Rietveld Quantitative Phase Analysis of Oil Well Cement: In Situ Hydration Study at 150 Bars and 150 °C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Laboratory Initial Characterization
2.3. Synchrotron X-ray Powder Diffraction (SXRPD) Experiment
2.4. SXRPD Data Analysis
3. Results and Discussion
3.1. Initial OWC Characterization
3.2. Qualitative Study
3.3. Quantitative Phase Analyses
4. Conclusions
Supplementary Materials
Additional Information
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ludwig, H.M.; Zhang, W. Research review of cement clinker chemistry. Cem. Concr. Res. 2015, 78, 24–37. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement chemistry, 2nd ed.; Acad. Press Publisher: London, UK, 1997. [Google Scholar]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Cuesta, A.; Zea-Garcia, J.D.; Londono-Zuluaga, D.; De la Torre, A.G.; Santacruz, I.; Vallcorba, O.; Dapiaggi, M.; Sanfélix, S.G.; Aranda, M.A.G. Multiscale understanding of tricalcium silicate hydration reactions. Sci. Rep. 2018, 8, 8544. [Google Scholar] [CrossRef] [PubMed]
- Lea, F.M.; Frederick, M.; Hewlett, P.C. Lea’s Chemistry of Cement and Concrete, 4th ed.; Elservier Butterworth-Heinemann: Oxford, UK; pp. 783–840.
- Jupe, A.C.; Wilkinson, A.P.; Funkhouser, G.P. Oil-Well Cement and C3S Hydration Under High Pressure as Seen by In Situ X-Ray Diffraction, Temperatures ≤80 °C with No Additives. J. Am. Ceram. Soc. 2011, 94, 1591–1597. [Google Scholar] [CrossRef]
- Jupe, A.C.; Wilkinson, A.P.; Funkhouser, G.P. The effect of pressure on tricalcium silicate hydration at different temperatures and in the presence of retarding additives. Cem. Concr. Res. 2012, 42, 1083–1087. [Google Scholar] [CrossRef]
- Lin, F.; Meyer, C. Hydration kinetics modeling of Portland cement considering the effects of curing temperature and applied pressure. Cem. Concr. Res. 2009, 39, 255–265. [Google Scholar] [CrossRef]
- Pang, X.; Meyer, C.; Darbe, R.; Funkhouser, G.P. Modeling the Effect of Curing Temperature and Pressure on Cement Hydration Kinetics. ACI Mater. J. 2013, 110, 137–148. [Google Scholar]
- Ma, S.; Yu, T.; Wang, Y.; Chaouche, M.; Kawashima, S. Phase Evolution of Oil Well Cements with Nano-additive at Elevated Temperature/Pressure. ACI Mater. J. 2016, 113, 571–578. [Google Scholar] [CrossRef]
