Study on Optimization Technology to Strengthen Ni-Based Composite Coating Electroplate Containing Nanodiamond
Abstract
:1. Introduction
2. Experimental
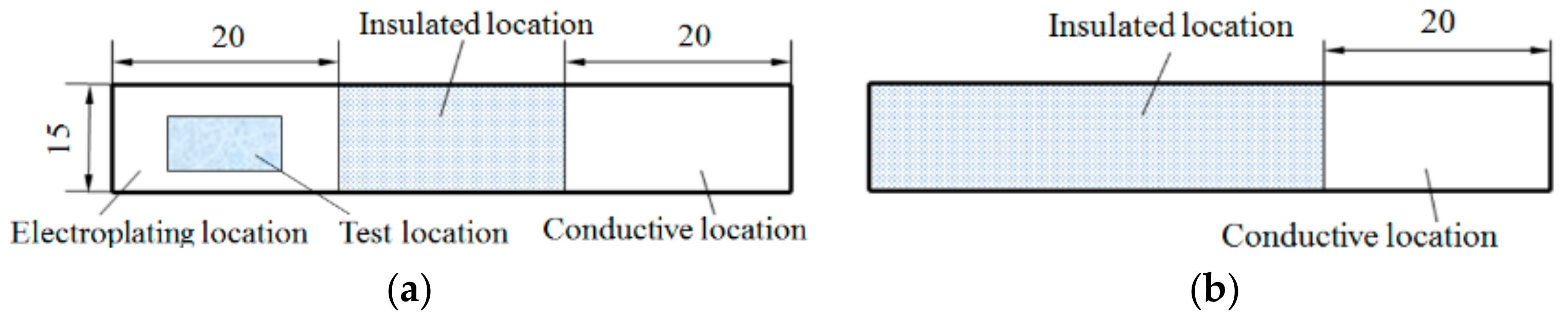
2.1. Substrate Specifications and Pretreatment
2.2. Nanodiamond Purification and Solution Preparation
2.3. Composition of Plating Solution and Process Conditions
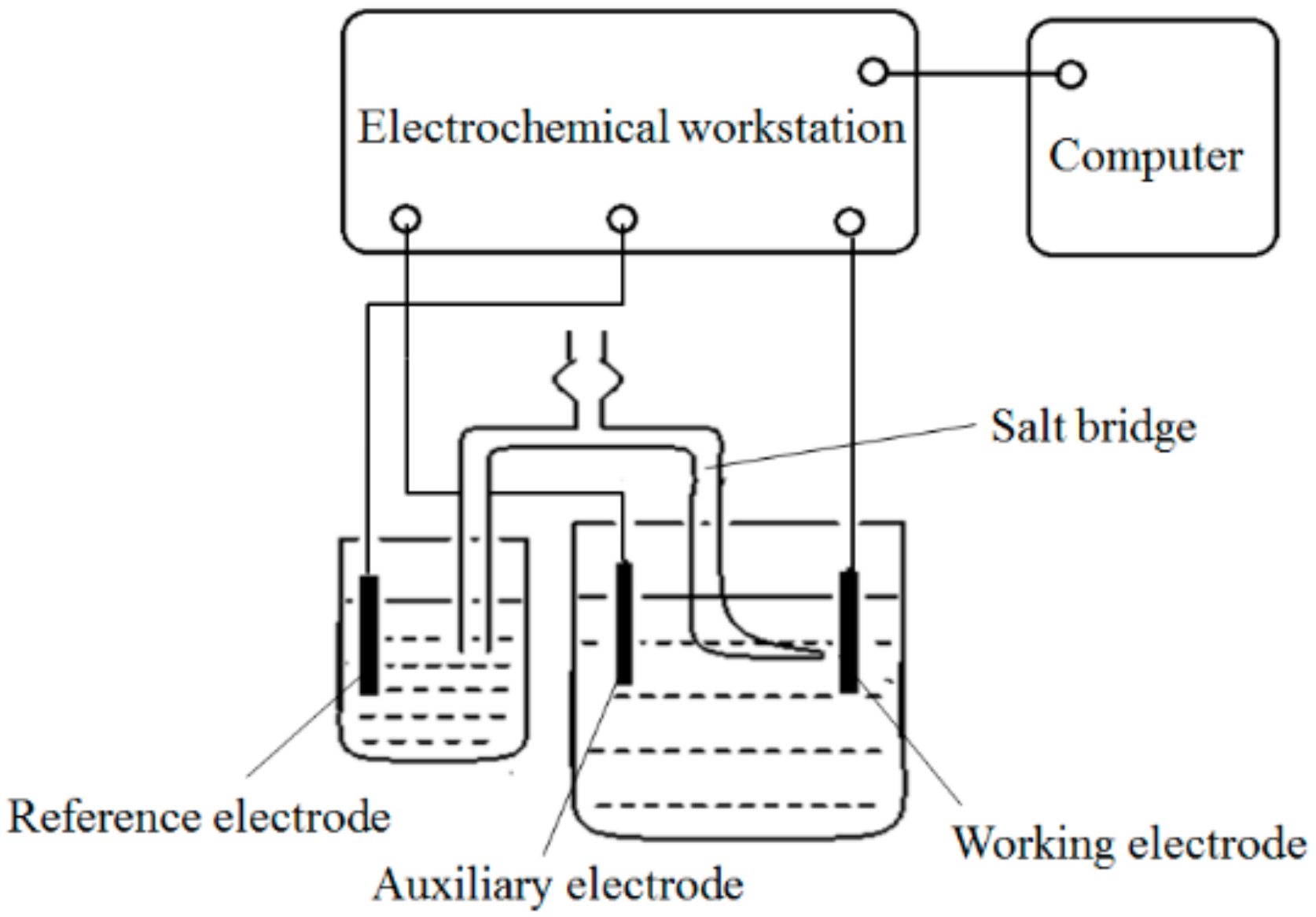
2.4. Electrochemical Tests of Composite Plating Bath
2.5. Characterization
3. Results and Discussion
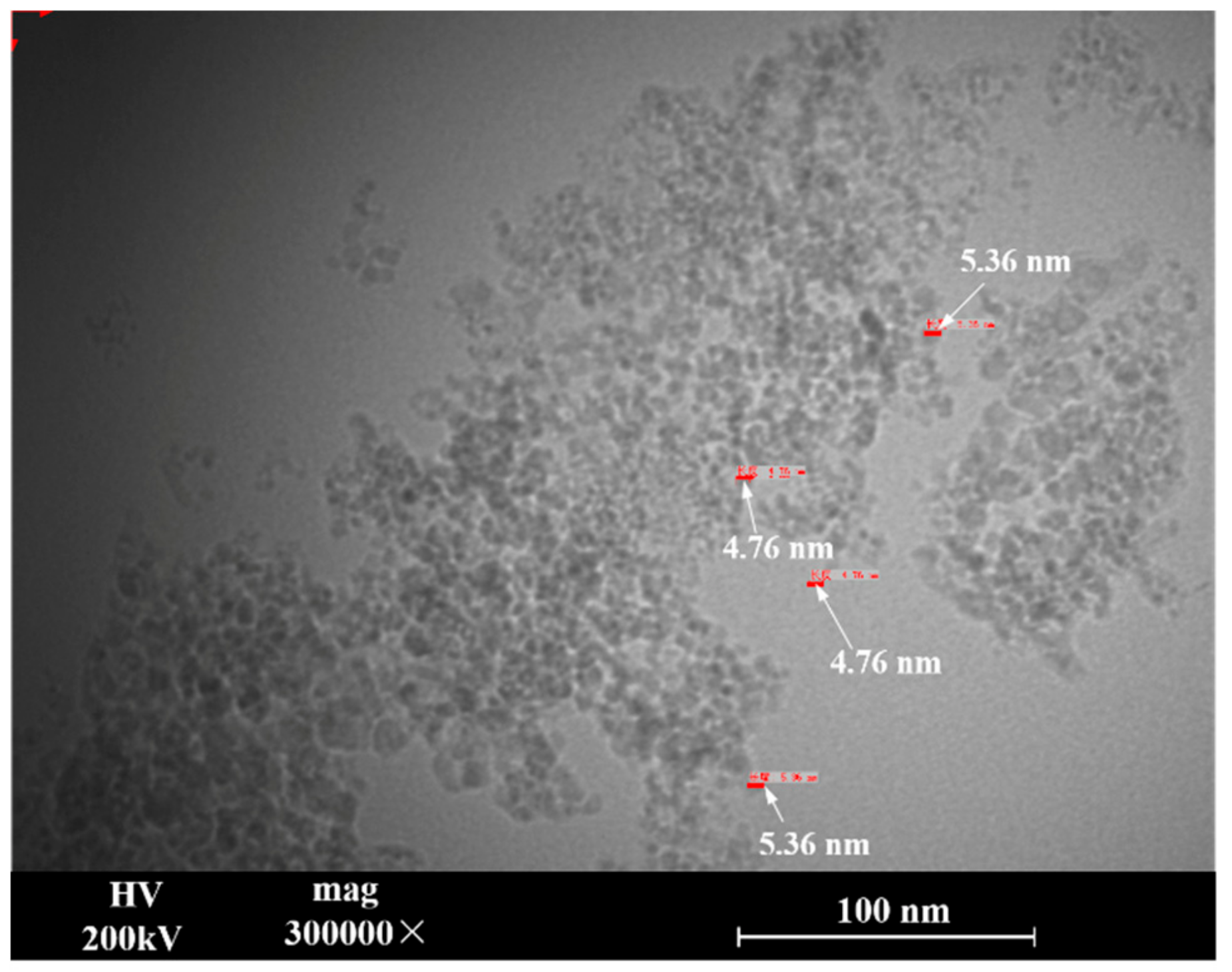
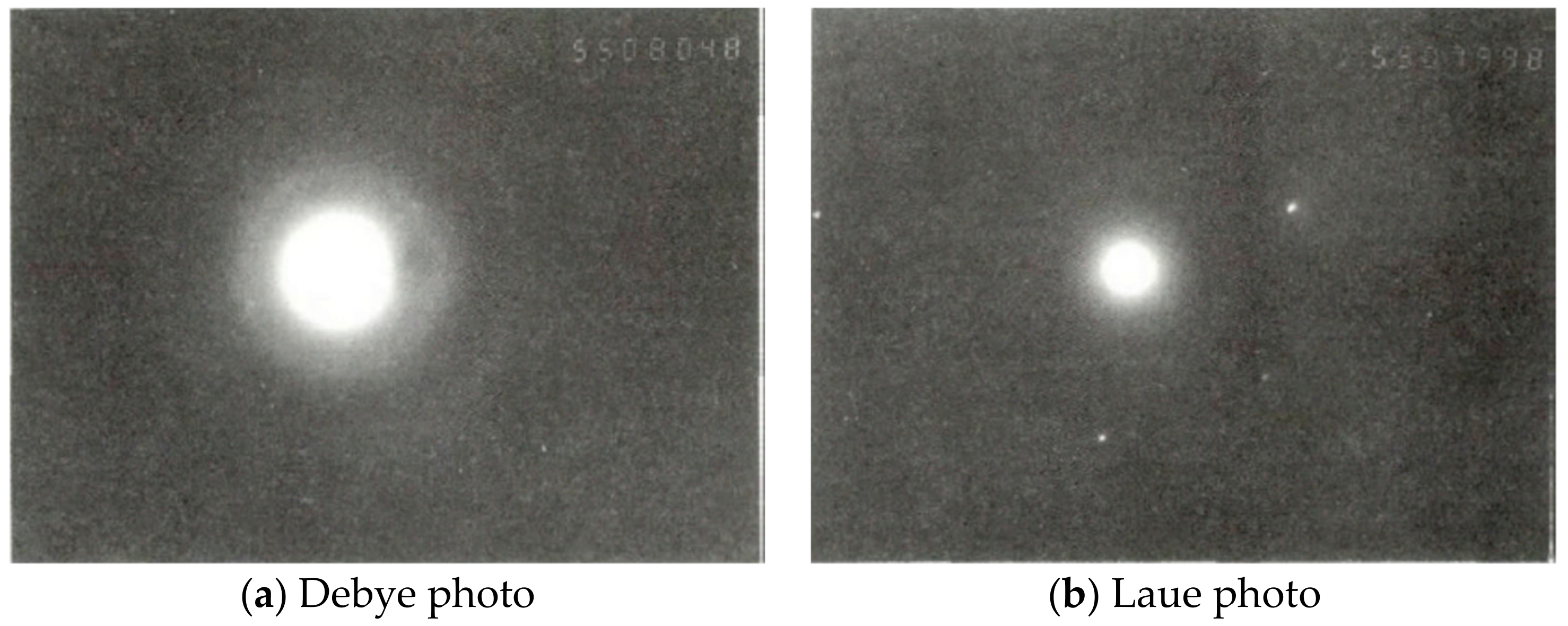
3.1. Nanodiamond Test
3.2. Electrochemical Test
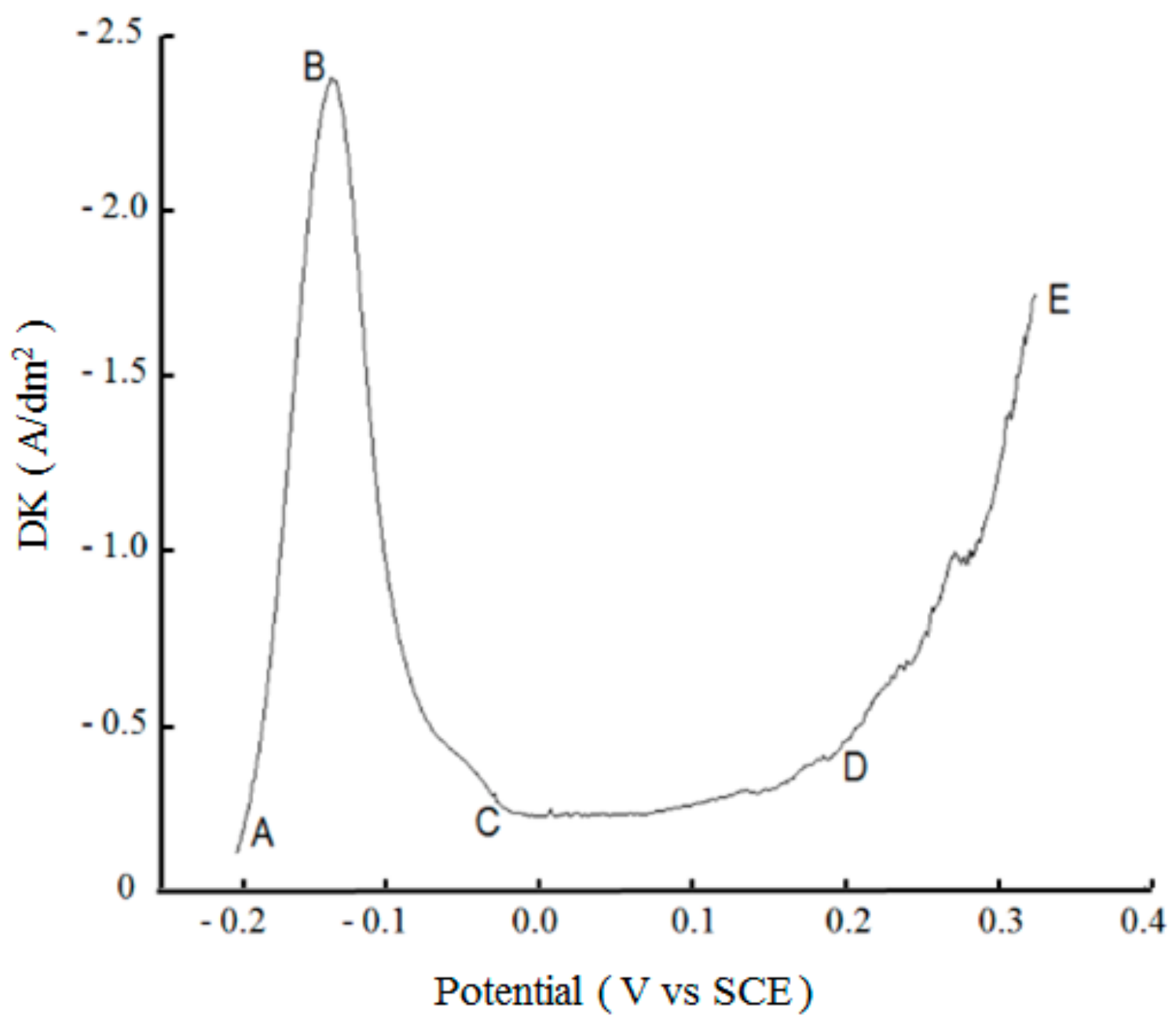
3.2.1. Anodic Polarization Process of Nickel Plate
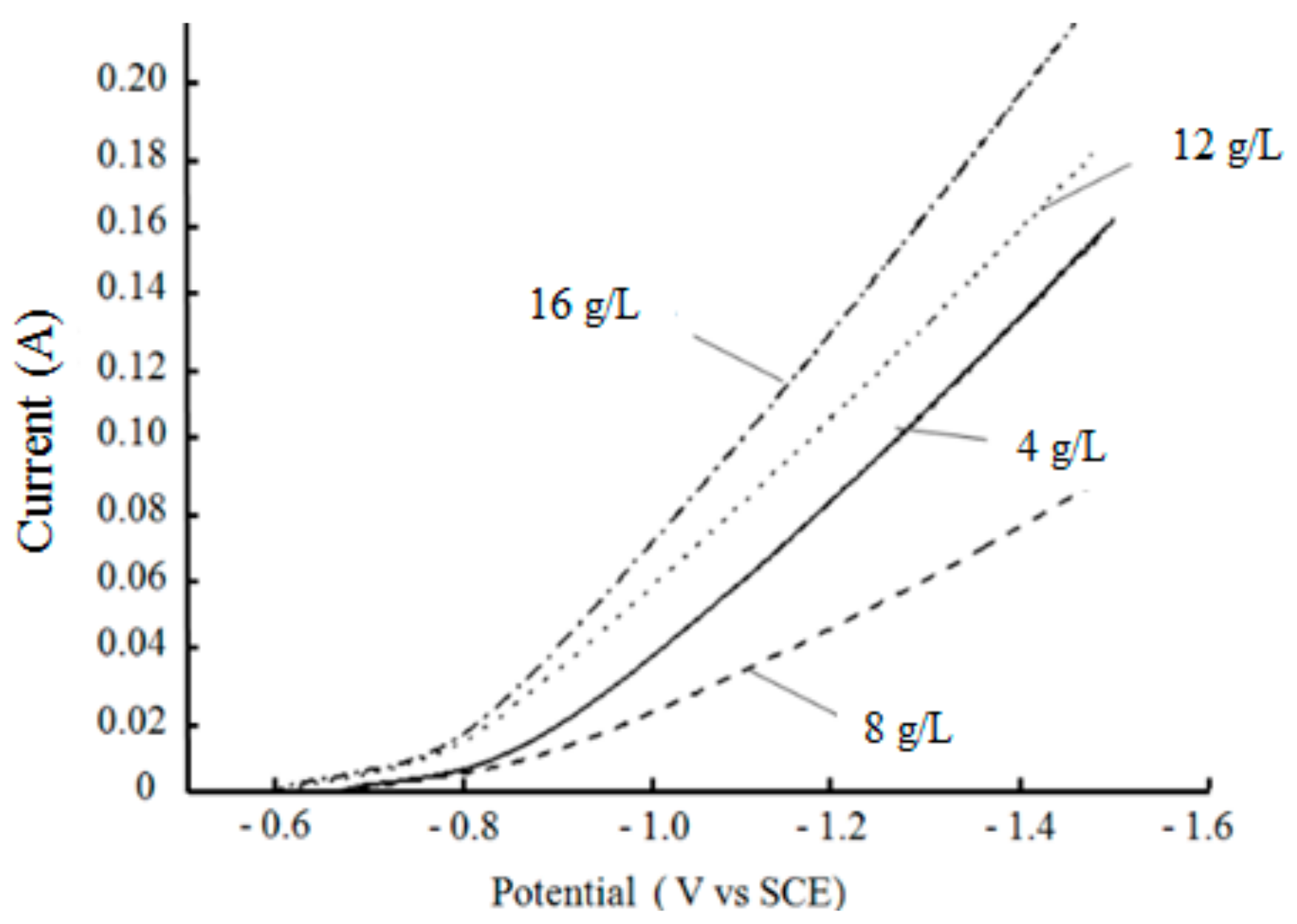
3.2.2. Effects of Nanodiamond Concentration on Cathodic Polarization Curves
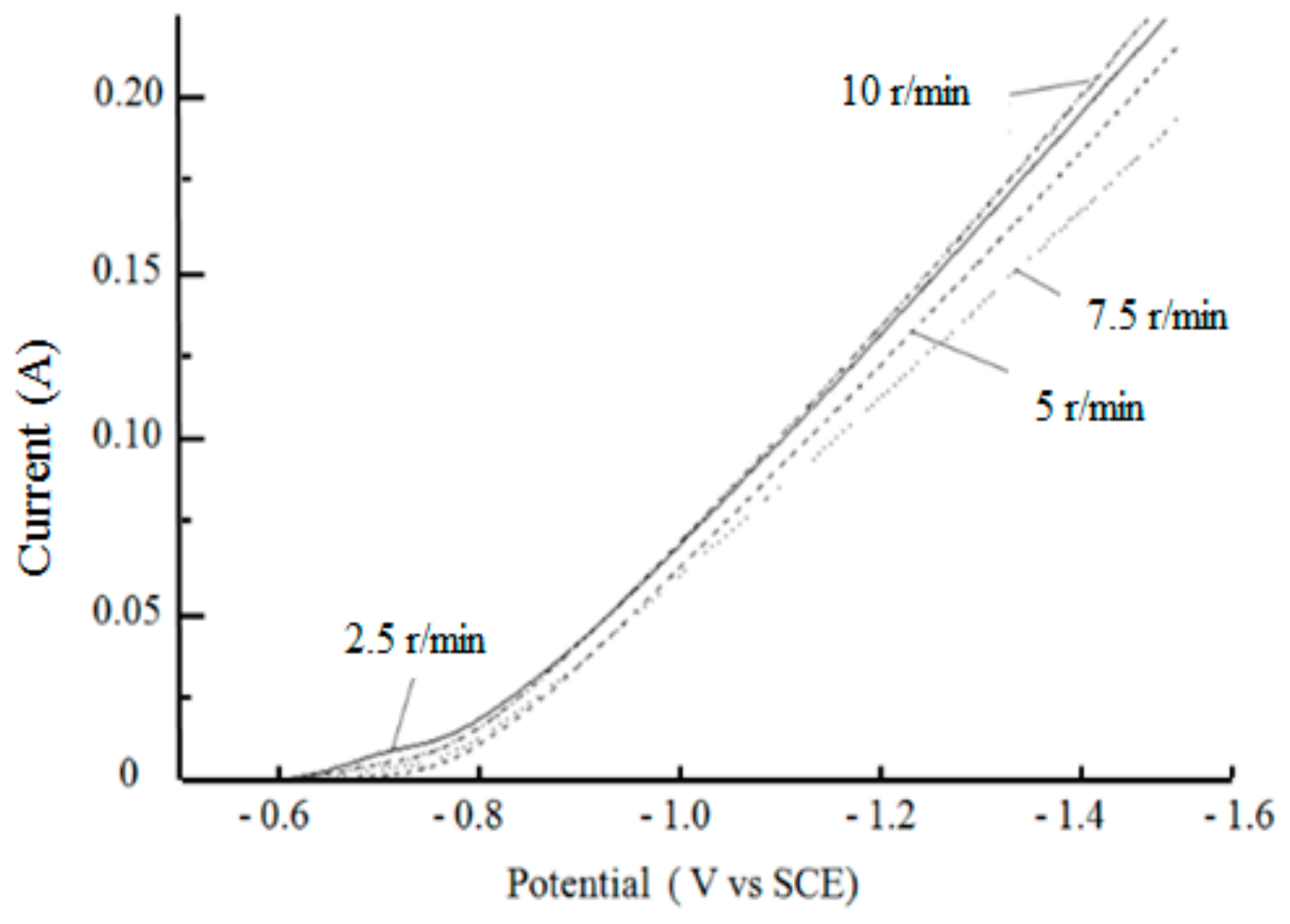
3.2.3. Effect of Stirring Speed on Cathodic Polarization Curves
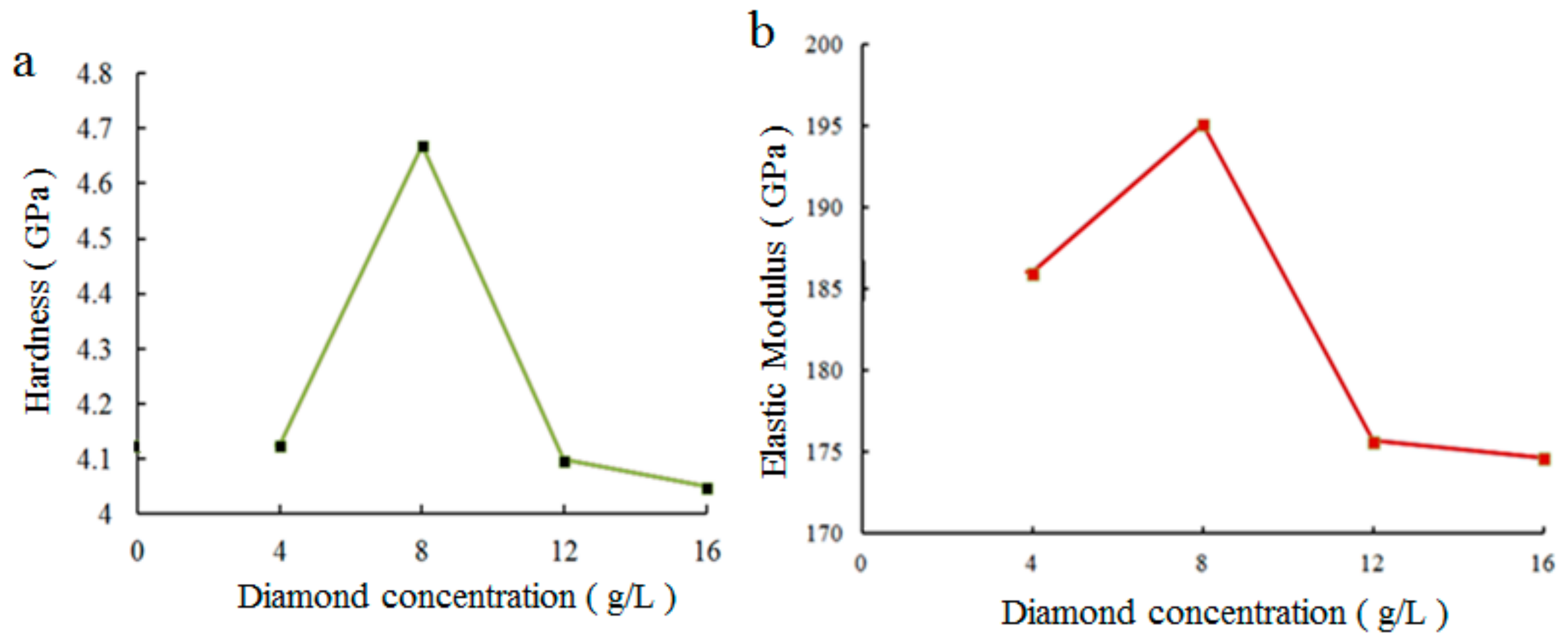
3.3. Effect of Nanodiamond Concentration in Bath on Properties of Composite Coatings Prepared by DC Electroplating
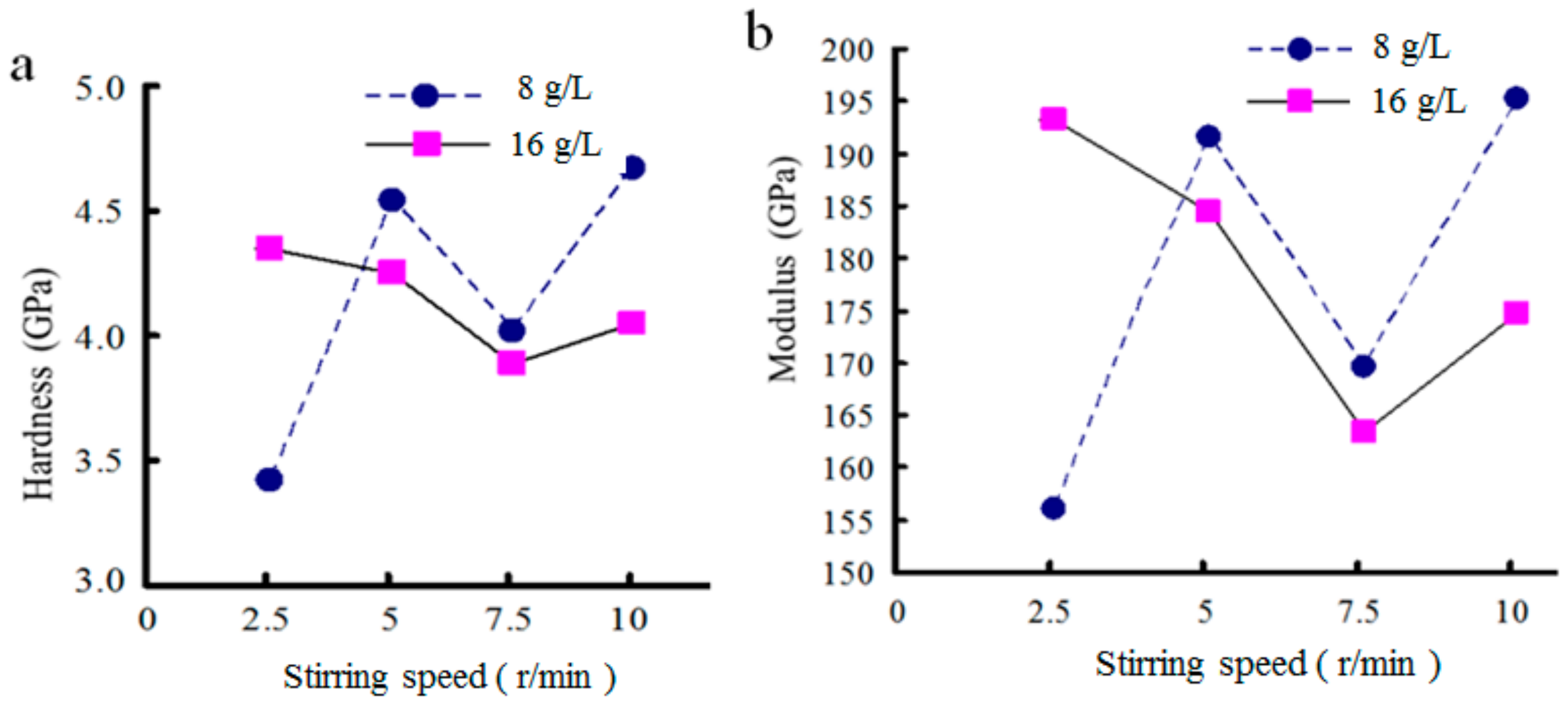
