A Review of The Lesser-Studied Microemulsion-Based Synthesis Methodologies Used for Preparing Nanoparticle Systems of The Noble Metals, Os, Re, Ir and Rh
Abstract
:1. Introduction
1.1. Noble Metal NPs
1.2. Fundamentals of The Microemulsion Technique
1.3. Preparation of Nanoparticles Using Microemulsion Techniques
1.4. Factors Affecting NPs Synthesis in a W/O Microemulsion
2. Recent Investigations on The Microemulsion-Based Synthesis of Re, Ir, Os, and Rh NPs
2.1. Microemulsion-Based Methodologies for Generating Re NPs
2.2. Microemulsion-Based Methodologies for Generating Ir NPs
2.3. Microemulsion-Based Methodologies for The Generation of Rh NPs Synthesis
3. Conclusions and Recommendations for Future Investigation
Funding
Acknowledgments
Conflicts of Interest
References
- Faraday, M. The bakerian lecture-experimental relations of gold (and other metals) to light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar]
- Brody, H. Gold. Nature 2013, 495, S1. [Google Scholar] [CrossRef] [PubMed]
- Jellinek, J. Nanoalloys: Tuning properties and characteristics through size and composition. Faraday Discuss. 2008, 138, 11–35. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G. Clusters and Colloids: from Theory to Applications; Wiley-VCH: Weinheim, Germany, 1994. [Google Scholar]
- Morrison, I.D.; Ross, S. Colloidal Dispersions: Suspensions, Emulsions, and Foams; Wiley-Interscience: New York, NY, USA, 2002; p. 656. [Google Scholar]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakumar, M. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arabian J. Chem. 2017. [Google Scholar] [CrossRef]
- Wang, W.Z.; Wang, G.; Wang, X.S.; Zhan, Y.; Liu, Y.; Zheng, C.L. Synthesis and characterization of Cu2O nanowires by a novel reduction route. Adv. Mater. 2002, 14, 67–69. [Google Scholar] [CrossRef]
- Dang, T.M.D.; Le, T.T.T.; Fribourg-Blanc, E.; Dang, M.C. Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 015009. [Google Scholar] [CrossRef]
- Chou, K.S.; Ren, C.Y. Synthesis of nanosized silver particles by chemical reduction method. Mater. Chem. Phys. 2000, 64, 241–246. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Mucalo, M.R.; Babu, K.M.; Wu, K.S.W. In situ characterisation of the aqueous gold colloid interface using CO as a surface probe: IR spectroscopic studies. J. Colloid Interface Sci. 2007, 310, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Mucalo, M.R.; Bullen, C.R. Rhenium-based hydrosols: preparation and properties. J. Colloid Interface Sci. 2001, 239, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Bhargava, A.; Gupta, R.; Jain, N.; Panwar, J. Synthesis and applications of noble metal nanoparticles: a review. Adv. Sci. Eng. Med. 2017, 9, 527–544. [Google Scholar] [CrossRef]
- Abedini, A.; Bakar, A.A.A.; Larki, F.; Menon, P.S.; Islam, M.S.; Shaari, S. Recent advances in shape-controlled synthesis of noble metal nanoparticles by radiolysis route. Nanoscale Res. Lett. 2016, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Rogach, A.L.; Jäckel, F.; Klar, T.A.; Feldmann, J. Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv. Mater. 2010, 22, 1805–1825. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhao, M.; Yang, T.H.; Xia, Y. Decahedral nanocrystals of noble metals: Synthesis, characterization, and applications. Mater. Today 2018, 22, 108–131. [Google Scholar] [CrossRef]
- Sharma, M.K.; Shah, D.O. Introduction to macro- and microemulsions. In Macro- and Microemulsions, Theory and Applications; Shah, D.O., Ed.; American Chemical Society: Florida, FL, USA, 1985; Volume 272, pp. 1–18. [Google Scholar]
- Tadros, T.F. Emulsion Science and Technology; Wileyion Science and TechnolKGaA: Weinheim, Germany, 2009. [Google Scholar]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials: 1st Nano Update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef]
- Hussain, T.; Batool, R. Microemulsion route for the synthesis of nano-structured catalytic materials. In Properties and Uses of Microemulsions; Karunaratne, D.N., Pamunuwa, G., Ranatunga, U., Eds.; IntechOpen: London Bridge Street, London, UK, 2017. [Google Scholar]
