Multifunctional Metallic Nanowires in Advanced Building Applications
Abstract
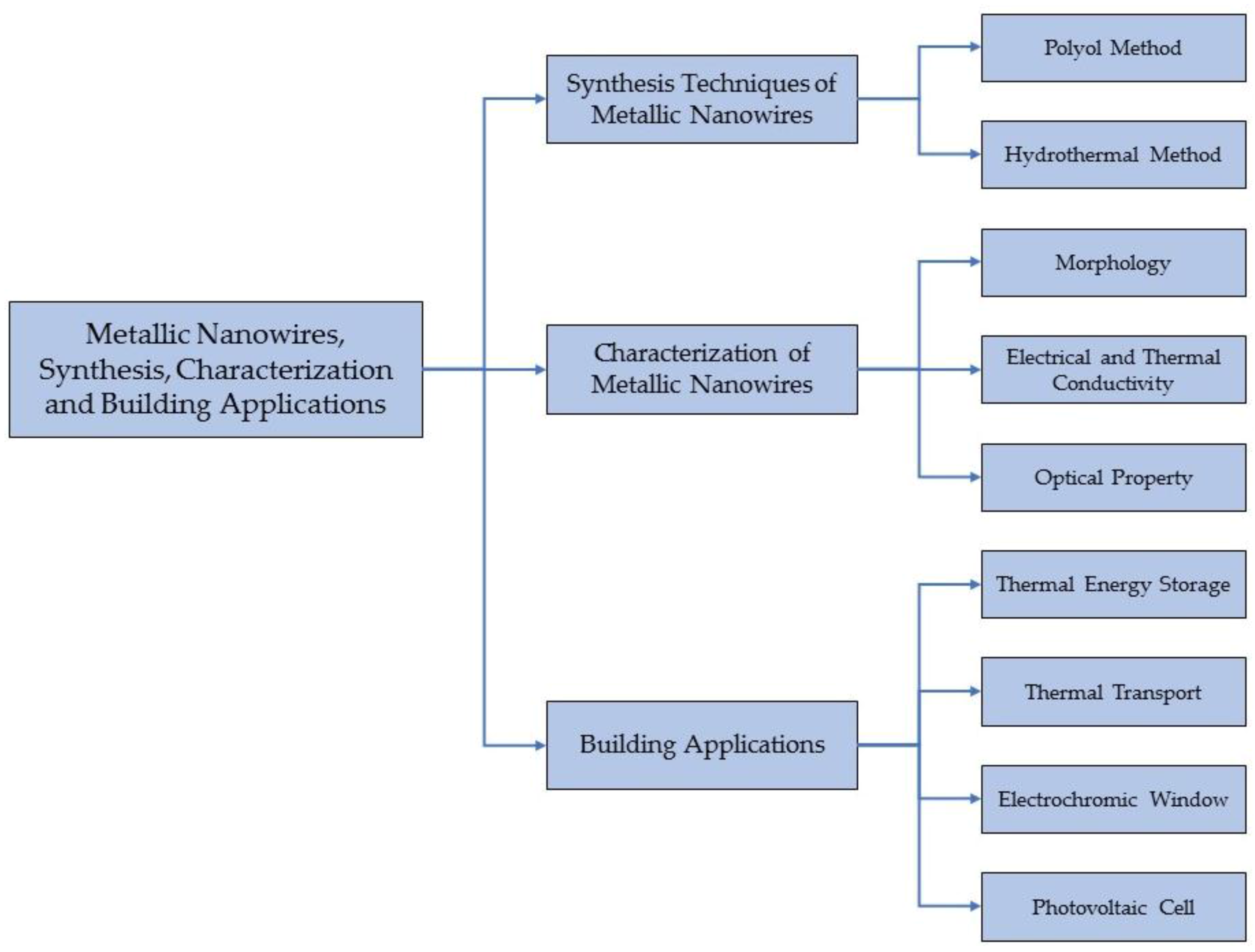
:1. Introduction
2. Synthesis Techniques of Metallic NWs
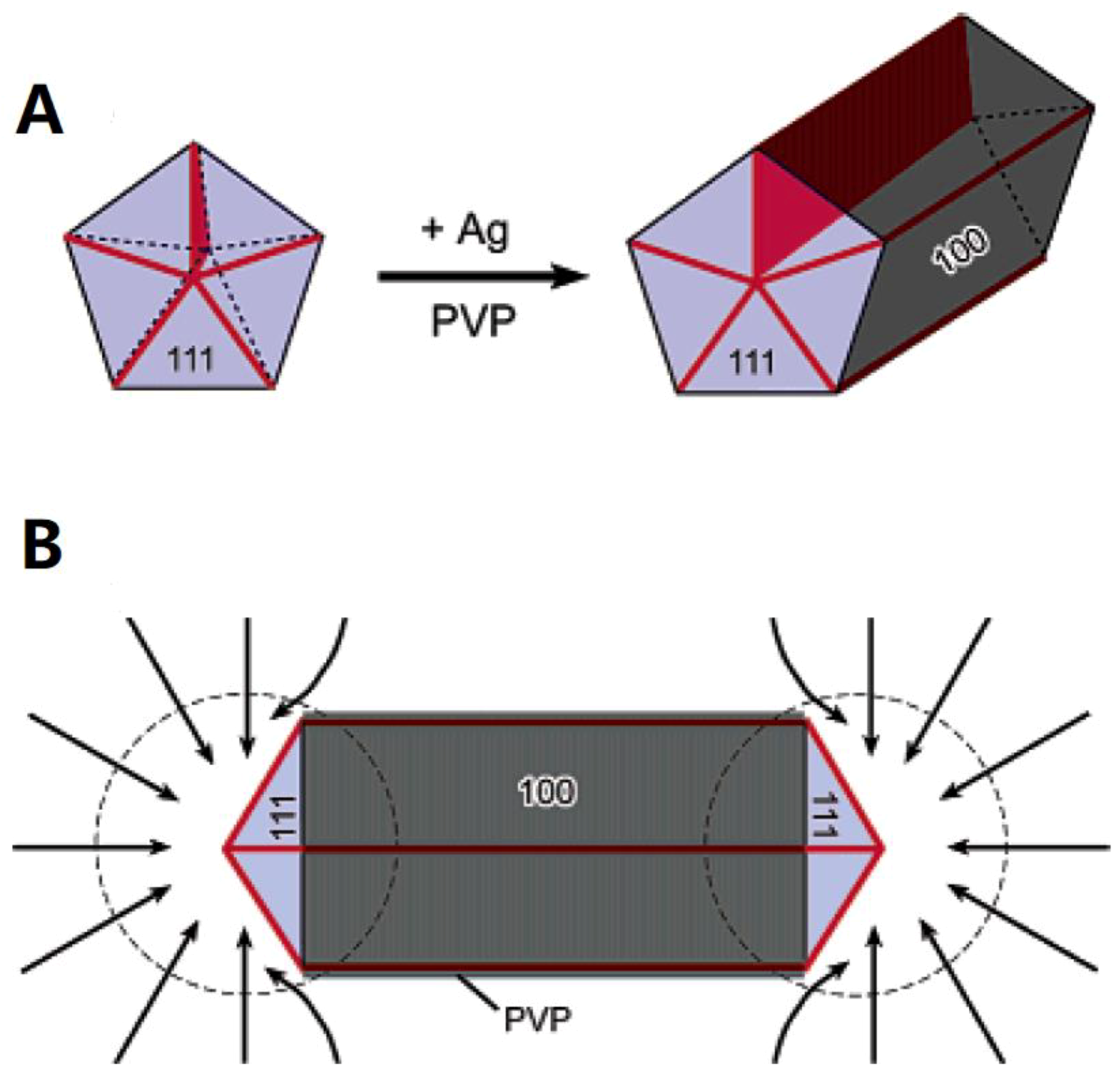
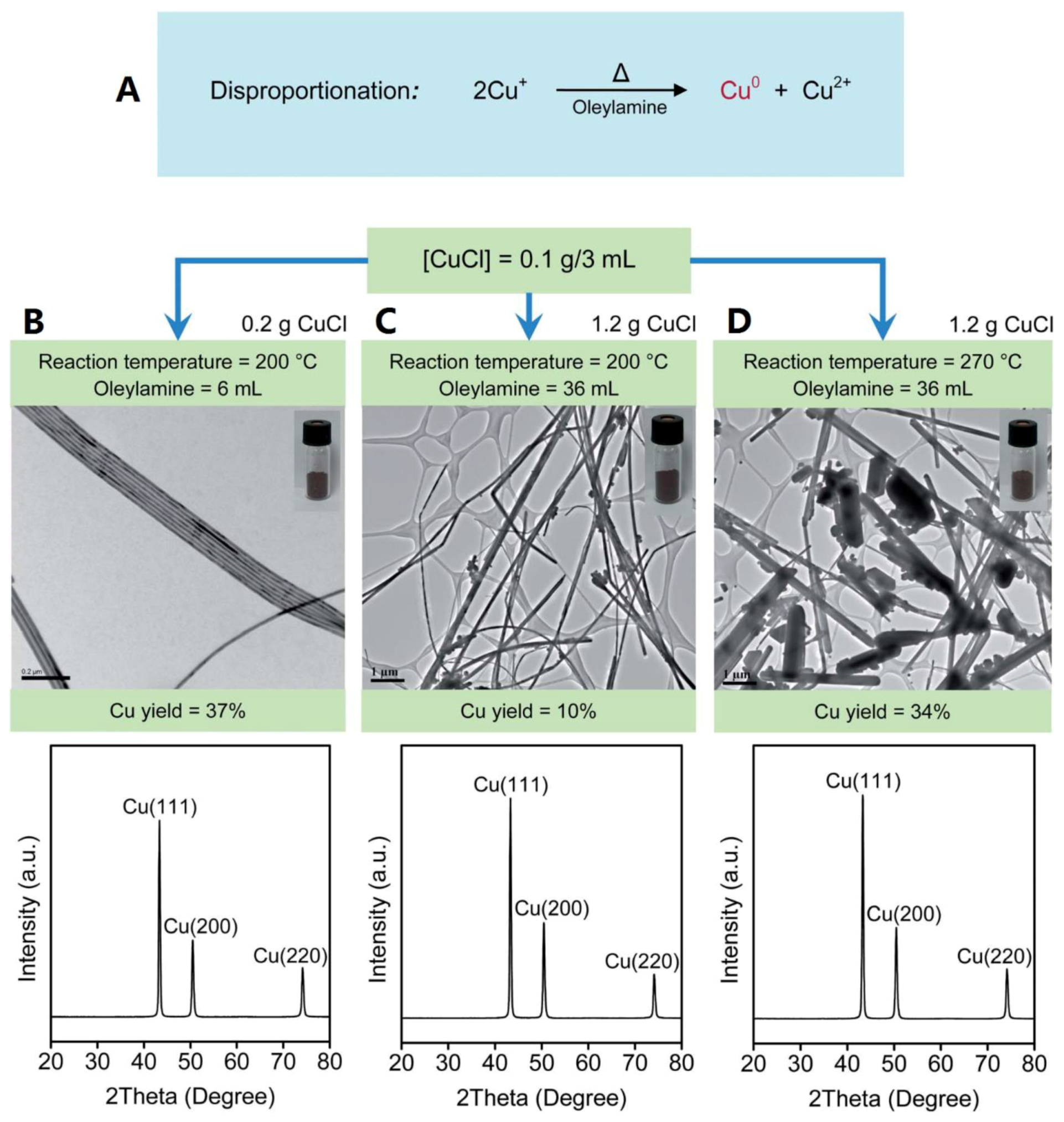
2.1. Polyol Method
2.2. Hydrothermal Method
3. Characterization of Metallic NWs
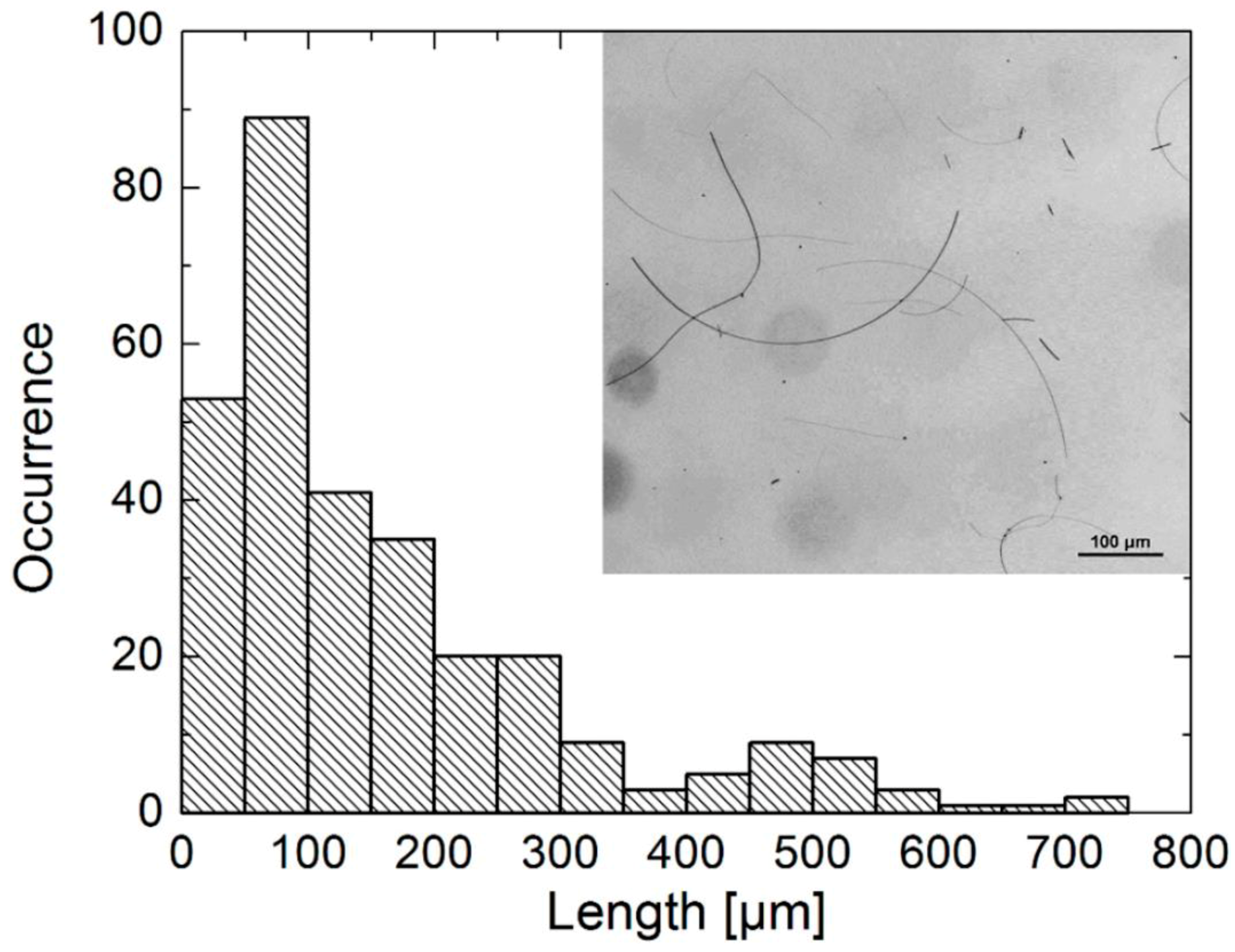
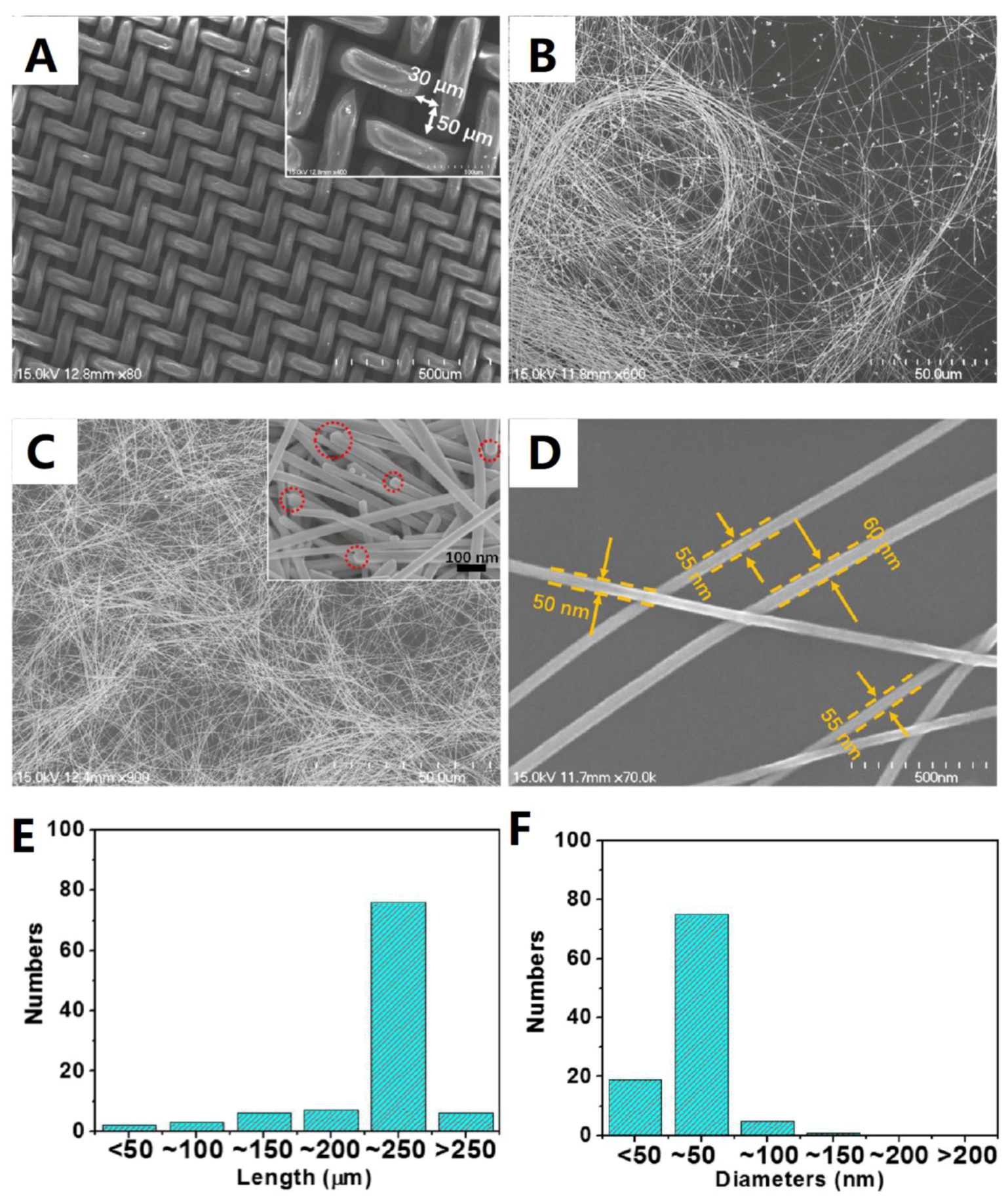
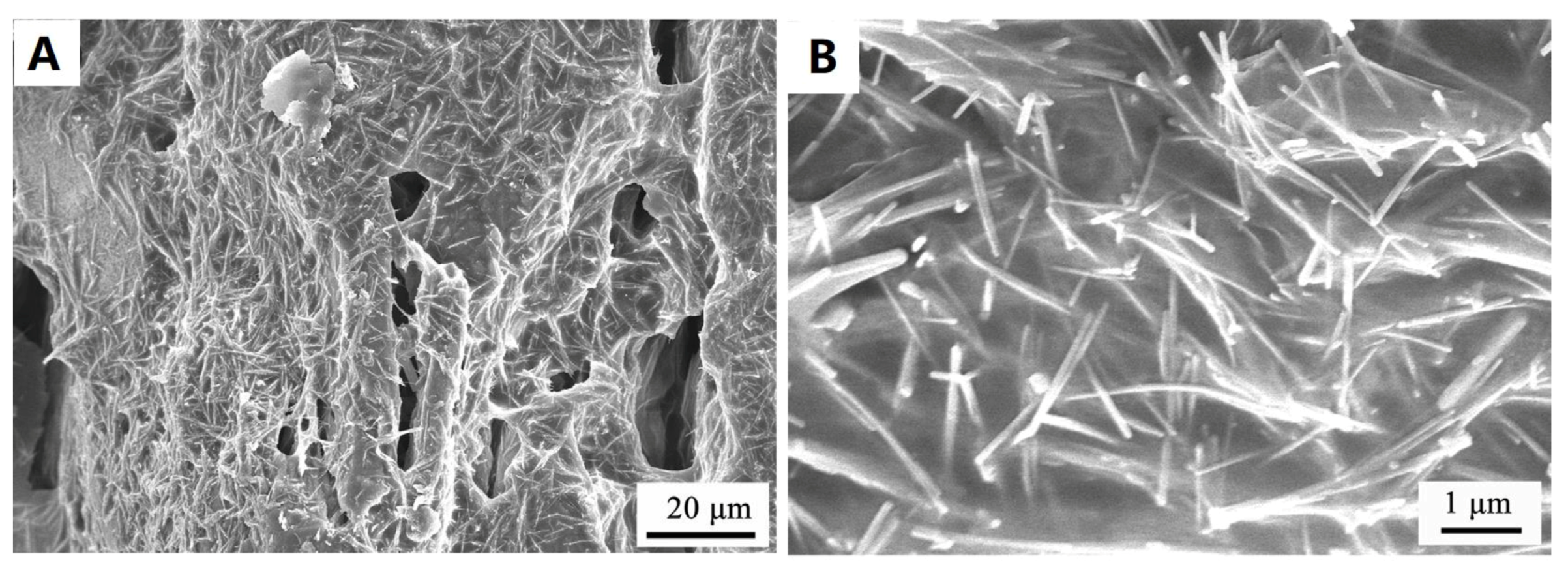
3.1. Morphology
3.2. Electrical and Thermal Conductivity
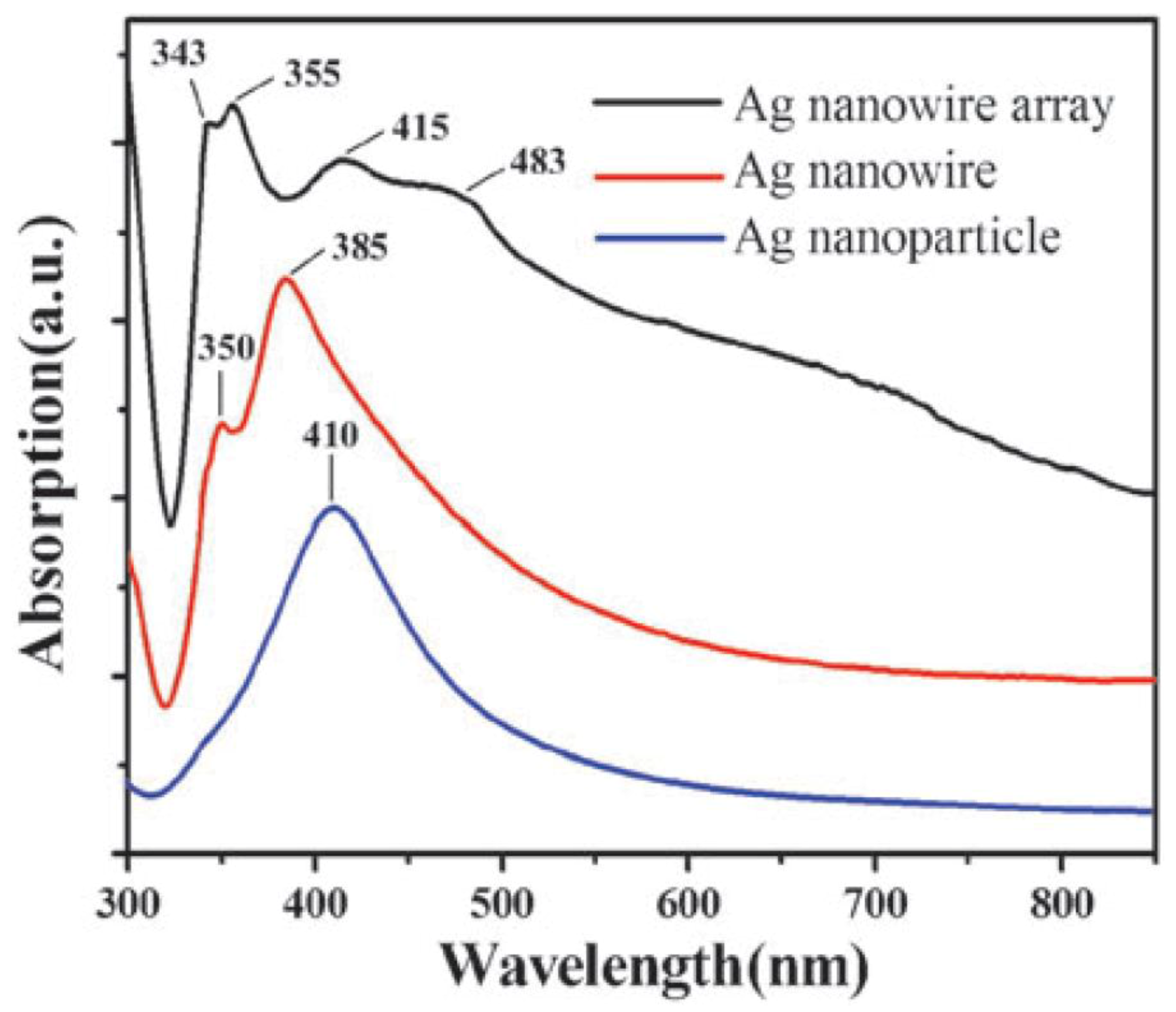
3.3. Optical Property
4. Building Applications Using Metallic NWs
4.1. Thermal Energy Storage
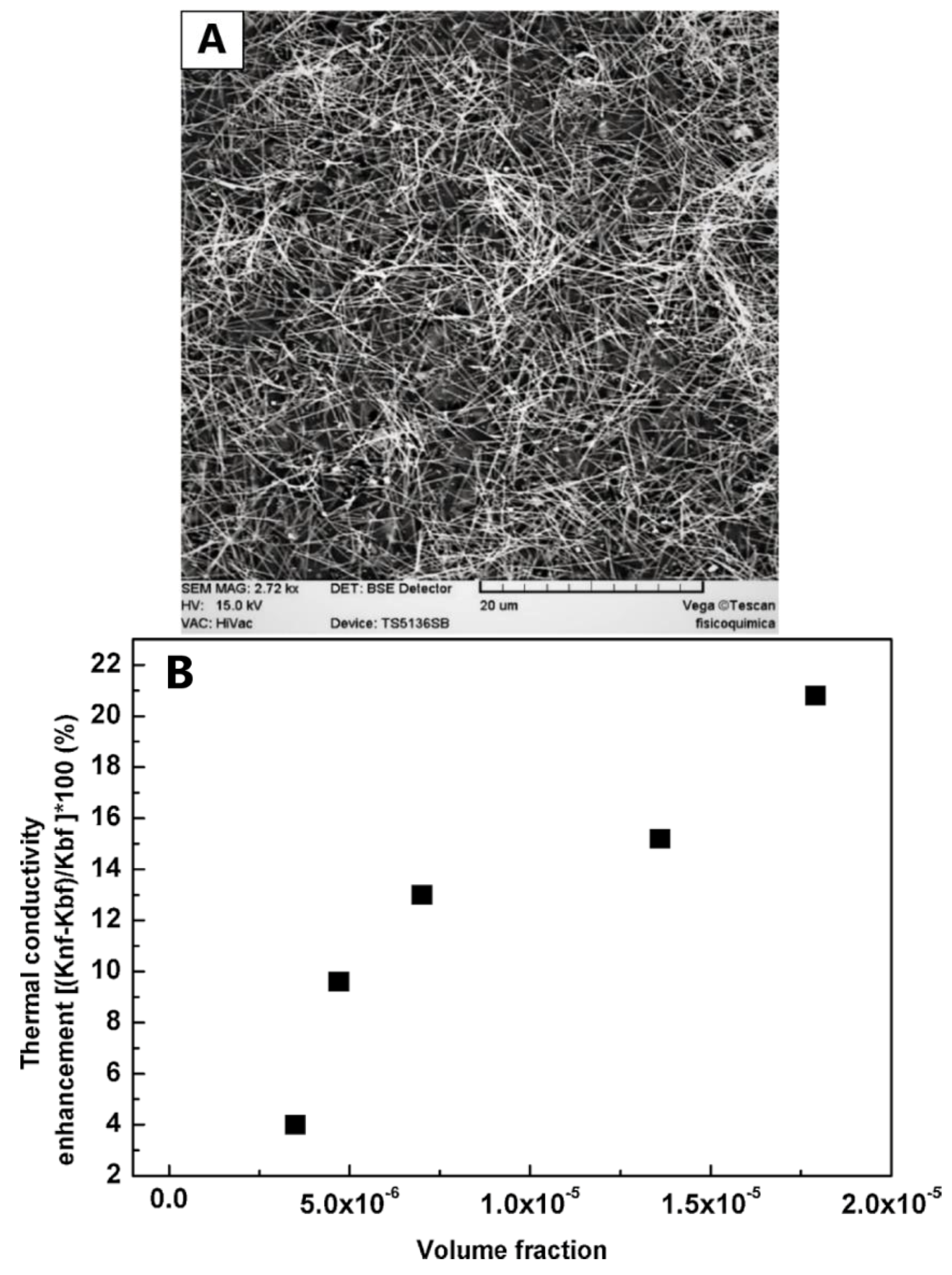
4.2. Thermal Transport
4.3. Electrochromic Window
4.4. Photovoltaic Cell
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Li, Q.-K.; Li, M. Internal stress and its effect on mechanical strength of metallic glass nanowires. Acta Mater. 2015, 91, 174–182. [Google Scholar] [CrossRef]
- Basarir, F.; Irani, F.S.; Kosemen, A.; Camic, B.T.; Oytun, F.; Tunaboylu, B.; Shin, H.J.; Nam, K.Y.; Choi, H. Recent progresses on solution-processed silver nanowire based transparent conducting electrodes for organic solar cells. Mater. Today Chem. 2017, 3, 60–72. [Google Scholar] [CrossRef]
- Shah, K.W.; Lu, Y. Morphology, large scale synthesis and building applications of copper nanomaterials. Constr. Build. Mater. 2018, 180, 544–578. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, S.; Wang, R.; Sun, J.; Gao, L. Copper nanowire based transparent conductive films with high stability and superior stretchability. J. Mater. Chem. C 2014, 2, 5309–5316. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Wiley, B.J. The synthesis and coating of long, thin copper nanowires to make flexible, transparent conducting films on plastic substrates. Adv. Mater. 2011, 23, 4798–4803. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Solution-processed metallic conducting polymer films as transparent electrode of optoelectronic devices. Adv. Mater. 2012, 24, 2436–2440. [Google Scholar]
- Lonjon, A.; Caffrey, I.; Carponcin, D.; Dantras, E.; Lacabanne, C. High electrically conductive composites of Polyamide 11 filled with silver nanowires: Nanocomposites processing, mechanical and electrical analysis. J. Non-Cryst. Solids 2013, 376, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.-C.; Chang, Y.-C.; Lin, Y.; Chang, S.-H.; Chang, W.-C.; Li, G.-A.; Tuan, H.-Y. Spray-Deposited Large-Area Copper Nanowire Transparent Conductive Electrodes and Their Uses for Touch Screen Applications. ACS Appl. Mater. Interfaces 2016, 8, 13009–13017. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, P.; Lee, H.; Lee, D.; Lee, S.S.; Ko, S.H. Very long Ag nanowire synthesis and its application in a highly transparent, conductive and flexible metal electrode touch panel. Nanoscale 2012, 4, 6408–6414. [Google Scholar] [CrossRef]
- Oo, T.Z.; Mathews, N.; Xing, G.; Wu, B.; Xing, B.; Wong, L.H.; Sum, T.C.; Mhaisalkar, S.G. Ultrafine Gold Nanowire Networks as Plasmonic Antennae in Organic Photovoltaics. J. Phys. Chem. C 2012, 116, 6453–6458. [Google Scholar]
- Otnes, G.; Borgström, M.T. Towards high efficiency nanowire solar cells. Nano Today 2017, 12, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Du, L.; Tan, S.; Zang, Z.; Zhao, C.; Mai, W. Flexible electrochromic supercapacitor hybrid electrodes based on tungsten oxide films and silver nanowires. Chem. Commun. 2016, 52, 6296–6299. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, H.; Lee, J.; Kwon, J.; Han, S.; Suh, Y.D.; Cho, H.; Shin, J.; Yeo, J.; Ko, S.H. Highly Stretchable and Transparent Metal Nanowire Heater for Wearable Electronics Applications. Adv. Mater. 2015, 27, 4744–4751. [Google Scholar] [CrossRef]
- Sreethawong, T.; Shah, K.W.; Zhang, S.-Y.; Ye, E.; Lim, S.H.; Maheswaran, U.; Mao, W.Y.; Han, M.-Y. Optimized production of copper nanostructures with high yields for efficient use as thermal conductivity-enhancing PCM dopant. J. Mater. Chem. A 2014, 2, 3417–3423. [Google Scholar] [CrossRef]
- Shah, K.W. A review on enhancement of phase change materials—A nanomaterials perspective. Energy Build. 2018, 175, 57–68. [Google Scholar] [CrossRef]