3.4. Effect of Stirring Speed on Properties of Composite Coatings Prepared by DC Electroplating
3.5. Effect of Electroplating Mode on Properties of Composite Coatings
3.5.1. Effect on the Hardness and Elastic Modulus of Composite Coatings
3.5.2. Effect on Surface Roughness Parameter Ra of Composite Coatings
3.5.3. Effect on Surface Morphology of Composite Coatings
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, W.; Zhao, Y.; Wang, X. Preparing a high-particle-content Ni/diamond composite coating with strong abrasive ability. Surf. Coat. Technol. 2013, 235, 489–494. [Google Scholar] [CrossRef]
- Vaezi, M.R.; Sadrnezhaad, S.K.; Nikzad, L. Electrodeposition of Ni–SiC nano-composite coatings and evaluation of wear and corrosion resistance and electroplating characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 176–182. [Google Scholar] [CrossRef]
- Walsh, F.C.; Low, C.T.; Bello, J.O. Influence of Surfactants on Electrodeposition of a Ni-Nanoparticulate SiC Composite Coating. Trans. Inst. Mater. Finish. 2015, 93, 147–156. [Google Scholar] [CrossRef]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Takebe, T.; Saji, T. Effect of particle size on the co-deposition of diamond with nickel in presence of a redox-active surfactant and mechanical property of the coatings. Diam. Relat. Mater. 2006, 15, 1570–1575. [Google Scholar] [CrossRef]
- Grosjean, A.; Rezrazi, M.; Takadoum, J.; Bercot, P. Hardness, friction and wear characteristics of nickel-SiC electroless composite deposits. Surf. Coat. Technol. 2001, 137, 92–96. [Google Scholar] [CrossRef]
- Hamed, M.; Saeed, A. Deposition characterization and electrochemical evaluation of Ni-P-nano diamond composite coatings. Appl. Surf. Sci. 2012, 258, 4574–4580. [Google Scholar]
- Jappes, J.W.; Ramamoorthy, B.; Nair, P.K. Novel approaches on the study of wear performance of electroless Ni-P/diamond composite deposites. J. Mater. Process. Technol. 2009, 209, 1004–1010. [Google Scholar] [CrossRef]
- Wun-Hsing, L.; Sen-Cheh, T.; Kung-Cheng, C. Effects of direct current and pulse–plating on the co-deposition of nickel and nanometer diamond powder. Surf. Coat. Technol. 1999, 120–121, 607–611. [Google Scholar]