- Li, Y.; Park, C.W. Particle size distribution in the synthesis of nanoparticles using microemulsions. Langmuir 1999, 15, 952–956. [Google Scholar] [CrossRef]
- Kronberg, B.; Holmberg, K.; Lindman, B. Types of surfactants, their synthesis, and applications. In Surface Chemistry of Surfactants and Polymers, 1st ed.; Kronberg, B., Holmberg, K., Lindman, B., Eds.; John Wiley & Sons, Ltd.: New York, NY, USA, 2014. [Google Scholar]
- Giannakas, A.E.; Vaimakis, T.C.; Ladavos, A.K.; Trikalitis, P.N.; Pomonis, P.J. Variation of surface properties and textural features of spinel ZnAl2O4 and perovskite LaMnO3 nanoparticles prepared via CTAB–butanol–octane–nitrate salt microemulsions in the reverse and bicontinuous states. J. Colloid Interface Sci. 2003, 259, 244–253. [Google Scholar] [CrossRef]
- Porta, F.; Prati, L.; Rossi, M.; Scarì, G. Synthesis of Au (0) nanoparticles from W/O microemulsions. Colloids Surf. A 2002, 211, 43–48. [Google Scholar] [CrossRef]
- Marchand, K.; Tarret, M.; Lechaire, J.; Normand, L.; Kasztelan, S.; Cseri, T. Investigation of AOT-based microemulsions for the controlled synthesis of MoSx nanoparticles: An electron microscopy study. Colloids Surf. A 2003, 214, 239–248. [Google Scholar] [CrossRef]
- Simmons, B.A.; Li, S.; John, V.T.; McPherson, G.L.; Bose, A.; Zhou, W.; He, J. Morphology of CdS Nanocrystals Synthesized in a Mixed Surfactant System. Nano Lett. 2002, 2, 263–268. [Google Scholar] [CrossRef]
- Pang, Q.; Shi, J.; Liu, Y.; Xing, D.; Gong, M.; Xu, N. A novel approach for preparation of Y2O3:Eu3+ nanoparticles by microemulsion-microwave heating. Mater. Sci. Eng. B 2003, 1, 57–61. [Google Scholar] [CrossRef]
- Zhang, X.; Chan, K.Y. Water-in-oil microemulsion synthesis of platinum−ruthenium nanoparticles, their characterization and electrocatalytic properties. Chem. Mater. 2003, 15, 451–459. [Google Scholar] [CrossRef]
- Milovanovic, M.; Arsenijevic, A.; Milovanovic, J.; Kanjevac, T.; Arsenijevic, N. Chapter 14—Nanoparticles in antiviral therapy. In Antimicrobial Nanoarchitectonics; Grumezescu, A.M., Ed.; Elsevier-Health Sciences Division: Philadelphia, PA, USA, 2017; pp. 383–410. [Google Scholar]
- Bedia, J.; Calvo, L.; Lemus, J.; Quintanilla, A.; Casas, J.; Mohedano, A.; Zazo, J.; Rodriguez, J.; Gilarranz, M. Colloidal and microemulsion synthesis of rhenium nanoparticles in aqueous medium. Colloid Surf. A 2015, 469, 202–210. [Google Scholar] [CrossRef]
- Eriksson, S.; Nylén, U.; Rojas, S.; Boutonnet, M. Preparation of catalysts from microemulsions and their applications in heterogeneous catalysis. Appl. Catal. A 2004, 265, 207–219. [Google Scholar] [CrossRef]
- Boutonnet, M.; Kizling, J.; Stenius, P.; Maire, G. The preparation of monodisperse colloidal metal particles from microemulsions. Colloid Surf. 1982, 5, 209–225. [Google Scholar] [CrossRef]
- Ganguli, A.K.; Ganguly, A.; Vaidya, S. Microemulsion-based synthesis of nanocrystalline materials. Chem. Soc. Rev. 2010, 39, 474–485. [Google Scholar] [CrossRef]
- Kanwar, R.; Rathee, J.; Tanaji Patil, M.; Kumar Mehta, S. microemulsions as nanotemplates: A soft and versatile approach [Online First]. In Microemulsion—A Chemical Nanoreactor; Working Title; Mejuto, J.C., Ed.; IntechOpen: London Bridge Street, London, UK, 2018. [Google Scholar]
- Cid, A. Synthesis of NPs by microemulsion method. In Microemulsion—A Chemical Nanoreactor; Mejuto, J.C., Ed.; IntechOpen: London Bridge Street, London, UK, 2018. [Google Scholar]
- López-Quintela, M.A.; Tojo, C.; Blanco, M.C.; García Rio, L.; Leis, J.R. Microemulsion dynamics and reactions in microemulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 264–278. [Google Scholar] [CrossRef]
- Tojo, C.; Blanco, M.C.; López-Quintela, M.A. The influence of reactant excess and film flexibility on the mechanism of nanoparticle formation in microemulsions: a monte carlo simulation. Langmuir 1998, 14, 6835–6839. [Google Scholar] [CrossRef]