- Lee, J.; Mahendra, S.; Alvarez, P.J.J. Nanomaterials in the construction industry: A review of their applications and environmental health and safety considerations. ACS Nano 2010, 4, 3580–3590. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, Y.; Wang, R.; Sun, J.; Gao, L. Highly thermal conductive copper nanowire composites with ultralow loading: Toward applications as thermal interface materials. ACS Appl. Mater. Interfaces 2014, 6, 6481–6486. [Google Scholar] [CrossRef]
- Hu, L.; Kim, H.S.; Lee, J.-Y.; Peumans, P.; Cui, Y. Scalable coating and properties of transparent, flexible, silver nanowire electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wu, Y.; Wang, Z.; Zhao, Y.; Sun, L. Electrospinning of Ag Nanowires/polyvinyl alcohol hybrid nanofibers for their antibacterial properties. Mater. Sci. Eng. C 2017, 78, 706–714. [Google Scholar] [CrossRef]
- Kaimlová, M.; Nemogová, I.; Kolářová, K.; Slepička, P.; Švorčík, V.; Siegel, J. Optimization of silver nanowire formation on laser processed PEN: Surface properties and antibacterial effects. Appl. Surf. Sci. 2019, 473, 516–526. [Google Scholar] [CrossRef]
- Tang, C.; Sun, W.; Lu, J.; Yan, W. Role of the anions in the hydrothermally formed silver nanowires and their antibacterial property. J. Colloid. Interface Sci. 2014, 416, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Sofiah, A.G.N.; Samykano, M.; Kadirgama, K.; Mohan, R.V.; Lah, N.A.C. Metallic nanowires: Mechanical properties—Theory and experiment. Appl. Mater. Today 2018, 11, 320–337. [Google Scholar] [CrossRef]
- Bera, D.; Kuiry, S.C.; Seal, S.J.J. Synthesis of nanostructured materials using template-assisted electrodeposition. Jom 2004, 56, 49–53. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Liang, J.; Hu, Z.; Li, S.; Peng, S.; Qian, Y. Synthesis of Copper Nanowires via a Complex-Surfactant-Assisted Hydrothermal Reduction Process. J. Phys. Chem. B 2003, 107, 12658–12661. [Google Scholar] [CrossRef]
- Zheng, X.-J.; Jiang, Z.-Y.; Xie, Z.-X.; Zhang, S.-H.; Mao, B.-W.; Zheng, L.-S. Growth of silver nanowires by an unconventional electrodeposition without template. Electrochem. Commun. 2007, 9, 629–632. [Google Scholar] [CrossRef]
- Liu, L.; He, C.; Li, J.; Guo, J.; Yang, D.; Wei, J. Green synthesis of silver nanowires via ultraviolet irradiation catalyzed by phosphomolybdic acid and their antibacterial properties. New J. Chem. 2013, 37, 2179–2185. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; Huang, L.; Pan, D. Large-scale synthesis of well-dispersed copper nanowires in an electric pressure cooker and their application in transparent and conductive networks. Inorg. Chem. 2014, 53, 4440–4444. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Gu, W.; Briceno, M.; Robertson, I.M.; Choi, H.; Kim, K. Copper Nanowires with a Five-Twinned Structure Grown by Chemical Vapor Deposition. Adv. Mater. 2008, 20, 1859–1863. [Google Scholar] [CrossRef]
- Zhang, P.; Wyman, I.; Hu, J.; Lin, S.; Zhong, Z.; Tu, Y.; Huang, Z.; Wei, Y. Silver nanowires: Synthesis technologies, growth mechanism and multifunctional applications. Mater. Sci. Eng. B 2017, 223, 1–23. [Google Scholar] [CrossRef]
- Hu, Z.; Kong, C.; Han, Y.; Zhao, H.; Yang, Y.; Wu, H. Large-scale synthesis of defect-free silver nanowires by electrodeless deposition. Mater. Lett. 2007, 61, 3931–3934. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, W.L.; McCaughy, B.F.; Hampsey, J.E.; Ji, X.; Jiang, Y.-B.; Xu, H.; Tang, J.; Schmehl, R.H.; O’Connor, C.; et al. Electrodeposition of Metallic Nanowire Thin Films Using Mesoporous Silica Templates. Adv. Mater. 2003, 15, 130–133. [Google Scholar] [CrossRef]
- Govindaraj, A.; Satishkumar, B.C.; Nath, M.; Rao, C.N.R. Metal Nanowires and Intercalated Metal Layers in Single-Walled Carbon Nanotube Bundles. Chem. Mater. 2000, 12, 202–205. [Google Scholar] [CrossRef]
- Lazzara, T.D.; Bourret, G.R.; Lennox, R.B.; van de Ven, T.G.M. Polymer Templated Synthesis of AgCN and Ag Nanowires. Chem. Mater. 2009, 21, 2020–2026. [Google Scholar] [CrossRef]