- Luan, X.W.; Wang, M.Z.; Zhao, Y.C. Study on electroplated nickel-nanodiamond composite coating. Diam. Abras. Eng. 2005, 150, 20–22. [Google Scholar]
- Petrov, I.; Detkov, P.; Drovosekov, A.; Ivanov, M.V.; Tyler, T.; Shenderova, O.; Voznecova, N.P.; Toporov, Y.P.; Schulz, D. Nickel galvanic coatings co-deposited with fractions of detonation nanodiamond. Diam. Relat. Mater. 2006, 15, 2035–2038. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Liu, H.; Xue, Q.; Xu, T. Effects of bivalent Co ion on the co-deposition of nickel and nano-diamond particles. Surf. Coat. Technol. 2005, 191, 1–6. [Google Scholar] [CrossRef]
- Medeliené, V.; Stankevič, V.; Bikulčius, G. The influence of artificial diamond additions on the formation and properties of an electroplated copper metal matrix coating. Surf. Coat. Technol. 2003, 168, 161–168. [Google Scholar] [CrossRef]
- Mandich, N.V.; Dennis, J.K. Codeposition of nanodiamonds with chromioum. Plat. Surf. Finish. 2001, 6, 117. [Google Scholar]
- Wang, L.P.; Gao, Y.; Xue, Q.J.; Liu, H.W.; Xu, T. Effect of nano-diamond particulates on the microstructure and wear-resistance of electrodeposited Ni-matrix coatings. Tribology 2004, l24, 488–492. [Google Scholar]