- Sanchez-Dominguez, M.; Koleilat, H.; Boutonnet, M.; Solans, C. Synthesis of Pt nanoparticles in oil-in-water microemulsions: phase behavior and effect of formulation parameters on nanoparticle characteristics. J. Dispers. Sci. Technol. 2011, 32, 1765–1770. [Google Scholar] [CrossRef]
- Sanchez-Dominguez, M.; Pemartin, K.; Boutonnet, M. Preparation of inorganic nanoparticles in oil-in-water microemulsions: A soft and versatile approach. Curr. Opin. Colloid Interface Sci. 2012, 17, 297–305. [Google Scholar] [CrossRef]
- Eastoe, J.; Hollamby, M.J.; Hudson, L. Recent advances in nanoparticle synthesis with reversed micelles. Adv. Colloid Interface Sci. 2006, 128–130, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Chandradass, J.; Balasubramanian, M.; Bae, D.S.; Kim, J.; Kim, K.H. Effect of water to surfactant ratio (R) on the particle size of MgAl2O4 nanoparticle prepared via reverse micelle process. J. Alloys Compd. 2010, 491, L25–L28. [Google Scholar] [CrossRef]
- Granata, G.; Pagnanelli, F.; Nishio-Hamane, D.; Sasaki, T. Effect of surfactant/water ratio and reagents’ concentration on size distribution of manganese carbonate nanoparticles synthesized by microemulsion mediated route. Appl. Surf. Sci. 2015, 331, 463–471. [Google Scholar] [CrossRef]
- Inaba, R.; Fukahori, T.; Hamamoto, M.; Ohno, T. Synthesis of nanosized TiO2 particles in reverse micelle systems and their photocatalytic activity for degradation of toluene in gas phase. J. Mol. Catal. A Chem. 2006, 260, 247–254. [Google Scholar] [CrossRef]
- Solanki, J.N.; Sengupta, R.; Murthy, Z. Synthesis of copper sulphide and copper nanoparticles with microemulsion method. Solid State Sci. 2010, 12, 1560–1566. [Google Scholar] [CrossRef]
- He, Y.; Yang, B.; Cheng, G.; Pan, H. Influence of the thermodynamic stability of microemulsion on the size of nanoparticles prepared by a coupling route of microemulsion with homogeneous precipitation. Mater. Lett. 2004, 58, 2019–2022. [Google Scholar] [CrossRef]
- Lisiecki, I.; Pileni, M.P. Synthesis of Well-defined and low size distribution cobalt nanocrystals: the limited influence of reverse micelles. Langmuir 2003, 19, 9486–9489. [Google Scholar] [CrossRef]
- Maillard, M.; Giorgio, S.; Pileni, M.P. Tuning the size of silver nanodisks with similar aspect ratios: synthesis and optical properties. J. Phys. Chem. B 2003, 107, 2466–2470. [Google Scholar] [CrossRef]
- Chen, D.H.; Wu, S.H. Synthesis of nickel nanoparticles in water-in-oil microemulsions. Chem. Mater. 2000, 12, 1354–1360. [Google Scholar] [CrossRef]
- Solanki, J.N.; Murthy, Z.V.P. Reduction of nitro aromatic compounds over Ag/Al2O3 nanocatalyst prepared in water-in-oil microemulsion: effects of water-to-surfactant mole ratio and type of reducing agent. Ind. Eng. Chem. Res. 2011, 50, 7338–7344. [Google Scholar] [CrossRef]
- Escudero, M.J.; Hontañón, E.; Schwartz, S.; Boutonnet, M.; Daza, L. Development and performance characterisation of new electrocatalysts for PEMFC. J. Power Sources 2002, 106, 206–214. [Google Scholar] [CrossRef]
- Kim, W.Y.; Hayashi, H.; Kishida, M.; Nagata, H.; Wakabayashi, K. Methanol synthesis from syngas over supported palladium catalysts prepared using water-in-oil microemulsion. Appl. Catal. A 1998, 169, 157–164. [Google Scholar] [CrossRef]
- Pocoroba, E.; Pettersson, L.J.; Agrell, J.; Boutonnet, M.; Jansson, K. Exhaust gas catalysts for heavy-duty applications: influence of the Pd particle size and particle size distribution on the combustion of natural gas and biogas. Top. Catal. 2001, 16, 407–412. [Google Scholar] [CrossRef]
- Rymeš, J.; Ehret, G.; Hilaire, L.; Boutonnet, M.; Jirátová, K. Microemulsions in the preparation of highly active combustion catalysts. Catal. Today 2002, 75, 297–303. [Google Scholar] [CrossRef]
- Fernández-García, M.; Martínez-Arias, A.; Iglesias-Juez, A.; Hungría, A.B.; Anderson, J.A.; Conesa, J.C.; Soria, J. New Pd/CexZr1−xO2/Al2O3 three-way catalysts prepared by microemulsion: Part 1. Characterization and catalytic behavior for CO oxidation. Appl. Catal. B 2001, 31, 39–50. [Google Scholar]