- Abbasi, N.M.; Yu, H.; Wang, L.; Amer, W.A.; Akram, M.; Khalid, H.; Chen, Y.; Saleem, M.; Sun, R. Preparation of silver nanowires and their application in conducting polymer nanocomposites. Mater. Chem. Phys. 2015, 166, 1–15. [Google Scholar] [CrossRef]
- Song, Y.; Garcia, R.M.; Dorin, R.M.; Wang, H.; Qiu, Y.; Coker, E.N.; Steen, W.A.; Miller, J.E.; Shelnutt, J.A. Synthesis of Platinum Nanowire Networks Using a Soft Template. Nano Lett. 2007, 7, 3650–3655. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, L.; Ma, J.; Cheng, H. Formation of Silver Nanowires in Aqueous Solutions of a Double-Hydrophilic Block Copolymer. Chem. Mater. 2001, 13, 2753–2755. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Liao, X.-H.; Zhao, X.-N.; Chen, H.-Y. Preparation of silver nanorods by electrochemical methods. Mater. Lett. 2001, 49, 91–95. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.; Herricks, T.; Xia, Y. Polyol Synthesis of Uniform Silver Nanowires: A Plausible Growth Mechanism and the Supporting Evidence. Nano Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Chen, J.; Wiley, B.J.; Xia, Y. One-dimensional nanostructures of metals: Large-scale synthesis and some potential applications. Langmuir 2007, 23, 4120–4129. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, Y.; Yang, L.; Han, D.; Lü, Z. A novel solution-phase route for the synthesis of crystalline silver nanowires. Mater. Res. Bull. 2005, 40, 1796–1801. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Green Synthesis of Ag and Pd Nanospheres, Nanowires, and Nanorods Using Vitamin: Catalytic Polymerisation of Aniline and Pyrrole. J. Nanomater. 2008, 2008, 3. [Google Scholar] [CrossRef]
- Sławiński, G.W.; Zamborini, F.P. Synthesis and Alignment of Silver Nanorods and Nanowires and the Formation of Pt, Pd, and Core/Shell Structures by Galvanic Exchange Directly on Surfaces. Langmuir 2007, 23, 10357–10365. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, Y. Large-Scale Synthesis of Uniform Silver Nanowires Through a Soft, Self-Seeding, Polyol Process. Adv. Mater. 2002, 14, 833–837. [Google Scholar] [CrossRef]
- Seshadri, I.; Esquenazi, G.L.; Cardinal, T.; Borca-Tasciuc, T.; Ramanath, G. Microwave synthesis of branched silver nanowires and their use as fillers for high thermal conductivity polymer composites. Nanotechnology 2016, 27, 175601. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, M.; Matsumoto, K.; Miyamae, N.; Tsuji, T.; Zhang, X. Rapid Preparation of Silver Nanorods and Nanowires by a Microwave-Polyol Method in the Presence of Pt Catalyst and Polyvinylpyrrolidone. Cryst. Growth Des. 2007, 7, 311–320. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Jiang, G.; Yang, Q.; Wang, J.; Yu, H.; Chen, T.; Wang, C.; Chen, X. The influence of seeding conditions and shielding gas atmosphere on the synthesis of silver nanowires through the polyol process. Nanotechnology 2006, 17, 466–474. [Google Scholar] [CrossRef]
- Coskun, S.; Aksoy, B.; Unalan, H.E. Polyol Synthesis of Silver Nanowires: An Extensive Parametric Study. Cryst. Growth Des. 2011, 11, 4963–4969. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Xia, Y. Polyol synthesis of silver nanostructures: Control of product morphology with Fe(II) or Fe(III) species. Langmuir 2005, 21, 8077–8080. [Google Scholar] [CrossRef]
- Korte, K.E.; Skrabalak, S.E.; Xia, Y. Rapid synthesis of silver nanowires through a CuCl- or CuCl2-mediated polyol process. J. Mater. Chem. 2008, 18, 437–441. [Google Scholar] [CrossRef]
- Lee, E.-J.; Chang, M.-H.; Kim, Y.-S.; Kim, J.-Y. High-pressure polyol synthesis of ultrathin silver nanowires: Electrical and optical properties. Apl. Mater. 2013, 1, 042118. [Google Scholar] [CrossRef] [Green Version]
- Jiu, J.; Murai, K.; Kim, K.; Kim, D.; Suganuma, K. Preparation of Ag nanorods with high yield by polyol process. Mater. Chem. Phys. 2009, 114, 333–338. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Yan, G. Review: Recent research progress on preparation of silver nanowires by soft solution method and their applications. Cryst. Res. Technol. 2011, 46, 427–438. [Google Scholar] [CrossRef]
- Choi, H.; Park, S.-H. Seedless Growth of Free-Standing Copper Nanowires by Chemical Vapor Deposition. J. Am. Chem. Soc. 2004, 126, 6248–6249. [Google Scholar] [CrossRef]