- Xie, H.B.; Zhang, L.X.; Ma, X.K. A study on properties and composite electroplating process of Cr-nanodiamond. Mater. Protect. 2006, 39, 31–33. [Google Scholar]
- Wang, B.C.; Zhu, Y.W.; Xu, X.Y.; Xie, S.Z. Preparation of Nanodiamond Suspension for Composite Cr-nanodiamond Coating. Surf. Technol. 2004, 33, 4–7. [Google Scholar]
- Yang, D.; Zhang, H.; Tong, X.; Zhang, Z.; Zheng, Z.; Fan, D. Tribological Bahavior of Chromium Based Nano-diamond composite coating. Heat Treat. Met. 2002, 27, 16–18. [Google Scholar]
- Zhao, Y.; Liu, M.H.; Feng, L.; Meng, Y.; Li, F.; Chen, Z. The process optimization for Ni coating electrodeposited on Q235A. Mater. Protect. 2014, 9, 32–35. (In Chinese) [Google Scholar]
- Yuan, X.; Wang, Y.; Sun, D.; Yu, H. Influence of pulse parameters on the microstructure and microhardness of nickel electrodeposits. Mater. Sci. Technol. 2010, 18, 90–95. [Google Scholar]
- Feng, L.M.; Wang, Y. Electroplating Technology; Chemical Industry Press: Beijing, China, 2010. (In Chinese) [Google Scholar]
- Liu, H.J.; Shi, X.P. On research of HT-I reagent for controlling pinpoint hole roof when nickel plating. Tianjin Chem. Ind. 2006, 20, 37–38. (In Chinese) [Google Scholar]