- Martínez-Arias, A.; Fernández-García, M.; Iglesias-Juez, A.; Hungría, A.B.; Anderson, J.A.; Conesa, J.C.; Soria, J. New Pd/CexZr1−xO2/Al2O3 three-way catalysts prepared by microemulsion: Part 2. In situ analysis of CO oxidation and NO reduction under stoichiometric CO+NO+O2. Appl. Catal. B 2001, 31, 51–60. [Google Scholar]
- Zhang, D.Y.; Zheng, Y.; Zhang, H.; Sun, J.H.; Tan, C.P.; He, L.; Zhang, W.; Ji, L.N.; Mao, Z.W. Delivery of phosphorescent anticancer iridium(iii) complexes by polydopamine nanoparticles for targeted combined photothermal-chemotherapy and thermal/photoacoustic/lifetime imaging. Adv. Sci. 2018, 5, 1800581. [Google Scholar] [CrossRef]
- Kang, S.; Shin, W.; Choi, M.H.; Ahn, M.; Kim, Y.K.; Kim, S.; Min, D.H.; Jang, H. Morphology-controlled synthesis of rhodium nanoparticles for cancer phototherapy. ACS Nano 2018, 12, 6997–7008. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, G.; Li, B.; Zhao, X.; Ji, T.; Song, G.; Xiao, Z.; Cao, Q.; Xiao, J.; Huang, X.; et al. Degradable rhenium trioxide nanocubes with high localized surface plasmon resonance absorbance like gold for photothermal theranostics. Biomaterials 2018, 159, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yan, X.; Hu, Y.; Liu, Y.; Guo, R.; Liao, M.; Shao, B.; Tang, Q.; Guo, X.; Chai, R.; et al. Two-photon image tracking of neural stem cells via iridium complexes encapsulated in polymeric nanospheres. ACS Biomater. Sci. Eng. 2019, 5, 1561–1568. [Google Scholar] [CrossRef]
- Kapkowski, M.; Popiel, J.; Siudyga, T.; Dzida, M.; Zorębski, E.; Musiał, M.; Sitko, R.; Szade, J.; Balin, K.; Klimontko, J.; et al. Mono- and bimetallic nano-Re systems doped Os, Mo, Ru, Ir as nanocatalytic platforms for the acetalization of polyalcohols into cyclic acetals and their applications as fuel additives. Appl. Catal. B 2018, 239, 154–167. [Google Scholar] [CrossRef]
- Fan, Z.; Luo, Z.; Chen, Y.; Wang, J.; Li, B.; Zong, Y.; Zhang, H. Synthesis of 4H/fcc-Au@M (M = Ir, Os, IrOs) Core-Shell nanoribbons for electrocatalytic oxygen evolution reaction. Small 2016, 12, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Adzic, R.R.; Zhang, J.; Shao, M.; Sasaki, K.; Vukmirovic, M.; Uribe, F.A. Platinum and mixed platinum-metal monolayer fuel cell electrocatalysts: design, activity and long-term performance stability. ECS Trans. 2006, 3, 31–36. [Google Scholar]
- Yang, Z.; Zhang, Y.; Wu, R. High stability and reactivity of pt-based core–shell nanoparticles for oxygen reduction reaction. J. Phys. Chem. C 2012, 116, 13774–13780. [Google Scholar] [CrossRef]
- Ohde, M.; Ohde, H.; Wai, C.M. Catalytic hydrogenation of arenes with rhodium nanoparticles in a water-in-supercritical CO2 microemulsion. Chem. Commun. 2002, 2388–2389. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Pitto-Barry, A.; Romero-Canelón, I.; Tran, J.; Soldevila-Barreda, J.J.; Hands-Portman, I.; Smith, C.J.; Kirby, N.; Dove, A.P.; O’Reilly, R.K.; et al. Precious metal carborane polymer nanoparticles: characterisation of micellar formulations and anticancer activity. Faraday Discuss. 2014, 175, 229–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, N.P.E.; Pitto-Barry, A.; Sanchez, A.M.; Dove, A.P.; Procter, R.J.; Soldevila-Barreda, J.J.; Kirby, N.; Hands-Portman, I.; Smith, C.J.; O’Reilly, R.K.; et al. Fabrication of crystals from single metal atoms. Nat. Commun. 2014, 5, 3851. [Google Scholar] [CrossRef] [Green Version]
