell, T.H.; Luo, J.-L.; Thangadurai, V.; Sarkar, P. High performance tubular solid oxide fuel cell based on Ba0.5Sr0.5Ce0.6Zr0.2Gd0.1Y0.1O3-δ proton conducting electrolyte. J. Electrochem. Soc. 2018, 165, F764–F769. [Google Scholar] [CrossRef]
- Li, F.; Tao, Z.; Dai, H.; Xi, X.; Ding, H. A high-performing proton-conducting solid oxide fuel cell with layered perovskite cathode in intermediate temperatures. Int. J. Hydrogen Energy 2018, 43, 19757–19762. [Google Scholar] [CrossRef]
- Lyagaeva, J.; Danilov, N.; Tarutin, A.; Vdovin, G.; Medvedev, D.; Demin, A.; Tsiakaras, P. Designing a protonic ceramic fuel cell with novel electrochemically active oxygen electrodes based on doped Nd0.5Ba0.5FeO3−δ. Dalton Trans. 2018, 47, 5149–8157. [Google Scholar] [CrossRef] [PubMed]
- Lyagaeva, J.; Vdovin, G.; Hakimova, L.; Medvedev, D.; Demin, A.; Tsiakaras, P. BaCe0.5Zr0.3Y0.2−xYbxO3−δ proton-conducting electrolytes for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2017, 251, 554–561. [Google Scholar] [CrossRef]
- Gui, L.; Ling, Y.; Li, G.; Wang, Z.; Wan, Y.; Wang, R.; He, B.; Zhao, L. Enhanced sinterability and conductivity of BaZr0.3Ce0.5Y0.2O3−δ by addition of bismuth oxide for proton conducting solid oxide fuel cells. J. Power Sources 2016, 301, 369–375. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C. BaFe0.6Co0.3Ce0.1O3−δ as cathode materials for proton-conducting solid oxide fuel cells. Ceram. Int. 2016, 42, 10511–10515. [Google Scholar] [CrossRef]
- Danilov, N.A.; Tarutin, A.P.; Lyagaeva, J.G.; Pikalova, E.Y.; Murashkina, A.A.; Medvedev, D.A.; Patrakeev, M.V.; Demin, A.K. Affinity of YBaCo4O7+δ-based layered cobaltites with protonic conductors of cerate-zirconate family. Ceram. Int. 2017, 43, 15418–15423. [Google Scholar] [CrossRef]
- Danilov, N.; Pikalova, E.; Lyagaeva, J.; Antonov, B.; Medvedev, D.; Demin, A.; Tsiakaras, P. Grain and grain boundary transport in BaCe0.5Zr0.3Ln0.2O3−δ (Ln-Y or lanthanide) electrolytes attractive for protonic ceramic fuel cells application. J. Power Sources 2017, 366, 161–168. [Google Scholar] [CrossRef]











| Electrolyte | Electrode | T, °C | Rp, Ω cm2 | Ref. |
|---|---|---|---|---|
| BaCe0.5Zr0.3Dy0.2O3−δ (BCZD) | Pr1.9Ba0.1NiO4+δ–BCZD | 600 | 0.39 | This work |
| 700 | 0.12 | |||
| BaCe0.7Zr0.1Y0.2O3−δ (BCZY1) | Pr1.8Sr0.2NiO4+δ | 600 | 2.17 | [50] |
| 700 | 0.33 | |||
| BaCe0.6Zr0.2Y0.2O3−δ (BCZY2) | Pr2NiO4+δ–BCZY2 | 600 | 0.21 | [51] |
| 700 | 0.06 | |||
| BCZY1 | (Pr0.9La0.1)2Ni0.74Cu0.21Nb0.05O4+δ– BCZY1 (infiltration) | 600 | 0.32 | [52] |
| 700 | 0.13 | |||
| BaCe0.9Y0.1O3−δ | Pr2NiO4+δ | 600 | 0.80 | [53] |
| Oxygen Electrode 2 | Electrolyte 3 | h, μm | T, °C | E, V | RO, Ω cm2 | Rp, Ω cm2 | ti,av | σav, mS cm−1 | σi,av, mS cm−1 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| PBN | BCZD | 25 | 600 | 1.076 | 0.52 | 0.39 | 0.97 | 4.8 | 4.7 | This work |
| 700 | 1.006 | 0.47 | 0.12 | 0.92 | 5.3 | 4.9 | ||||
| SFM | BZCY8 | 1200 | 700 | 0.96 | 38.09 | 3.59 | 0.87 | 3.2 | 2.7 | [65] |
| SFM | BZCY8 | 20 | 600 | 1.03 | 0.80 | 1.47 | 0.97 | 2.5 | 2.4 | [66] |
| 700 | 0.96 | 0.57 | 0.33 | 0.91 | 3.5 | 3.2 | ||||
| PLNCN | BCZY1 | 12 | 600 | 0.99 | 0.33 | 0.32 | 0.94 | 3.6 | 3.4 | [52] |