- Cui, F.; Yu, Y.; Dou, L.; Sun, J.; Yang, Q.; Schildknecht, C.; Schierle-Arndt, K.; Yang, P. Synthesis of Ultrathin Copper Nanowires Using Tris(trimethylsilyl)silane for High-Performance and Low-Haze Transparent Conductors. Nano Lett. 2015, 15, 7610–7615. [Google Scholar] [CrossRef]
- Haase, D.; Hampel, S.; Leonhardt, A.; Thomas, J.; Mattern, N.; Büchner, B. Facile one-step-synthesis of carbon wrapped copper nanowires by thermal decomposition of Copper (II)–acetylacetonate. Surf. Coat. Technol. 2007, 201, 9184–9188. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Bergin, S.M.; Hua, Y.-L.; Li, Z.-Y.; Wiley, B.J. The Growth Mechanism of Copper Nanowires and Their Properties in Flexible, Transparent Conducting Films. Adv. Mater. 2010, 22, 3558–3563. [Google Scholar] [CrossRef]
- Shi, Y.; Li, H.; Chen, L.; Huang, X. Obtaining ultra-long copper nanowires via a hydrothermal process. Sci. Technol. Adv. Mater. 2005, 6, 761–765. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-Q.; Zhang, M.; Wang, F.-X.; Pan, G.-B. Facile microwave-assisted synthesis of uniform single-crystal copper nanowires with excellent electrical conductivity. RSC Adv. 2012, 2, 11235–11237. [Google Scholar] [CrossRef]
- Meléndrez, M.F.; Medina, C.; Solis-Pomar, F.; Flores, P.; Paulraj, M.; Pérez-Tijerina, E. Quality and high yield synthesis of Ag nanowires by microwave-assisted hydrothermal method. Nanoscale Res. Lett. 2015, 10, 48. [Google Scholar] [CrossRef]
- Kumar, D.V.; Kim, I.; Zhong, Z.; Kim, K.; Lee, D.; Moon, J. Cu(II)-alkyl amine complex mediated hydrothermal synthesis of Cu nanowires: Exploring the dual role of alkyl amines. Phys. Chem. Chem. Phys. 2014, 16, 22107–22115. [Google Scholar] [CrossRef]
- Cwik, M.; Buczynska, D.; Sulowska, K.; Rozniecka, E.; Mackowski, S.; Niedziolka-Jonsson, J. Optical Properties of Submillimeter Silver Nanowires Synthesized Using the Hydrothermal Method. Materials 2019, 12, 721. [Google Scholar] [CrossRef]
- Bari, B.; Lee, J.; Jang, T.; Won, P.; Ko, S.H.; Alamgir, K.; Arshad, M.; Guo, L.J. Simple hydrothermal synthesis of very-long and thin silver nanowires and their application in high quality transparent electrodes. J. Mater. Chem. A 2016, 4, 11365–11371. [Google Scholar]
- Zhang, Y.; Guo, J.; Xu, D.; Sun, Y.; Yan, F. One-Pot Synthesis and Purification of Ultralong Silver Nanowires for Flexible Transparent Conductive Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 25465–25473. [Google Scholar] [CrossRef]
- Zeng, J.-L.; Zhu, F.-R.; Yu, S.-B.; Zhu, L.; Cao, Z.; Sun, L.-X.; Deng, G.-R.; Yan, W.-P.; Zhang, L. Effects of copper nanowires on the properties of an organic phase change material. Sol. Energy Mater. Sol. Cells 2012, 105, 174–178. [Google Scholar] [CrossRef]
- Bid, A.; Bora, A.; Raychaudhuri, A.K. Temperature dependence of the resistance of metallic nanowires of diameter ≥ 15 nm: Applicability of Bloch-Grüneisen theorem. Phys. Rev. B 2006, 74, 035426. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, L.; Xu, S.; Lu, M.; Wang, X. Temperature Dependence of Electrical and Thermal Conduction in Single Silver Nanowire. Sci. Rep. 2015, 5, 10718. [Google Scholar] [CrossRef]
- Rojo, M.M.; Calero, O.C.; Lopeandia, A.F.; Rodriguez-Viejo, J.; Martín-Gonzalez, M. Review on measurement techniques of transport properties of nanowires. Nanoscale 2013, 5, 11526–11544. [Google Scholar] [PubMed]
- He, G.-C.; Lu, H.; Dong, X.-Z.; Zhang, Y.-L.; Liu, J.; Xie, C.-Q.; Zhao, Z.-S. Electrical and thermal properties of silver nanowire fabricated on a flexible substrate by two-beam laser direct writing for designing a thermometer. RSC Adv. 2018, 8, 24893–24899. [Google Scholar] [CrossRef] [Green Version]
- Sawtelle, S.D.; Reed, M.A. Temperature-dependent thermal conductivity and suppressed Lorenz number in ultrathin gold nanowires. Phys. Rev. B 2019, 99, 054304. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Govorov, A.O.; Dulka, J.; Kotov, N.A. Bioconjugates of CdTe Nanowires and Au Nanoparticles: Plasmon−Exciton Interactions, Luminescence Enhancement, and Collective Effects. Nano Lett. 2004, 4, 2323–2330. [Google Scholar]