- Pitto-Barry, A.; Geraki, K.; Horbury, M.D.; Stavros, V.G.; Mosselmans, J.F.W.; Walton, R.I.; Sadler, P.J.; Barry, N.P.E. Controlled fabrication of osmium nanocrystals by electron, laser and microwave irradiation and characterisation by microfocus X-ray absorption spectroscopy. Chem. Commun. 2017, 53, 12898–12901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitto-Barry, A.; Perdigao, L.M.; Walker, M.; Lawrence, J.; Costantini, G.; Sadler, P.J.; Barry, N.P. Synthesis and controlled growth of osmium nanoparticles by electron irradiation. Dalton Trans. 2015, 44, 20308–20311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, S.V.; Ramarao, C.; Lee, A.L.; Østergaard, N.; Smith, S.C.; Shirley, I.M. Microencapsulation of osmium tetroxide in polyurea. Org. Lett. 2003, 5, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Gyger, F.; Bockstaller, P.; Gerthsen, D.; Feldmann, C. Ammonia-in-oil-microemulsions and their application. Angew. Chem. Int. Ed. 2013, 52, 12443–12447. [Google Scholar] [CrossRef] [PubMed]
- Revina, A.; Kuznetsov, M.; Chekmarev, A. Physicochemical properties of rhenium nanoparticles obtained in reverse micelles. Dokl. Chem. 2013, 450, 119–121. [Google Scholar] [CrossRef]
- Revina, A.A.; Kuznetsov, M.A.; Chekmarev, A.M.; Boyakov, E.E.; Zolotarevskii, V.I. Synthesis and physicochemical properties of rhenium nanoparticles. Prot. Met. Phys. Chem. Surf. 2018, 54, 43–50. [Google Scholar] [CrossRef]
- Babu, K.M.; Mucalo, M.R. XPS studies of freshly prepared rhenium nanoparticle dispersions from hydrazinium hydrate and borohydride reduction of hexachlororhenate solutions. J. Mater. Sci. Lett. 2003, 22, 1755–1757. [Google Scholar] [CrossRef]
- Boutonnet, M.; Lögdberg, S.; Elm Svensson, E. Recent developments in the application of nanoparticles prepared from W/O microemulsions in heterogeneous catalysis. Curr. Opin. Colloid Interface Sci. 2008, 13, 270–286. [Google Scholar] [CrossRef]
- Asrami, M.R.; Nejati, B.; Tavasoli, A.; Karimi, A. Performance Enhancement of Pt-Re/Al2O3 Naphtha Reforming Nanocatalysts Using Microemulsion Technique. Pet. Coal 2016, 58. [Google Scholar]
- Logvinenko, V.; Fedorov, V.; Mironov, Y.; Drebushchak, V. Kinetic and thermodynamic stability of cluster compounds under heating. J. Therm. Anal. Calorim. 2007, 88, 687–692. [Google Scholar] [CrossRef]
- Naumov, N.; Kim, S.; Virovets, A.; Mironov, Y.; Fedorov, V. New rhenium octahedral cluster sulfido-cyanide chain polymer: the synthesis and crystal structure of Cs4[{Re6S8}(CN)4S2/2]. Bull. Korean Chem. Soc. 2006, 27, 635. [Google Scholar] [CrossRef]
- Naumov, N.G.; Ledneva, A.Y.; Kim, S.J.; Fedorov, V.E. New trans-[Re6S8(CN)4L2]n− rhenium cluster complexes: Syntheses, crystal structures and properties. J. Clust. Sci. 2009, 20, 225–239. [Google Scholar] [CrossRef]
- Aubert, T.; Ledneva, A.Y.; Grasset, F.; Kimoto, K.; Naumov, N.G.; Molard, Y.; Saito, N.; Haneda, H.; Cordier, S. Synthesis and characterization of A4[Re6Q8L6]@SiO2 red-emitting silica nanoparticles based on Re6 metal atom clusters (A = Cs or K, Q = S or Se, and L = OH or CN). Langmuir 2010, 26, 18512–18518. [Google Scholar] [CrossRef] [PubMed]
- Kolb, M.; Maier, W.; Stöwe, K. High-throughput syntheses of nano-scaled mixed metal sulphides. Catal. Today 2011, 159, 64–73. [Google Scholar] [CrossRef]
- Lu, T.; Wei, H.; Yang, X.; Li, J.; Wang, X.; Zhang, T. Microemulsion-controlled synthesis of one-dimensional Ir nanowires and their catalytic activity in selective hydrogenation of o-chloronitrobenzene. Langmuir 2014, 31, 90–95. [Google Scholar] [CrossRef]
- Yang, F.; Fu, L.; Cheng, G.; Chen, S.; Luo, W. Ir-oriented nanocrystalline assemblies with high activity for hydrogen oxidation/evolution reactions in an alkaline electrolyte. J. Mater. Chem. A 2017, 5, 22959–22963. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, J.Y.; Zhao, Y.S.; Yao, J. Organic core–shell nanostructures: microemulsion synthesis and upconverted emission. Chem. Commun. 2010, 46, 4959–4961. [Google Scholar] [CrossRef]
- Ricciardi, L.; Martini, M.; Tillement, O.; Sancey, L.; Perriat, P.; Ghedini, M.; Szerb, E.I.; Yadav, Y.J.; La Deda, M. Multifunctional material based on ionic transition metal complexes and gold–silica nanoparticles: Synthesis and photophysical characterization for application in imaging and therapy. J. Photochem. Photobiol. B 2014, 140, 396–404. [Google Scholar] [CrossRef]