- Luo, H.X.; Huang, X.G.; Mei, Y.X. Causes and solutions of pinholes fault on the Nickel coating. Hydraul. Pneum. Seals 2014, 2, 62–63. [Google Scholar]
- Guglielmi, N. Kinetics of the deposition of inert particles from electrolytic baths. J. Electrochem. Soc. 1972, 119, 1009–1012. [Google Scholar] [CrossRef]
- Xiao, X.; Chu, R.B. Causes and trouble–shootings for pores and pits on nickel electrode posits. Electroplat. Finish. 2004, 23, 53–58. [Google Scholar]
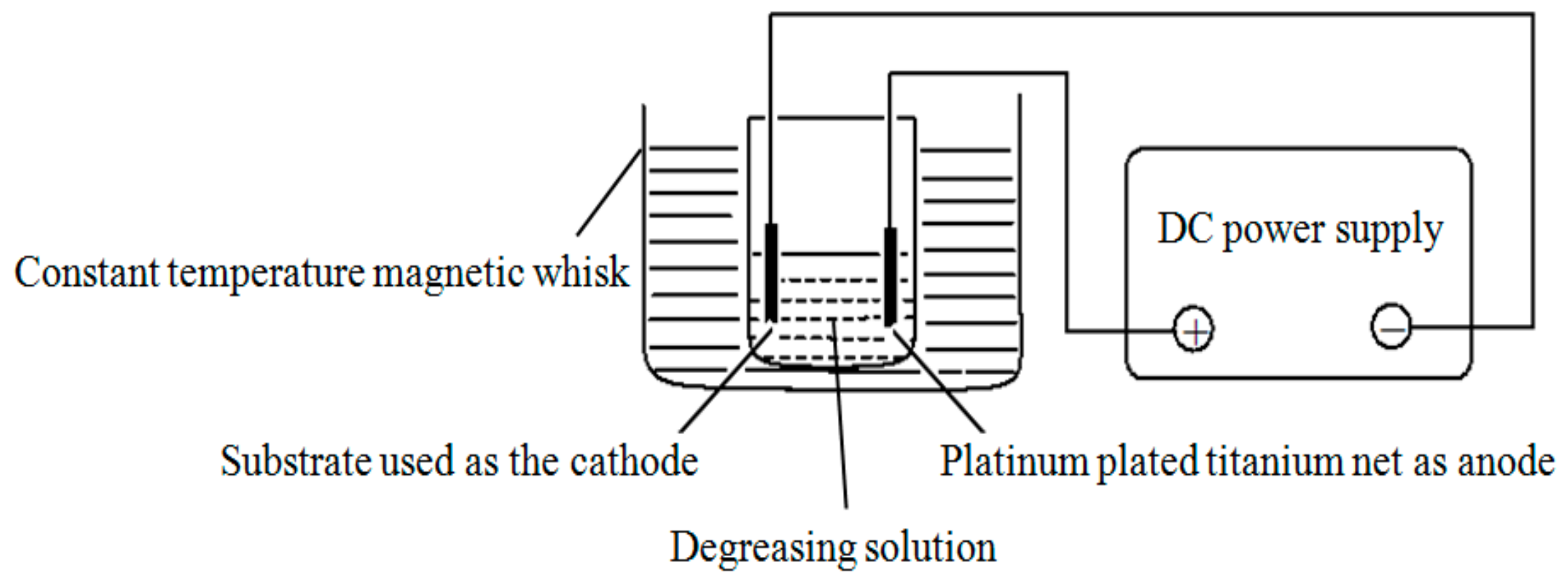




| Chemicals and Parameters | Chemical Degreasing | Electrochemical Degreasing |
|---|---|---|
| NaOH (g/L) | 65 | 15 |
| Na2CO3 (g/L) | 17.5 | 55 |
| Na3PO4·12H2O (g/L) | 17.5 | 35 |
| Na2SiO3·9H2O (g/L) | 5 | 7.5 |
| Temperature (°C) | 70 | 70 |
| Time (min) | 2 | 2 |
| Current (A) | – | 0.2 |
| Parameter | Experiment 1 | Experiment 2 | ||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| Pulse frequency (Hz) | 1000 | 1000 | 1000 | 1000 |
| Average current (A) | 0.06 | 0.02 | 0.06 | 0.01 |
| Operating time (ms) | 50 | 10 | 100 | 10 |