- Na, S.-I.; Jo, J.; Kim, S.-S.; Nah, Y.-C.; Kim, D.-Y. Plasmon enhanced performance of organic solar cells using electrodeposited Ag nanoparticles. Appl. Phys. Lett. 2008, 93, 073307. [Google Scholar]
- Duan, J.L.; Cornelius, T.W.; Liu, J.; Karim, S.; Yao, H.J.; Picht, O.; Rauber, M.; Müller, S.; Neumann, R. Surface Plasmon Resonances of Cu Nanowire Arrays. J. Phys. Chem. C 2009, 113, 13583–13587. [Google Scholar] [CrossRef]
- Wiley, B.J.; Im, S.H.; Li, Z.-Y.; McLellan, J.; Siekkinen, A.; Xia, Y. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J. Phys. Chem. B 2006, 110, 15666–15675. [Google Scholar] [CrossRef]
- Bi, Y.; Hu, H.; Lu, G. Highly ordered rectangular silver nanowire monolayers: Water-assisted synthesis and galvanic replacement reaction with HAuCl4. Chem. Commun. 2010, 46, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Xiao, H.; Zeng, Q.; Wang, B.; Wen, S.; Li, B.; Ding, Y.; Yu, H. Direct transformation of metallic copper to copper nanostructures by simple alcohol thermal treatment and their photoactivity. RSC Adv. 2015, 5, 98344–98349. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.-R.; Zhang, L.; Zeng, J.-L.; Zhu, L.; Zhu, Z.; Zhu, X.-Y.; Li, R.-H.; Xiao, Z.-L.; Cao, Z. Preparation and thermal properties of palmitic acid/polyaniline/copper nanowires form-stable phase change materials. J. Therm. Anal. Calorim. 2013, 115, 1133–1141. [Google Scholar] [CrossRef]
- Zeng, J.L.; Cao, Z.; Yang, D.W.; Sun, L.X.; Zhang, L. Thermal conductivity enhancement of Ag nanowires on an organic phase change material. J. Therm. Anal. Calorim. 2009, 101, 385–389. [Google Scholar]
- Deng, Y.; Li, J.; Qian, T.; Guan, W.; Li, Y.; Yin, X. Thermal conductivity enhancement of polyethylene glycol/expanded vermiculite shape-stabilized composite phase change materials with silver nanowire for thermal energy storage. Chem. Eng. J. 2016, 295, 427–435. [Google Scholar] [CrossRef]
- Carbajal-Valdéz, R.; Rodríguez-Juárez, A.; Jiménez-Pérez, J.L.; Sánchez-Ramírez, J.F.; Cruz-Orea, A.; Correa-Pacheco, Z.N.; Macias, M.; Luna-Sánchez, J.L. Experimental investigation on thermal properties of Ag nanowire nanofluids at low concentrations. Thermochim. Acta 2019, 671, 83–88. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, W.; Zhu, D.; Xie, H.; Huang, G. Enhanced Thermal Conductivity for Nanofluids Containing Silver Nanowires with Different Shapes. J. Nanomater. 2017, 2017, 5802016. [Google Scholar] [CrossRef]
- Şimşek, E.; Coskun, S.; Okutucu-Özyurt, T.; Unalan, H.E. Heat transfer enhancement by silver nanowire suspensions in microchannel heat sinks. Int. J. Therm. Sci. 2018, 123, 1–13. [Google Scholar] [CrossRef]
- Dussault, J.-M.; Gosselin, L. Office buildings with electrochromic windows: A sensitivity analysis of design parameters on energy performance, and thermal and visual comfort. Energy Build. 2017, 153, 50–62. [Google Scholar] [CrossRef]
- Clear, R.D.; Inkarojrit, V.; Lee, E.S. Subject responses to electrochromic windows. Energy Build. 2006, 38, 758–779. [Google Scholar] [CrossRef] [Green Version]
- Yuksel, R.; Sarioba, Z.; Cirpan, A.; Hiralal, P.; Unalan, H.E. Transparent and Flexible Supercapacitors with Single Walled Carbon Nanotube Thin Film Electrodes. ACS Appl. Mater. Interfaces 2014, 6, 15434–15439. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhou, C. The Race To Replace Tin-Doped Indium Oxide: Which Material Will Win? ACS Nano 2010, 4, 11–14. [Google Scholar] [CrossRef]
- Lin, S.; Bai, X.; Wang, H.; Wang, H.; Song, J.; Huang, K.; Wang, C.; Wang, N.; Li, B.; Lei, M.; et al. Roll-to-Roll Production of Transparent Silver-Nanofiber-Network Electrodes for Flexible Electrochromic Smart Windows. Adv. Mater. 2017, 29, 1703238. [Google Scholar]
- Liu, L.; Layani, M.; Yellinek, S.; Kamyshny, A.; Ling, H.; Lee, P.S.; Magdassi, S.; Mandler, D. “Nano to nano” electrodeposition of WO3 crystalline nanoparticles for electrochromic coatings. J. Mater. Chem. A 2014, 2, 16224–16229. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Kim, H. Highly transparent conductive reduced graphene oxide/silver nanowires/silver grid electrodes for low-voltage electrochromic smart windows. ACS Appl. Mater. Interfaces 2018, 11, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, Y.; Choi, D.; Chu, W.S.; Ahn, S.-H.; Chun, D.-M.; Lee, C.S. Kinetic spraying of silver nanowire blended graphite powder to fabricate transparent conductive electrode and their application in electrochromic device. Appl. Surf. Sci. 2018, 456, 19–24. [Google Scholar] [CrossRef]