- Félix-Navarro, R.M.; Salazar-Gastélum, M.I.; Beltrán-Gastélum, M.; Reynoso-Soto, E.; Lin, S.W.; Pérez-Sicairos, S.; Paraguay-Delgado, F.; Alonso-Núñez, G. Development of a Pt-Ir bimetallic nanoparticulated electrocatalyst deposited on MWCNT for an electro-fenton process. J. Electrochem. Soc. 2014, 161, H845–H853. [Google Scholar] [CrossRef]
- Szumełda, T.; Drelinkiewicz, A.; Kosydar, R.; Góral-Kurbiel, M.; Gurgul, J.; Duraczyńska, D. Formation of Pd-group VIII bimetallic nanoparticles by the “water-in-oil” microemulsion method. Colloids Surf. A. 2017, 529, 246–260. [Google Scholar] [CrossRef]
- Sanchez-Dominguez, M.; Boutonnet, M.; Solans, C. A novel approach to metal and metal oxide nanoparticle synthesis: the oil-in-water microemulsion reaction method. J. Nanopart. Res. 2009, 11, 1823. [Google Scholar] [CrossRef]
- Yoon, B.; Pan, H.B.; Wai, C.M. Relative catalytic activities of carbon nanotube-supported metallic nanoparticles for room-temperature hydrogenation of benzene. J. Phys. Chem. C 2009, 113, 1520–1525. [Google Scholar] [CrossRef]
- Liao, Y.J.; Pan, H.B.; Wai, C.M. Pt, Rh and Pt-Rh nanoparticles on modified single-walled carbon nanotubes for hydrogenation of benzene at room temperature. J. Nanosci. Nanotechnol. 2011, 11, 8580–8585. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Wang, K.; Liang, H. Photochemical generation of catalytically active shape selective rhodium nanocubes. J. Phys. Chem. C 2009, 113, 18570–18577. [Google Scholar] [CrossRef]
- Montes, V.; Checa, M.; Marinas, A.; Boutonnet, M.; Marinas, J.; Urbano, F.; Järas, S.; Pinel, C. Synthesis of different ZnO-supported metal systems through microemulsion technique and application to catalytic transformation of glycerol to acetol and 1, 2-propanediol. Catal. Today 2014, 223, 129–137. [Google Scholar] [CrossRef]
- Sergeev Mihail, O.; Revina Alexandra, A.; Busev Sergey, A.; Zolotarevskiy Victor, I.; Zhavoronkova Kseniya, N.; Boeva Olga, A. Catalytic properties of monometallic and bimetallic palladium and rhodium nanoparticles obtained in reverse micellar systems. Nanotechnol. Rev. 2014, 3, 515. [Google Scholar] [CrossRef]
- Revina, A.; Kuznetsov, M.; Busev, S.; Boyakov, E.; Mikhaylov, A.; Chekmarev, A. Synthesis properties and electrocatalytic activities of Pd: Ru, Rh nanoparticles. Int. J. Adv. Res. Chem. Sci. 2015, 2, 7–21. [Google Scholar]
- Guo, X.; Liang, B.; Jian, J.; Zhang, Y.; Ye, X. Glucose biosensor based on a platinum electrode modified with rhodium nanoparticles and with glucose oxidase immobilized on gold nanoparticles. Microchim. Acta 2014, 181, 519–525. [Google Scholar] [CrossRef]
- Suryawanshi, Y.R.; Chakraborty, M.; Jauhari, S.; Mukhopadhyay, S.; Shenoy, K.T. Selective hydrogenation of 4’,4”(5”)-Di-Tert-Butyldibenzo-18-Crown-6 ether over Rh/γ-Al2O3 nanocatalyst. Int. J. Chem. React. Eng. 2016, 15. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, J.; Lee, J.M. Small size Rh nanoparticles in micelle nanostructure by ionic liquid/CTAB for acceptorless dehydrogenation of alcohols only in pure water. ACS Sustain. Chem. Eng. 2017, 5, 2056–2060. [Google Scholar] [CrossRef]
- Jiang, B.; Li, C.; Dag, Ö.; Abe, H.; Takei, T.; Imai, T.; Hossain, M.S.A.; Islam, M.T.; Wood, K.; Henzie, J. Mesoporous metallic rhodium nanoparticles. Nat. Commun. 2017, 8, 15581. [Google Scholar] [CrossRef]
- Zahedifar, M.; Zhiani, R.; Sadeghzadeh, S.M.; Shamsa, F. Nanofibrous rhodium with a new morphology for the hydrogenation of CO2 to formate. New J. Chem. 2019, 43, 4489–4496. [Google Scholar] [CrossRef]
- Sanders, T.; Papas, P.; Veser, G. Supported nanocomposite catalysts for high-temperature partial oxidation of methane. Chem. Eng. J. 2008, 142, 122–132. [Google Scholar] [CrossRef]