- Bresson, B.; Meducin, F.; Zanni, H.; Noik, C. Hydration of tricalcium silicate (C3S) at high temperature and high pressure. J. Mater. Sci. 2002, 37, 5355–5365. [Google Scholar] [CrossRef]
- Meducin, F.; Zanni, H.; Noik, C.; Hamel, G.; Bresson, B. Tricalcium silicate (C3S) hydration under high pressure at ambient and high temperature (200 °C). Cem. Concr. Res. 2008, 38, 320–324. [Google Scholar] [CrossRef]
- Palou, M.; Živica, V.; Ifka, T.; Boháč, M.; Zmrzlý, M. Effect of hydrothermal curing on early hydration of G-Oil well cement. J. Therm. Anal. Calorim. 2014, 116, 597–603. [Google Scholar] [CrossRef]
- Palou, M.T.; Šoukal, F.; Boháč, M.; Šiler, P.; Ifka, T.; Živica, V. Performance of G-Oil Well cement exposed to elevated hydrothermal curing conditions. J. Therm. Anal. Calorim. 2014, 118, 865–874. [Google Scholar] [CrossRef]
- Jupe, A.C.; Wilkinson, A.P.; Luke, K.; Funkhouser, G.P. Class H cement hydration at 180 °C and high pressure in the presence of added silica. Cem. Concr. Res. 2008, 38, 660–666. [Google Scholar] [CrossRef]
- Kuzielová, E.; Žemlička, M.; Másilko, J.; Palou, M.T. Pore structure development of blended G-oil well cement submitted to hydrothermal curing conditions. Geothermics 2017, 68, 86–93. [Google Scholar] [CrossRef]
- Kuzielová, E.; Žemlička, M.; Másilko, J.; Palou, M.T. Development of G-oil well cement phase composition during long therm hydrothermal curing. Geothermics 2019, 80, 129–137. [Google Scholar] [CrossRef]
- Aranda, M.A.G. Recent studies of cements and concretes by synchrotron radiation crystallographic and cognate methods. Crystallogr. Rev. 2016, 22, 150–196. [Google Scholar] [CrossRef]
- Fraga, E.; Yáñez, A.; Zea-Garcia, J.D.; De la Torre, Á.G.; Cuesta, A.; Valcárcel-Fernández, R.; Farré-París, F.; Malfois, M.; Aranda, M.A.G. High pressure and temperature spinning capillary cell for in situ synchrotron X-ray powder diffraction. J. Synchrotron Radiat. 2019, 26. [Google Scholar] [CrossRef]
- Jupe, A.C.; Wilkinson, A.P. Sample cell for powder x-ray diffraction at up to 500 bars and 200 °C. Rev. Sci. Instrum. 2006, 77, 113901. [Google Scholar] [CrossRef]
- Ashiotis, G.; Deschildre, A.; Nawaz, Z.; Wright, J.P.; Karkoulis, D.; Picca, F.E.; Kieffer, J. The fast azimuthal integration Python library: pyFAI. J. Appl. Crystallogr. 2015, 48, 510–519. [Google Scholar] [CrossRef]