- Yuksel, R.; Ataoglu, E.; Turan, J.; Alpugan, E.; Ozdemir Hacioglu, S.; Toppare, L.; Cirpan, A.; Emrah Unalan, H.; Gunbas, G. A new high-performance blue to transmissive electrochromic material and use of silver nanowire network electrodes as substrates. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1680–1686. [Google Scholar]
- Zhou, K.; Wang, H.; Zhang, S.; Jiu, J.; Liu, J.; Zhang, Y.; Yan, H. Electrochromic modulation of near-infrared light by WO3 films deposited on silver nanowire substrates. J. Mater. Sci. 2017, 52, 12783–12794. [Google Scholar]
- Sampaio, P.G.V.; González, M.O.A.; de Vasconcelos, R.M.; dos Santos, M.A.T.; de Toledo, J.C.; Pereira, J.P.P. Photovoltaic technologies: Mapping from patent analysis. Renew. Sustain. Energy Rev. 2018, 93, 215–224. [Google Scholar] [CrossRef]
- Osseweijer, F.J.W.; van den Hurk, L.B.P.; Teunissen, E.J.H.M.; van Sark, W.G.J.H.M. A comparative review of building integrated photovoltaics ecosystems in selected European countries. Renew. Sustain. Energy Rev. 2018, 90, 1027–1040. [Google Scholar] [CrossRef]
- Sannicolo, T.; Lagrange, M.; Cabos, A.; Celle, C.; Simonato, J.-P.; Bellet, D. Metallic Nanowire-Based Transparent Electrodes for Next Generation Flexible Devices: A Review. Small 2016, 12, 6052–6075. [Google Scholar] [CrossRef]
- Lu, Y.; Chang, R.; Shabunko, V.; Lay Yee, A.T. The implementation of building-integrated photovoltaics in Singapore: Drivers versus barriers. Energy 2019, 168, 400–408. [Google Scholar] [CrossRef]
- Kim, C.-H.; Cha, S.-H.; Kim, S.C.; Song, M.; Lee, J.; Shin, W.S.; Moon, S.-J.; Bahng, J.H.; Kotov, N.A.; Jin, S.-H. Silver Nanowire Embedded in P3HT:PCBM for High-Efficiency Hybrid Photovoltaic Device Applications. ACS Nano 2011, 5, 3319–3325. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Lin, J.; Wu, N.; Nie, S.; Luo, Q.; Ma, C.-Q.; Cui, Z. Inkjet printed silver nanowire network as top electrode for semi-transparent organic photovoltaic devices. Appl. Phys. Lett. 2015, 106, 093302. [Google Scholar]
- Maisch, P.; Tam, K.C.; Lucera, L.; Egelhaaf, H.-J.; Scheiber, H.; Maier, E.; Brabec, C.J. Inkjet printed silver nanowire percolation networks as electrodes for highly efficient semitransparent organic solar cells. Org. Electron. 2016, 38, 139–143. [Google Scholar] [CrossRef]
- Park, Y.; Bormann, L.; Müller-Meskamp, L.; Vandewal, K.; Leo, K. Efficient flexible organic photovoltaics using silver nanowires and polymer based transparent electrodes. Org. Electron. 2016, 36, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Stoichkov, V.; Horie, M.; Brousseau, E.; Kettle, J. Spray coated silver nanowires as transparent electrodes in OPVs for Building Integrated Photovoltaics applications. Sol. Energy Mater. Sol. Cells 2016, 157, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.-H.; Song, T.-B.; Bob, B.; Zhu, R.; Duan, H.-S.; Yang, Y. Silver Nanowire Composite Window Layers for Fully Solution-Deposited Thin-Film Photovoltaic Devices. Adv. Mater. 2012, 24, 5499–5504. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Prasher, P.; Kim, J. Solution processed silver-nanowire/zinc oxide based transparent conductive electrode for efficient photovoltaic performance. Nano-Struct. Nano-Objects 2018, 16, 151–155. [Google Scholar] [CrossRef]
- Teymouri, Z.; Naji, L.; Fakharan, Z. The influences of polyol process parameters on the optoelectronic characteristics of AgNWs-based flexible electrodes and their application in ITO-free polymer solar cells. Org. Electron. 2018, 62, 621–629. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Q.-L.; Wan, H.-J.; Zhang, Y.-N.; Zheng, S.-W.; Zhang, Y. A facile synthesis of segmented silver nanowires and enhancement of the performance of polymer solar cells. Phys. Chem. Chem. Phys. PCCP 2018, 20, 18837–18843. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.; Kim, A.; Lee, D.; Yang, W.; Woo, K.; Jeong, S.; Moon, J. Annealing-free fabrication of highly oxidation-resistive copper nanowire composite conductors for photovoltaics. Npg Asia Mater. 2014, 6, e105. [Google Scholar] [CrossRef]