| Duty cycle (%) | 20 | 10 | 20 | 10 |
| Instrument | Model | Manufacturer |
|---|---|---|
| Electrochemical workstation | CHI660E | Beijing Huake Putian Science and Technology Co., Ltd. (Beijing, China) |
| Heat-collection thermostatic heating magnetic stirrer | DF-101S | Gongyi City Yuhua Instrument Co., Ltd. (Gongyi, China) |
| Working electrode (steel disc) | Q235A | – |
| Auxiliary electrode (nickel plate) | – | – |
| Reference electrode (saturated calomel electrode, SCE) | CHI150 | Tianjin Aida Hengsheng Technology Development Co., Ltd. (Tianjin, China) |
| Luggin capillary salt bridge | – | Self-made |
| Element | Fe | Cr | Si | Al | Na | K | Cu |
| Content | 0.150 | 0.070 | 0.300 | 0.005 | 0.030 | 0.002 | 0.005 |
| Element | Ca | Mg | Mn | Ti | Pb | Incombustible Substance | Total |
| Content | 0.002 | 0.005 | 0.001 | 0.010 | 0.001 | 0.95 | 1.531 |
| Crystal Face | (111) | (220) | (113) | (400) | (331) |
|---|---|---|---|---|---|
| Nanodiamond Standard diamond | 100 100 | 16 25 | 0.6 16 | 0.4 8 | 0.2 16 |
| Electroplating Mode | Hardness (GPa) | Elastic Modulus (GPa) | ||
|---|---|---|---|---|
| DC electroplating | 4.68 | 194.30 | ||