- Von Dreele, R.B.; Larson, A.C. General structure analysis system (GSAS). Los Alamos Natl. Lab. Rep. LAUR 2004, 748, 86–748. [Google Scholar]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld Refinement of Debye-Scherrer Synchrotron X-ray Data from A1203. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef]
- Aranda, M.A.G.; De la Torre, A.G.; Leon-Reina, L. Rietveld Quantitative Phase Analysis of OPC Clinkers, Cements and Hydration Products. Rev. Mineral. Geochem. 2012, 74, 169–209. [Google Scholar] [CrossRef] [Green Version]
- McCusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Louër, D.; Scardi, P. Rietveld refinement guidelines. J. Appl. Crystallogr. 1999, 32, 36–50. [Google Scholar] [CrossRef] [Green Version]
- De la Torre, A.G.; Santacruz, I.; Cuesta, A.; León-Reina, L.; Aranda, M.A.G. Diffraction and crystallography applied to anhydrous cements. In Cementitious Materials: Composition, Properties, Application; Pöllmann, H., Ed.; De Gruyter Publising: Berlin, Germany, 2017. [Google Scholar]
- De la Torre, A.G.; Aranda, M.A.G. Accuracy in Rietveld quantitative phase analysis of Portland cements. J. Appl. Crystallogr. 2003, 36, 1169–1176. [Google Scholar] [CrossRef]
- De La Torre, Á.G.; Bruque, S.; Campo, J.; Aranda, M.A.G. The superstructure of C3S from synchrotron and neutron powder diffraction and its role in quantitative phase analyses. Cem. Concr. Res. 2002, 32, 1347–1356. [Google Scholar] [CrossRef]
- Mumme, W.G.; Hill, R.J.; Bushnell-Wye, G.; Segnit, E.R. Rietveld crystal structure refinements, crystal chemistry and calculated powder diffraction data for the polymorphs of dicalcium silicate and related phases. Neues Jahrb. fuer Mineral. 1995, 169, 35–68. [Google Scholar]
- Colville, A.A.; Geller, S. The Crystal Structure of Brownmillerite, Ca2FeAlO5. Acta Crystallogr. B. 1971, 27, 2311–2315. [Google Scholar] [CrossRef]
- Takéuchi, Y.; Nishi, F. Crystal-chemical characterization of the 3CaO·Al2O3—Na2O solid-solution series. Cryst. Mater. 1980, 152, 259–308. [Google Scholar]
- De la Torre, Á.G.; López-Olmo, M.-G.; Álvarez-Rua, C.; García-Granda, S.; Aranda, M.A.G. Structure and microstructure of gypsum and its relevance to Rietveld quantitative phase analyses. Powder Diffr. 2004, 19, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Bezou, C.; Nonat, A.; Mutin, J.-C.; Christensen, A.N.; Lehmann, M.S. Investigation of the Crystal Structure of γ-CaSO4, CaSO4 · 0.5 H2O, and CaSO4 · 0.6 H2O by Powder Diffraction Methods. J. Solid State Chem. 1995, 117, 165–176. [Google Scholar] [CrossRef]