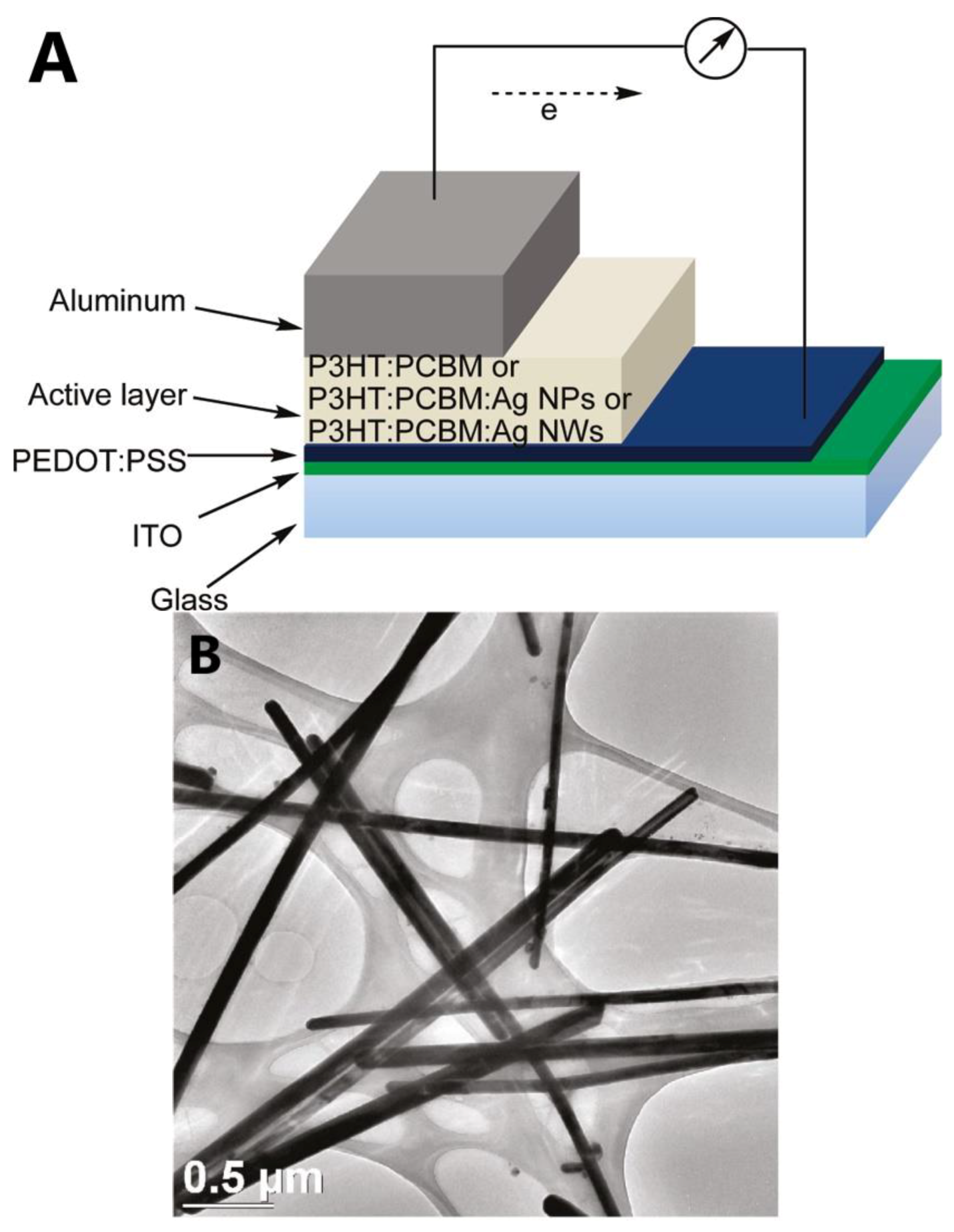
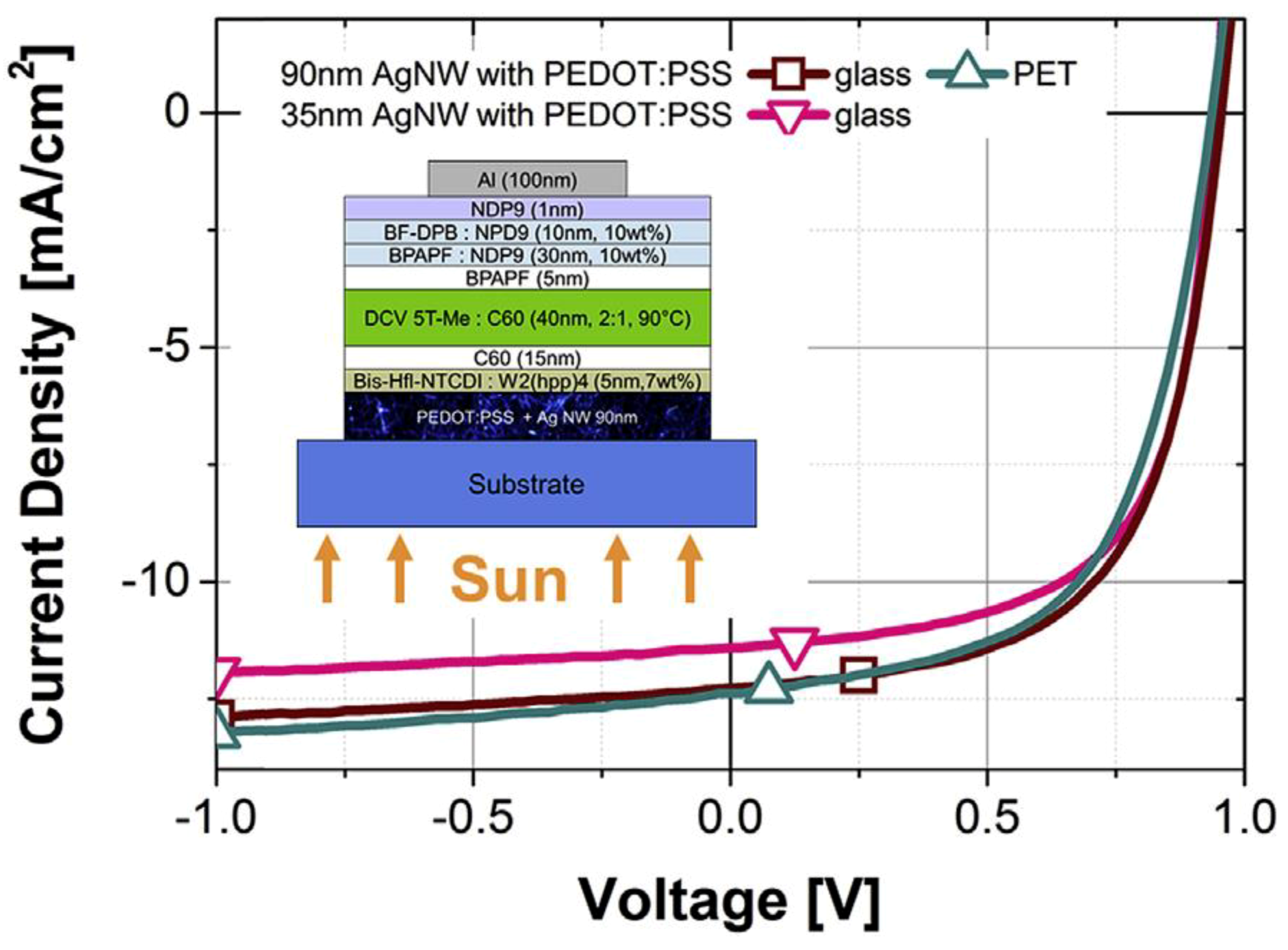
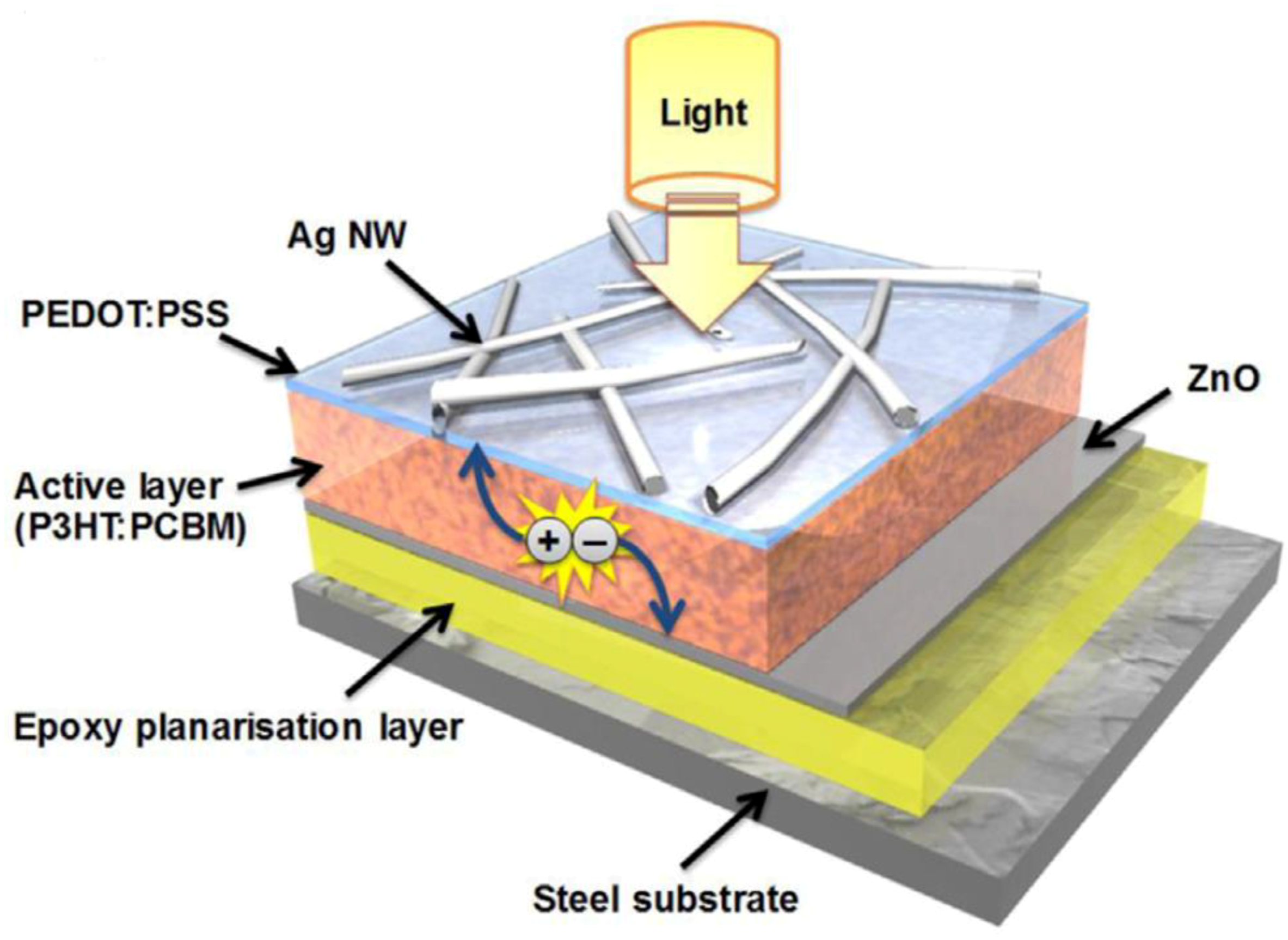
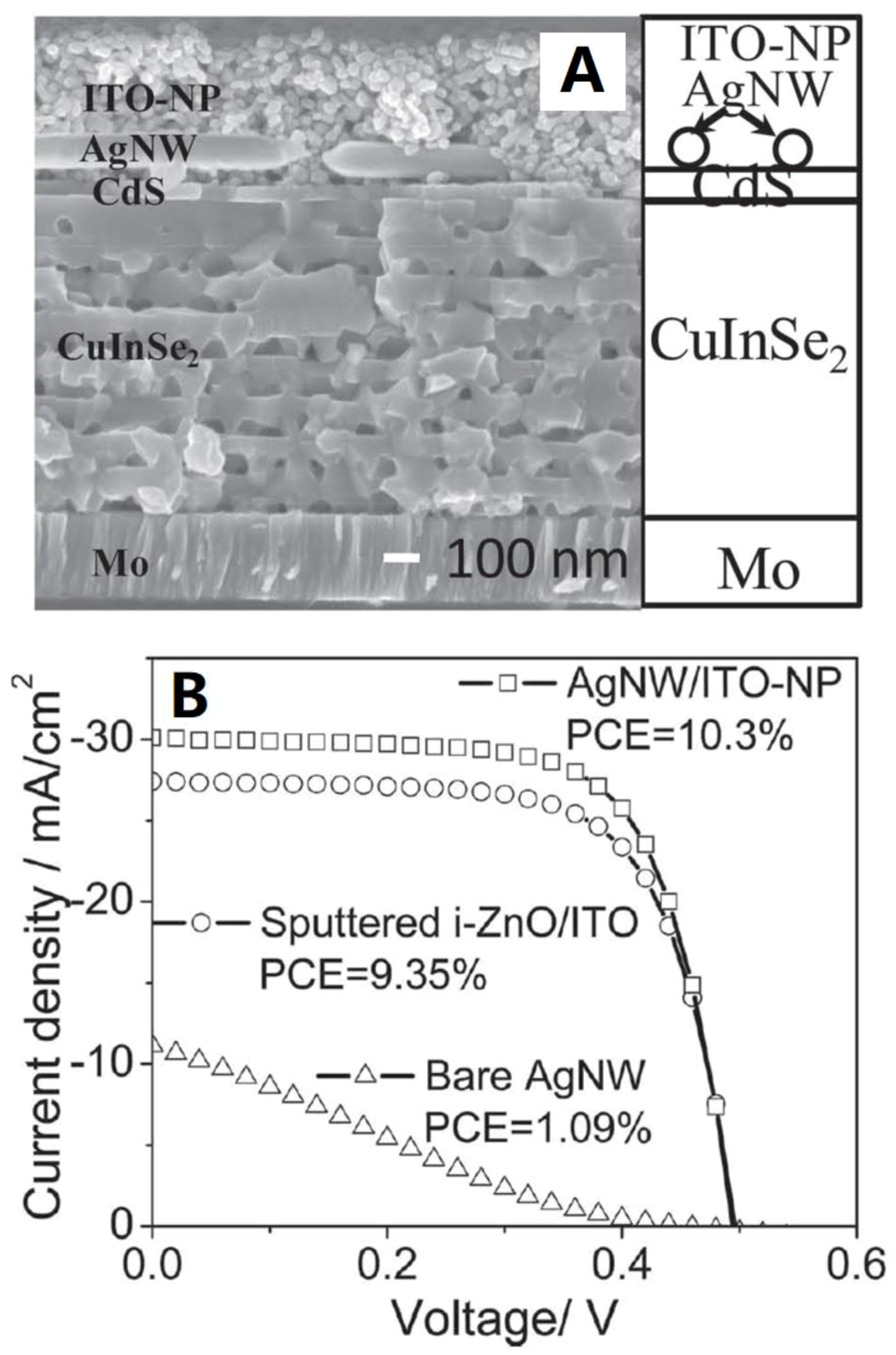
| Approaches | Main Feature | Advantages | Limitations |
|---|---|---|---|
| Hard template method | The hard template serves as a powerful scaffold to control the diameter and length of NWs | The dimensions of metallic NWs can be easily shaped with the morphology of the used hard template | The purification process is time-consuming and tedious because of the fabrication and subsequent removal of template The removal of template may damage the structure of metallic NWs The remover is usually not environmental-friendly |
| Soft template method | The soft templates can dissolve in solution | The purification process is easy without the removal of template Metallic NWs can be scaled up readily | It is hard to control the morphology, size, and yield of metallic NWs It requires subsequent immobilization on matrices for many devices Precursors need to be carefully selected |
| Polyol method | Polyol is used as both solvent and reducing agent | It is able to produce AgNWs both at large scales and of high quality | The injection rate of EG solution needs to be accurately controlled in the self-seeding polyol process Capping agents may interact strongly with the metal surface The distribution of synthesized AgNWs is not easily controllable |
| Hydrothermal method | Water is used as solvent | Environmentally friendly, feasibility of large-scale fabrication of ultralong AgNWs and CuNWs Fewer constraints regarding precursor selection and the conditions of the solvents and reactions | Surface oxidation of metallic NWs may occur |
| Microwave method | Microware radiation is used as heating source | Fast and large-scale synthesis for metallic NWs Easy and highly reproducible process | The morphology and distribution of metallic NWs are very susceptible to changes with reaction parameters, such as microwave power, concentration of reducing agent, and surfactant and reaction time |
| References | Author | Year | Potential Building Applications | Base PCM | NW | Performance |
|---|---|---|---|---|---|---|
| [14] | Shah et al. | 2014 | Passive heating and cooling Solar absorption cooling Evaporative cooling Radiative cooling Air conditioning system | CaCl2∙6H2O (Tm = 29 °C) | CuNWs | A highest CuNWs yield of 50% was obtained Doping 0.17 wt.% CuNWs resulted in 52% enhancement in the thermal conductivity of composite PCM |
| [77] | Zhu et al. | 2014 | Solar domestic hot water system Solar chimney Solar desalination Photovoltaic/thermal system | Palmitic acid (Tm = 64 °C) | CuNWs | Thermal conductivity of form-stable PCM was increased from 0.377 W m−1 K−1 to 0.455 W m−1 K−1 by doping 11.2 wt.% of CuNWs The latent heat of the CuNWs doped form-stable PCM could attain 149 J g−1 |
| [64] | Zeng et al. | 2012 | Solar air heating system District heating system Waste heat recovery | Tetradecanol (Tm = 40 °C) | CuNWs | Thermal conductivity of PCM was increased from 0.32 W m−1 K−1 to 2.86 W m−1 K−1 by doping 11.9 vol.% of CuNWs The latent heat of the CuNWs doped composite PCM could attain 86.95 J g−1 |
| [78] | Zeng et al. | 2010 | Solar air heating system District heating system Waste heat recovery | Tetradecanol (Tm = 40 °C) | AgNWs | Thermal conductivity of PCM was increased from 0.32 W m−1 K−1 to 1.46 W m−1 K−1 by doping 11.8 vol.% of AgNWs The latent heat of the AgNWs doped composite PCM could attain 76.5 J g−1 |
| [79] | Deng et al. | 2016 | Solar domestic hot water system Solar chimney Solar desalination Photovoltaic/thermal system | Expanded vermiculite (Tm = 60 °C) | AgNWs | Thermal conductivity of PCM was increased from 0.25 W m−1 K−1 to 0.68 W m−1 K−1 by doping 19.3 wt.% of AgNWs The latent heat of the AgNWs doped composite PCM could attain 99.1–96.4 J g−1 The supercooling degree of composite PCM was reduced by 7 °C |
| References | Author | Year | Potential Building Applications | Base Fluid | Performance |