| 700 | 0.95 | 0.21 | 0.13 | 0.91 | 5.8 | 5.2 | ||||
| PBC | BCZYYC | 10 | 600 | 1.00 | 0.37 | 0.34 | 0.94 | 2.7 | 2.5 | [67] |
| 700 | 0.99 | 0.26 | 0.12 | 0.92 | 3.8 | 3.5 | ||||
| SSC–BCZY1/ | BCZY1/ | 9 | 600 | 1.13 | 0.47 | 0.42 | 1.00 | 1.9 | 1.9 | [68] |
| 700 | 1.07 | 0.31 | 0.10 | 0.97 | 2.9 | 2.8 | ||||
| GBSC | BCZY1 | 20 | 600 | 1.01 | 0.65 | 0.39 | 0.93 | 3.1 | 2.8 | [69] |
| 700 | 1.02 | 0.52 | 0.08 | 0.92 | 3.8 | 3.6 | ||||
| NBFN | BCZY1 | 40 | 600 | 1.11 | 0.84 | 0.71 | 0.99 | 4.8 | 4.7 | [70] |
| LSCF | BCZY4 | 30 | 600 | 1.07 | 0.72 | 0.24 | 0.96 | 4.2 | 3.9 | [37] |
| 700 | 1.01 | 0.58 | 0.07 | 0.91 | 5.2 | 4.7 | ||||
| BCZY6 | 30 | 600 | 1.06 | 0.58 | 0.13 | 0.95 | 5.2 | 4.9 | ||
| 700 | 0.99 | 0.46 | 0.04 | 0.90 | 6.5 | 5.8 | ||||
| BCZY7 | 30 | 600 | 1.04 | 1.37 | 0.65 | 0.94 | 2.2 | 2.1 | ||
| 700 | 0.96 | 0.97 | 0.21 | 0.88 | 3.1 | 2.7 | ||||
| BZY | 30 | 600 | 0.93 | 1.34 | 0.82 | 0.89 | 2.2 | 1.9 | ||
| 700 | 0.84 | 1.13 | 0.29 | 0.80 | 2.7 | 2.1 | ||||
| BSCF | BCZY1/ | 6 | 600 | 1.04 | 0.37 | 0.85 | 0.98 | 1.6 | 1.6 | [71] |
| LSCF–BCZY3.5 | BCZY3.5 | 8 | 600 | 1.05 | 0.31 | 1.25 | 0.99 | 2.6 | 2.6 | [72] |
| 700 | 1.02 | 0.22 | 0.30 | 0.96 | 3.6 | 3.5 | ||||
| LSCF–BSCZGY | BSCZGY | 10 | 600 | 1.15 | 0.41 | 3.46 | 1.00 | 2.4 | 2.4 | [73] |
| 700 | 1.13 | 0.19 | 1.82 | 1.00 | 5.4 | 5.4 | ||||
| PBFM–SSC | BCZY1 | 25 | 600 | 1.01 | 0.41 | 0.54 | 0.95 | 6.1 | 5.8 | [74] |
| NBFC | BCZD | 30 | 600 | 1.05 | 0.68 | 0.66 | 0.96 | 4.4 | 4.2 | [75] |
| 700 | 1.01 | 0.42 | 0.24 | 0.94 | 7.1 | 6.7 | ||||
| NBFC’ | BCZYY | 25 | 600 | 1.04 | 1.04 | 0.83 | 0.95 | 2.4 | 2.3 | [76] |
| 700 | 1.01 | 0.65 | 0.22 | 0.92 | 3.8 | 3.6 | ||||
| PBC–BCZY | BCZY0.3 | 17 | 600 | 1.01 | 0.32 | 0.64 | 0.96 | 5.3 | 5.1 | [77] |
| BFCC | BCZYY’ | 30 | 600 | 1.06 | 0.49 | 0.28 | 0.96 | 6.1 | 5.9 | [78] |
| YBCZ | BCZD | 20 | 600 | 1.03 | 0.77 | 0.51 | 0.94 | 2.6 | 2.5 | [79] |
| 700 | 0.95 | 0.49 | 0.18 | 0.89 | 4.4 | 3.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarutin, A.; Lyagaeva, J.; Farlenkov, A.; Plaksin, S.; Vdovin, G.; Demin, A.; Medvedev, D. A Reversible Protonic Ceramic Cell with Symmetrically Designed Pr2NiO4+δ-Based Electrodes: Fabrication and Electrochemical Features. Materials 2019, 12, 118. https://doi.org/10.3390/ma12010118
Tarutin A, Lyagaeva J, Farlenkov A, Plaksin S, Vdovin G, Demin A, Medvedev D. A Reversible Protonic Ceramic Cell with Symmetrically Designed Pr2NiO4+δ-Based Electrodes: Fabrication and Electrochemical Features. Materials. 2019; 12(1):118. https://doi.org/10.3390/ma12010118
Chicago/Turabian StyleTarutin, Artem, Julia Lyagaeva, Andrey Farlenkov, Sergey Plaksin, Gennady Vdovin, Anatoly Demin, and Dmitry Medvedev. 2019. "A Reversible Protonic Ceramic Cell with Symmetrically Designed Pr2NiO4+δ-Based Electrodes: Fabrication and Electrochemical Features" Materials 12, no. 1: 118. https://doi.org/10.3390/ma12010118
APA StyleTarutin, A., Lyagaeva, J., Farlenkov, A., Plaksin, S., Vdovin, G., Demin, A., & Medvedev, D. (2019). A Reversible Protonic Ceramic Cell with Symmetrically Designed Pr2NiO4+δ-Based Electrodes: Fabrication and Electrochemical Features. Materials, 12(1), 118. https://doi.org/10.3390/ma12010118