- Petch, H.E. IUCr The hydrogen positions in portlandite, Ca(OH)2, as indicated by the electron distribution. Acta Crystallogr. 1961, 14, 950–957. [Google Scholar] [CrossRef]
- Goetz-Neunhoeffer, F.; Neubauer, J. Refined ettringite (Ca6Al2(SO4)3(OH)12∙26H2O) structure for quantitative X-ray diffraction analysis. Powder Diffr. 2006, 21, 4–11. [Google Scholar] [CrossRef]
- Yamnova, N.A.; Sarp, K.; Egorov-Tismenko, Y.K.; Pushcharovskii, D.Y. Crystal structure of jaffeite. Crystallogr. Rep. 1993, 38, 464–467. [Google Scholar]
- Sacerdoti, M.; Passaglia, E. The crystal structure of katoite and implications within the hydrogrossular group of minerals. Bull. Minéralogie 1985, 108, 1–8. [Google Scholar] [CrossRef]
- Yano, T.; Urabe, K.; Ikawa, H.; Teraushi, T.; Ishizawa, N.; Udagawa, S. Structure of α-dicalcium silicate hydrate. Acta Crystallogr. C. 1993, 49, 1555–1559. [Google Scholar] [CrossRef]
- Lothenbach, B.; Durdzinski, P.; De Weerdt, K. Thermogravimetric analysis. In A Practical Guide to Microstructural Analysis of Cementitious Materials; Scrivener, K., Snellings, R., Lothenbach, B., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Colston, S.L.; Barnes, P.; Jupe, A.C.; Jacques, S.D.M.; Hall, C.; Livesey, P.; Dransfield, J.; Meller, N.; Maitland, G.C. An in situ synchrotron energy-dispersive diffraction study of the hydration of oilwell cement systems under high temperature/autoclave conditions up to 130 °C. Cem. Concr. Res. 2005, 35, 2223–2232. [Google Scholar] [CrossRef]

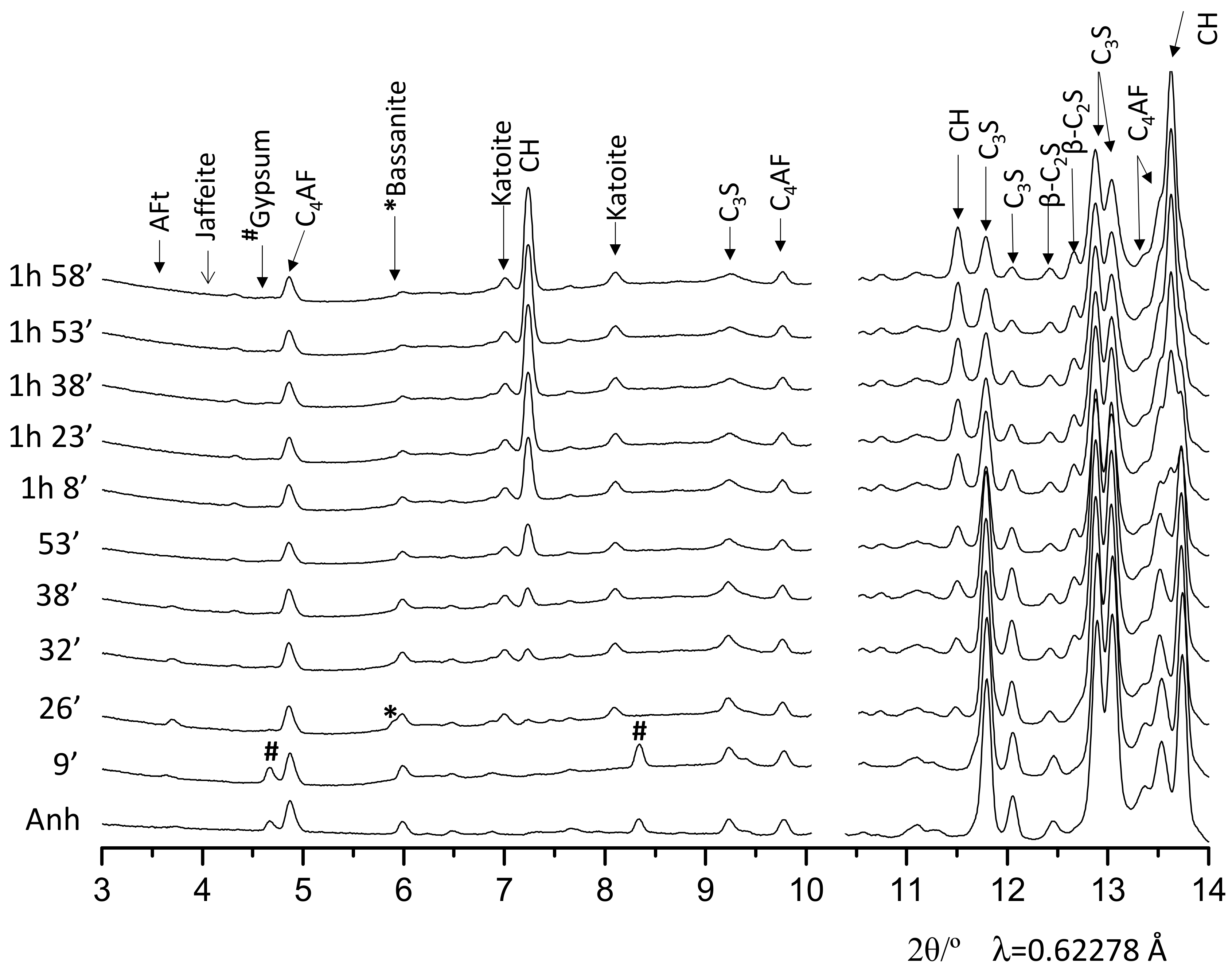
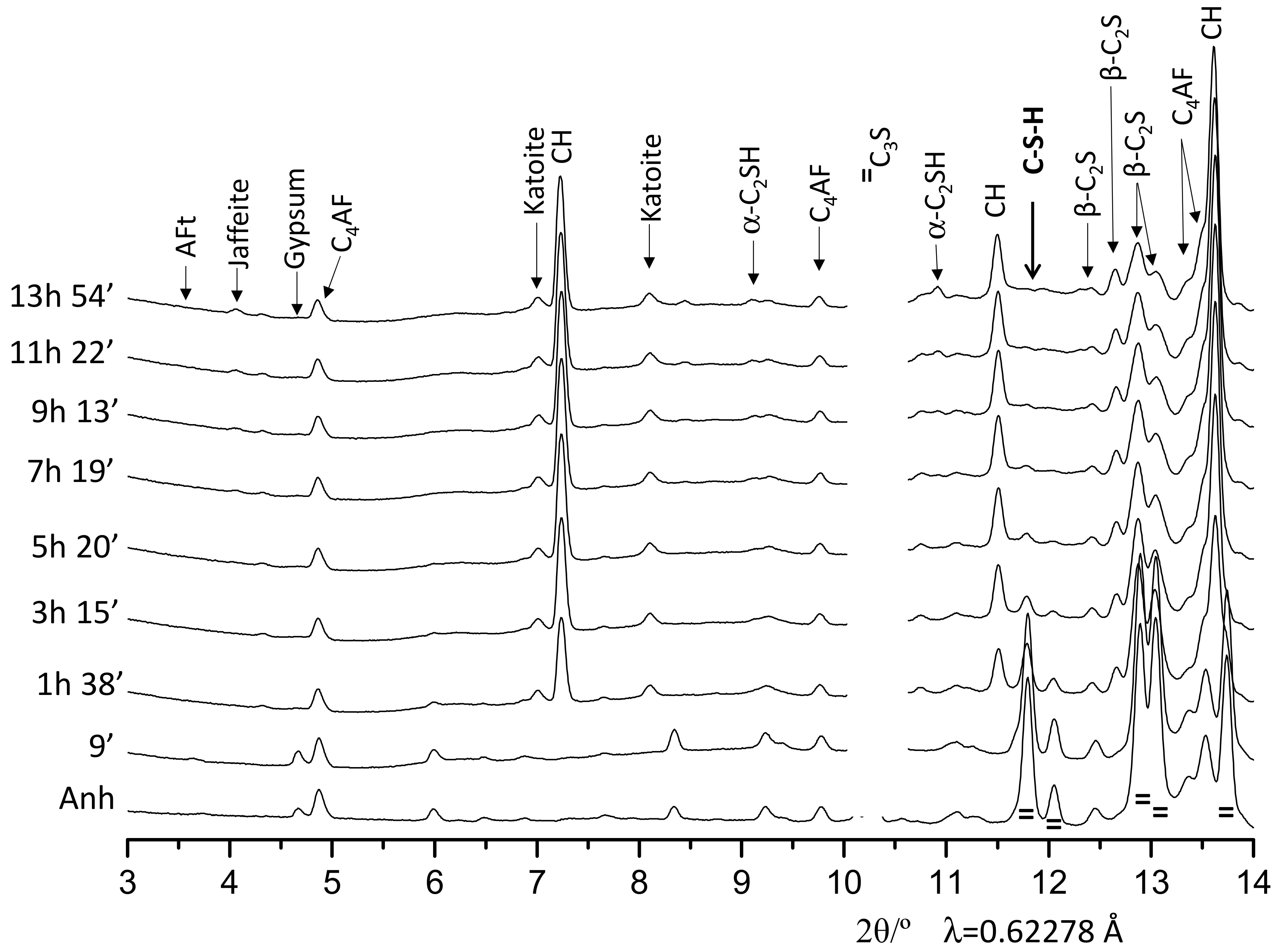



| Phase/wt% | t0 | 9′ | 26′ | 53′ | 1 h 38′ | 3 h 15′ | 5 h 20′ | 7 h 19′ | 9 h 13′ | 11 h 22′ | 13 h 54′ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C3S [28] | 41.6(1) | 40.1(1) | 34.6(1) | 29.9(1) | 15.6(2) | 8.2(2) | 4.9(3) | 3.3(2) | 2.5(3) | 2.1(3) | 1.5(3) |
| β-C2S [29] | 9.9(2) | 9.9(-) | 9.9(-) | 9.9(-) | 9.9(-) | 9.9(-) | 9.1(3) | 8.6(3) | 7.8(3) | 6.9(3) | 5.8(3) |
| C4AF [30] | 11.3(1) | 11.1(1) | 9.0(1) | 9.1(1) | 8.1(2) | 8.1(-) | 8.1(-) | 8.1(-) | 8.1(-) | 8.1(-) | 8.1(-) |