|---|---|---|---|---|---|
| [80] | Carbajal–Valdez et al. | 2019 | Air conditioning system including air handling unit, fan coil unit, cooling tower, chiller plant, etc. Solar energy harvesting system including solar panel, heat exchanger, water tank, etc. | DI water | Adding 1.74 × 10−4 vol.% AgNWs resulted in 20.8% enhancement in the thermal conductivity of nanofluid (from 0.613 W m−1 K−1 to 0.723 W m−1 K−1) |
| [81] | Zhang et al. | 2017 | EG | The thermal conductivity enhancement of 13.42% was achieved for the EG/AgNWs nanofluid by doping 0.46 vol.% of AgNWs (from 0.256 W m−1 K−1 to 0.284 W m−1 K−1) | |
| [82] | Şimşek et al. | 2018 | PVP–DI water | A maximum enhancement of 56% has been observed in the heat transfer coefficient with no increase in the pumping power |
| References | Author | Year | Potential Building Applications | Substrate | Performance |
|---|---|---|---|---|---|
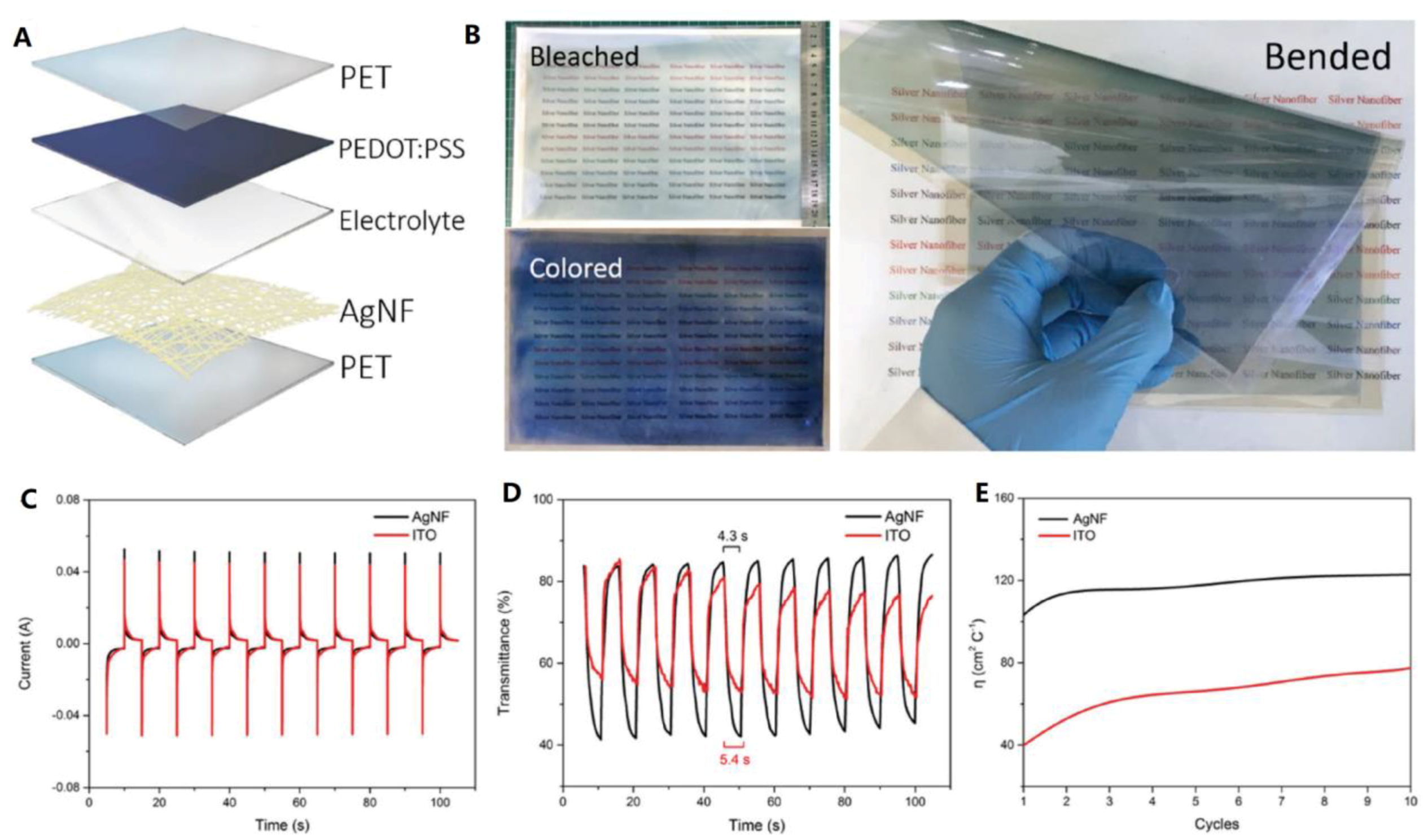
| [87] | Lin et al. | 2017 | Electrochromic windows (ECW) | PET | Sheet resistance of 15 Ω sq−1 and transmittance of 95% were obtained for the TCE Switching time of coloration of 4.3 s and coloration efficiency of 120 cm2 C−1 were achieved for the ECW |
| [89] | Mallikarjuna and Kim | 2018 | PET | Sheet resistance of 0.714 Ω sq−1 and transmittance of 90.9% at wavelength of 550 nm were obtained for the TCE Switching response (12 s for coloration and 10 s for bleaching), coloration efficiency of 33.4 cm2 C−1 were obtained and device responsivity of 90% were achieved for the ECD | |
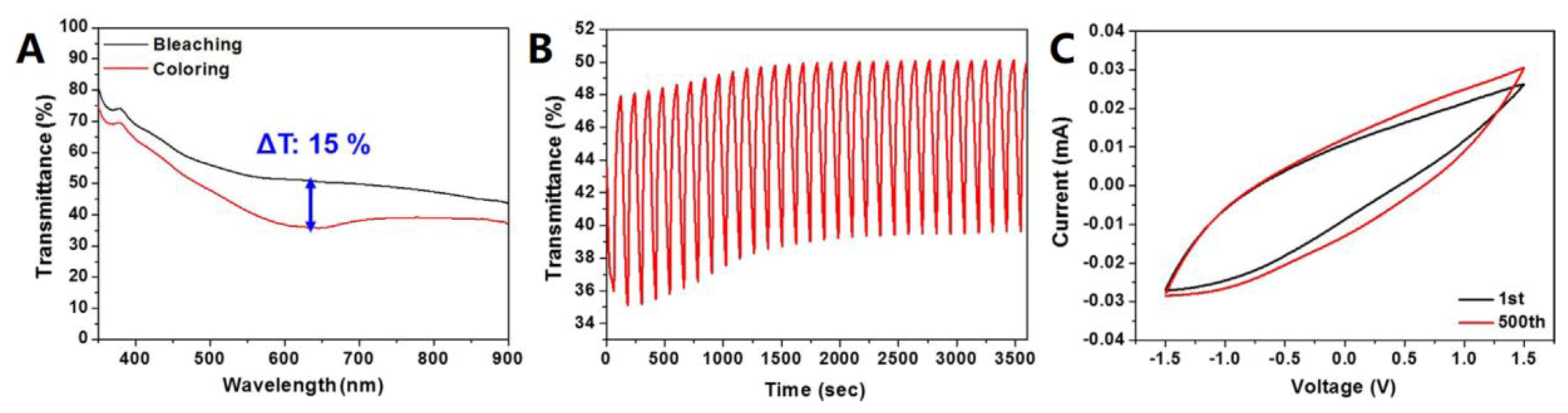
| [90] | Kim et al. | 2018 | Graphene | Sheet resistance of 24–28 Ω sq−1 and transmittance of 58–67% were obtained for the TCE A maximum optical contrast of 15% at wavelength of 630 nm was achieved for the electrochromic film | |
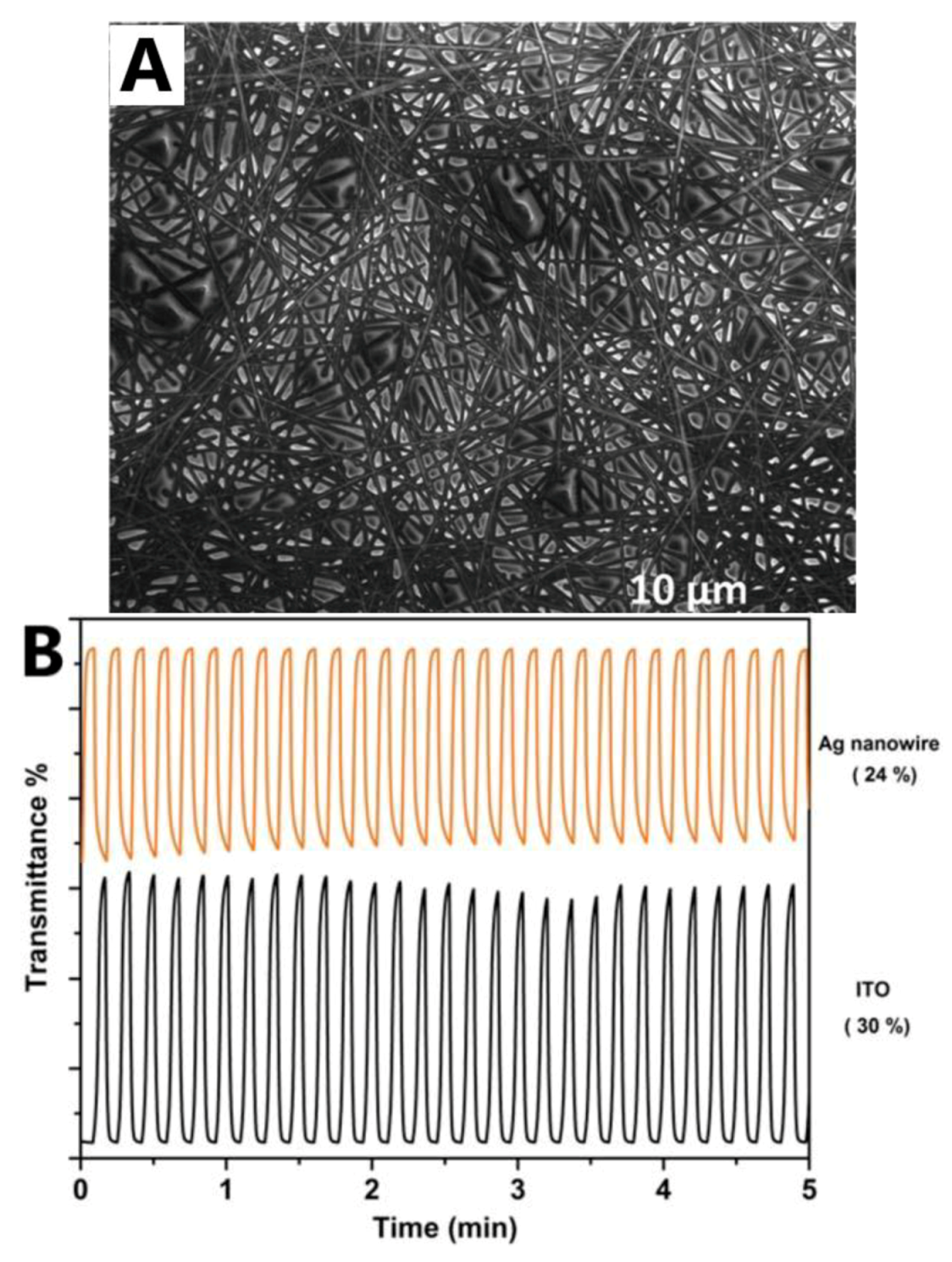
| [91] | Yuksel et al. | 2017 | PET | Very fast switching response of 0.86 s (coloration) and 0.57 s (bleaching) was obtained for the ECD | |
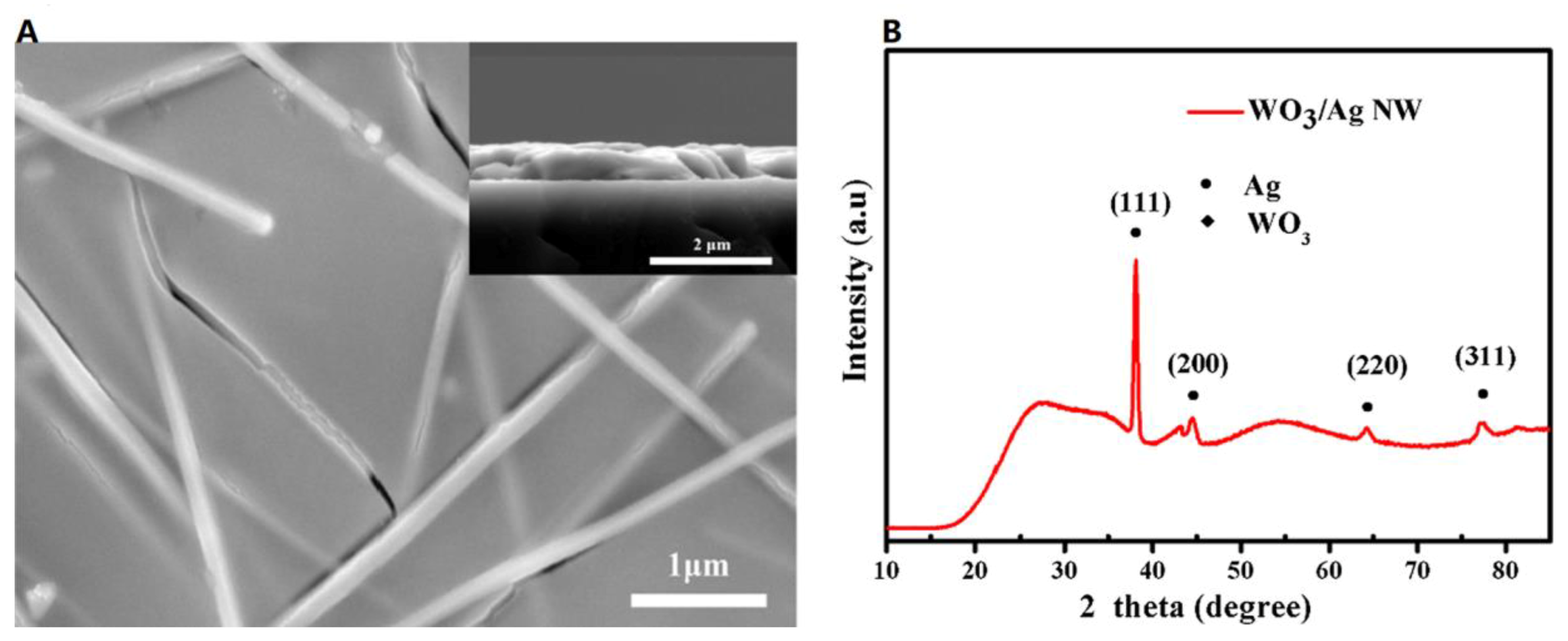
| [92] | Zhou et al. | 2017 | PVA | High coloration efficiency of 50.0 and 86.9 cm2 C−1 at 630 and 1100 nm and switching response of 17.5 s for coloration and bleaching were obtained for the ECD |
| References | Author | Year | Potential Building Applications | PV Device Structure | Performance | |||
|---|---|---|---|---|---|---|---|---|
| Power Conversion Efficiency (PCE) (%) | Short-Circuit Current Density (JSC) (mA cm−2) | Fill Factor (FF) (%) | Open-Circuit Voltage (VOC) (V) | |||||
| [97] | Kim et al. | 2011 | Building-integrated photovoltaic (BIPV) | Al/P3HT:PCBM:AgNWs/PEDOT:PSS/ITO/Glass | 3.9 | 9.3 | 63.3 | 0.66 |
| [98] | Lu et al. | 2015 | AgNWs/ PEDOT:PSS:MoO3/ P3HT:PCBM/ZnO/ITO/Glass | 2.7 | 8.4 | 54.0 | 0.60 | |
| [99] | Maisch et al. | 2016 | AgNWs/PEDOT:PSS/PV2000:PC70BM/ZnO/AgNWs/Glass | 4.3 | 10.7 | 52.8 | 0.76 | |
| [100] | Park et al. | 2016 | Al/NDP9/BF-DPB /BPAPF /BPAPF/DCV 5T-Me/C60/Bis-Hfl-NTCDI:W2(hpp)4/ PEDOT:PSS:AgNWs/Glass | 7.1 | 12.3 | 60.6 | 0.95 | |
| [101] | Ding et al. | 2016 | AgNWs/PEDOT:PSS/P3HT:PCBM/Epoxy/Steel | 2.3 | 8.2 | 53.0 | 0.53 | |
| [102] | Chung et al. | 2012 | ITO/AgNWs/CdS/CuInSe2/Mo/Glass | 10.3 | 30.1 | 69.3 | 0.49 | |
| [103] | Singh et al. | 2018 | ZnO/AgNWs/CdS/CuInSe2/Mo/Glass | 13.4 | 33.8 | 62.0 | 0.64 | |
| [104] | Teymouri et al. | 2019 | Al/P3HT:PCBM/PEDOT:PSS/PET:AgNWs | 3.1 | 9.1 | 68.0 | 0.51 | |
| [105] | Wei et al. | 2018 | PEDOT:PSS:AgNWs/P3HT:PC61BM/ZnO/Al | 3.3 | 9.9 | 55.7 | 0.59 | |
| [106] | Won et al. | 2014 | CuNW/AZO/i-ZnO/ZnS/CIGSSe/Mo/Glass | 7.1 | 33.7 | 36.0 | 0.58 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, K.W.; Xiong, T. Multifunctional Metallic Nanowires in Advanced Building Applications. Materials 2019, 12, 1731. https://doi.org/10.3390/ma12111731
Shah KW, Xiong T. Multifunctional Metallic Nanowires in Advanced Building Applications. Materials. 2019; 12(11):1731. https://doi.org/10.3390/ma12111731
Chicago/Turabian StyleShah, Kwok Wei, and Teng Xiong. 2019. "Multifunctional Metallic Nanowires in Advanced Building Applications" Materials 12, no. 11: 1731. https://doi.org/10.3390/ma12111731