- Kirchhoff, M.; Specht, U.; Veser, G. Engineering high-temperature stable nanocomposite materials. Nanotechnol. 2005, 16, S401–S408. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Veser, G. Exceptional high-temperature stability through distillation-like self-stabilization in bimetallic nanoparticles. Nat. Mater. 2010, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Fajardie, F.; Tempère, J.F.; Manoli, J.M.; Touret, O.; Blanchard, G.; Djéga-Mariadassou, G. Activity of Rhx+ species in CO oxidation and NO Reduction in a CO/NO/O2 stoichiometric mixture over a Rh/CeO2-ZrO2 catalyst. J. Catal. 1998, 179, 469–476. [Google Scholar] [CrossRef]
- Bion, N.; Epron, F.; Moreno, M.; Mariño, F.; Duprez, D. Preferential oxidation of carbon monoxide in the presence of hydrogen (PROX) over noble metals and transition metal oxides: advantages and drawbacks. Top. Catal. 2008, 51, 76. [Google Scholar] [CrossRef]
- Boaro, M.; Modafferi, V.; Pappacena, A.; Llorca, J.; Baglio, V.; Frusteri, F.; Frontera, P.; Trovarelli, A.; Antonucci, P.L. Comparison between Ni–Rh/gadolinia doped ceria catalysts in reforming of propane for anode implementations in intermediate solid oxide fuel cells. J. Power Sources 2010, 195, 649–661. [Google Scholar] [CrossRef]
- Cai, W.; Wang, F.; Van Veen, A.C.; Provendier, H.; Mirodatos, C.; Shen, W. Autothermal reforming of ethanol for hydrogen production over an Rh/CeO2 catalyst. Catal. Today 2008, 138, 152–156. [Google Scholar] [CrossRef]
- Galletti, C.; Fiorot, S.; Specchia, S.; Saracco, G.; Specchia, V. Activity of rhodium-based catalysts for CO preferential oxidation in H2-rich gases. Top. Catal. 2007, 45, 15–19. [Google Scholar] [CrossRef]
- Yee, A.; Morrison, S.J.; Idriss, H. The reactions of ethanol over M/CeO2 catalysts: Evidence of carbon–carbon bond dissociation at low temperatures over Rh/CeO2. Catal. Today 2000, 63, 327–335. [Google Scholar] [CrossRef]
- Kurnatowska, M.; Kepinski, L. Structure and thermal stability of nanocrystalline Ce1−xRhxO2−y in reducing and oxidizing atmosphere. Mater. Res. Bull. 2013, 48, 852–862. [Google Scholar] [CrossRef]










| Metal NP Composition | Microemulsion Type | Surfactant | Particle Size | Highlight(s) of Synthetic Method | Ref |
|---|---|---|---|---|---|
| Re NPs | Reverse microemulsion (a/o) | DDAB or DDAI | 2.2 nm | Useful for synthesizing oxidation-sensitive NPs. Needs very low temperature | [72] |
| Re NPs | Reverse microemulsion | AOT | 1–18 nm (depends on the synthesis conditions) | Control of the particle size with varying γ-irradiation doses. | [73,74] |
| Re/Re oxide NPs | Reverse microemulsion | AOT | 0.7–1.4 nm | Obtained very small sized particles with a narrow size distribution but metal NPs oxidation state was not studied. Reverse trend observed for W factor effect. | [75] |
| Pt-Re bimetallic NPs | Reverse microemulsion | Triton X-100 | 1.8–2.05 nm | Small bimetallic NPs obtained, Used a very low W value (=0.3) | [77] |
| Re6 cluster@SiO2 NPs | Reverse microemulsion | Brij 30 | 30 nm | NPs kept their luminescence properties in aqueous solutions (potential for biological applications) (need to form multinuclear Re cluster at high temperatures first) | [81] |
| Re sulfide NPs | Reverse microemulsion | NP5/NP10 or NP10/Triton X-45 | - | Introduced a novel high-throughput microemulsion synthesis device | [82] |
| Metal NP Composition | Microemulsion Type | Surfactant | Particle Size | Highlight(s) of Synthetic Method | Ref |
|---|---|---|---|---|---|
| Ir nanowires | Reverse microemulsion | Several surfactants such as CTAB, Triton X-100, Brij 30, etc. | 1.8 nm diameter (for the CTAB-micelles) | Presents a facile method for synthesizing 1D Ir nanomaterials and evaluating the effects of different surfactants on the morphology and size of prepared Ir NPs | [83,84] |
| Organoiridium (III) complex NPs | Normal microemulsion | CTAB | 30 nm | Uses an organometallic complex for a normal microemulsion NP synthesis. Reduction of perylene (which acts as a triplet annihilator) occurs outside the CTAB-micelles with the formation of core-shell Ir complex@perylene NPs | [85] |
| Ir(III)complex@GS NPs | Reverse microemulsion | Triton X-100 | 50 nm for Ru1@GSNPs (as a sample of all the M@GSNPs) | Synthesizes doped transition metal complexes into GS NPs with potential biomedical applications | [86] |