| Double-pulse electroplating | Operating time (ms) | 50 | 5.22 | 197.38 |
| 100 | 5.14 | 192.5 | ||
| Electrodeposition Mode | Surface Roughness Ra (µm) | ||
|---|---|---|---|
| DC electroplating | 1.184 | ||
| Double-pulse electroplating | Operating time (ms) | 50 | 0.792 |
| 100 | 1.006 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Wang, D.; Wang, H.; Shi, Y.; Liu, B.; Li, F.; Gong, Y.; Zhang, W. Study on Optimization Technology to Strengthen Ni-Based Composite Coating Electroplate Containing Nanodiamond. Materials 2019, 12, 1654. https://doi.org/10.3390/ma12101654
Liu M, Wang D, Wang H, Shi Y, Liu B, Li F, Gong Y, Zhang W. Study on Optimization Technology to Strengthen Ni-Based Composite Coating Electroplate Containing Nanodiamond. Materials. 2019; 12(10):1654. https://doi.org/10.3390/ma12101654
Chicago/Turabian StyleLiu, Meihua, Dongai Wang, Huaiwen Wang, Yan Shi, Bing Liu, Feihui Li, Yunlan Gong, and Wengang Zhang. 2019. "Study on Optimization Technology to Strengthen Ni-Based Composite Coating Electroplate Containing Nanodiamond" Materials 12, no. 10: 1654. https://doi.org/10.3390/ma12101654