| o-C3A [31] | 2.3(1) | 2.2(1) | 1.1(1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C H2 [32] | 2.9(2) | 3.1(1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C H0.5 [33] | 0.0 | 0.0 | 1.1(1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| CH [34] | 0.0 | 0.0 | 0.3(1) | 3.0(1) | 7.2(1) | 10.1(1) | 11.6(1) | 12.4(1) | 12.8(1) | 12.8(1) | 12.9(1) |
| AFt [35] | 0.0 | 0.6(1) | 0.6(1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Jaffeite [36] | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3(2) |
| Katoite [37] | 0.0 | 0.0 | 3.5(1) | 5.8(2) | 6.0(2) | 6.4(2) | 6.5(2) | 6.6(2) | 6.5(2) | 6.3(2) | 6.2(2) |
| α-C2SH [38] | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2(3) | 0.6(3) | 1.1(3) | 2.1(3) |
| FW $ | 32.0(-) # | 31.2 | 26.3 | 21.9 | 16.0 | 12.9 | 11.2 | 10.2 | 9.6 | 9.0 | 8.3 |
| Amorph. # | 1.7 | 13.5 | 20.4 | 37.2 | 44.3 | 48.6 | 50.7 | 52.1 | 53.7 | 54.7 |
| Type of Sample | Blaine/m2/kg | w/c Mass Ratio | T/°C | P/Bars | t50%(C3S)/min | t90%(C3S)/h | t50%(C2S)/h | Remarks | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Class G-HSR | 340 | 0.47 | 150 | 150 | 98 | 6.6 | ~15 | - | This work |
| Pure-C3S | n.r. | 0.44 | 160 | 400 | n.a. | 16 | - | $ | [11] |
| Class G | 310 | 0.40 | 130 | ~5 | 160 | ~6 | - | # | [40] |
| Class G | n.r. | 0.412 | 80 | 400 | 150 | >13 | - | @ | [6] |
| Class H | n.r. | 0.38 | 57 | “ | “ | - | - | & | [7] |
| Class A | 327 | n.r. | 60 | ~1000 | 90 | - | - | - | [10] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraga, E.; Cuesta, A.; Zea-Garcia, J.D.; De la Torre, A.G.; Yáñez-Casal, A.; Aranda, M.A.G. Rietveld Quantitative Phase Analysis of Oil Well Cement: In Situ Hydration Study at 150 Bars and 150 °C. Materials 2019, 12, 1897. https://doi.org/10.3390/ma12121897
Fraga E, Cuesta A, Zea-Garcia JD, De la Torre AG, Yáñez-Casal A, Aranda MAG. Rietveld Quantitative Phase Analysis of Oil Well Cement: In Situ Hydration Study at 150 Bars and 150 °C. Materials. 2019; 12(12):1897. https://doi.org/10.3390/ma12121897
Chicago/Turabian StyleFraga, Edmundo, Ana Cuesta, Jesus D. Zea-Garcia, Angeles G. De la Torre, Armando Yáñez-Casal, and Miguel A. G. Aranda. 2019. "Rietveld Quantitative Phase Analysis of Oil Well Cement: In Situ Hydration Study at 150 Bars and 150 °C" Materials 12, no. 12: 1897. https://doi.org/10.3390/ma12121897