| Ir and Pt-Ir bimetallic NPs anchored onto MWCNTs | Reverse microemulsion | Brij 30 | - | Prepares a stable and reusable cathode of bimetallic Pt-Ir NPs@MWCNTs | [87] |
| Ir and Pd-Ir bimetallic NPs supported on carbon | Reverse microemulsion | Triton X-114 | 4 nm for Ir/C and 4.2 nm for Pd-Ir/C NPs | The size of mono- and bimetallic NPs has been studied. The synthesized Pd-Ir-0.1/C NPs were much smaller than the Pd/C NPs. Among all the mono- and bimetallic/C synthesized NPs prepared, the Ir mono- and bimetallic/C NPs turned out to be the smallest. | [88] |
| Metal NP Composition | Microemulsion Type | Surfactant | Particle Size | Highlight(s) of Synthetic Method | Ref |
|---|---|---|---|---|---|
| Rh NPs | Normal microemulsion | Brij 96V | 3.9 nm | Synthesis of metal and metal oxide NPs including Rh by a normal microemulsion (less oil phase) | [89] |
| Rh mono- and bimetallic NPs supported on functionalized multi- and single wall CNTs | Reverse microemulsion | AOT | 5.6 nm and 4.6 nm for Rh and Pd-Rh, and 4.5 nm and 2.3 nm for Rh and Pt-Rh, respectively. | The bimetallic supported NPs showed a significant increase in their catalytic activity compared to monometallic supported NPs | [90,91] |
| Rh NPs supported on ZnO | Reverse microemulsion | Synperonic 13/6.5 | 2.1 nm | The selectivity of the microemulsion-synthesis Rh@ZnO NPs increased with respect to glycerol dehydrogenation but the activity compared to DP-solids decreased which could be caused by the presence of some remaining surfactant molecules around the particles after heating | [93] |
| Rh mono- and bimetallic NPs | Reverse microemulsion | AOT | 0.7–6.5 nm, and 2–4 nm | Smaller particles prepared by lower γ-irradiation doses | [94,95] |
| Rh NPs | Reverse microemulsion | AOT | 10 nm | Prepared a very good Rh NPs-modified Pt electrode which performed as an effective glucose biosensor in real blood samples | [96] |
| Rh NPs | Reverse microemulsion | AOT | 4 nm | Smaller particles with higher activity obtained by the microemulsion technique compared to MWv-method | [97] |
| Rh NPs | Normal microemulsion | CTAB | 1–2 nm | Using an ionic liquid to improve the micellization and the size control of the particles | [98] |
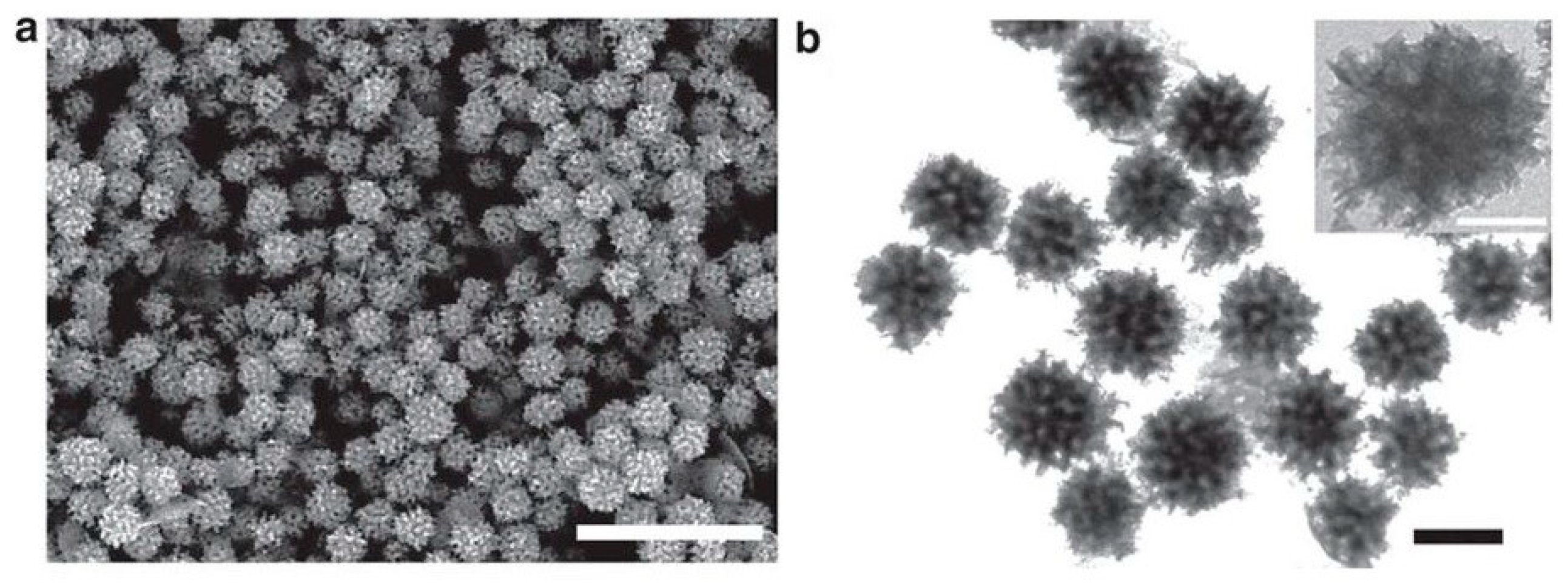
| Rh NPs | Polymer-micellar template | PEO-b-PMMA | 100 nm | Synthesizing mesoporous Rh NPs for the first time by applying a chemical reduction method | [99] |
| Rh NPs | Reverse microemulsion | CPB | 12 nm | Synthesizing a fibrous-structure of Rh NPs with high thermal and mechanical stability and high surface area | [100] |
| Pt-Rh@BHA NPs | Reverse microemulsion | (PPG-b-PEG-b-PPG) polymer | 4.1 nm | Prepared bimetallic Pt-Rh NPs with exceptionally high thermal stability and precise control of composition | [103] |
| Ce1−xRhxO2−y mixed oxide NPs | Reverse microemulsion | Triton X-100 | 4–5 nm | Prepared a range of Ce-Rh mixed oxide nanocrystals with a wide and higher range of Rh doping levels with their structural stability studied under oxidizing and reducing atmospheres | [110] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleimani Zohr Shiri, M.; Henderson, W.; Mucalo, M.R. A Review of The Lesser-Studied Microemulsion-Based Synthesis Methodologies Used for Preparing Nanoparticle Systems of The Noble Metals, Os, Re, Ir and Rh. Materials 2019, 12, 1896. https://doi.org/10.3390/ma12121896
Soleimani Zohr Shiri M, Henderson W, Mucalo MR. A Review of The Lesser-Studied Microemulsion-Based Synthesis Methodologies Used for Preparing Nanoparticle Systems of The Noble Metals, Os, Re, Ir and Rh. Materials. 2019; 12(12):1896. https://doi.org/10.3390/ma12121896
Chicago/Turabian StyleSoleimani Zohr Shiri, Mohammad, William Henderson, and Michael R. Mucalo. 2019. "A Review of The Lesser-Studied Microemulsion-Based Synthesis Methodologies Used for Preparing Nanoparticle Systems of The Noble Metals, Os, Re, Ir and Rh" Materials 12, no. 12: 1896. https://doi.org/10.3390/ma12121896





