Bitumen Recovery from Crude Bitumen Samples from Halfaya Oilfield by Single and Composite Solvents—Process, Parameters, and Mechanism
Abstract
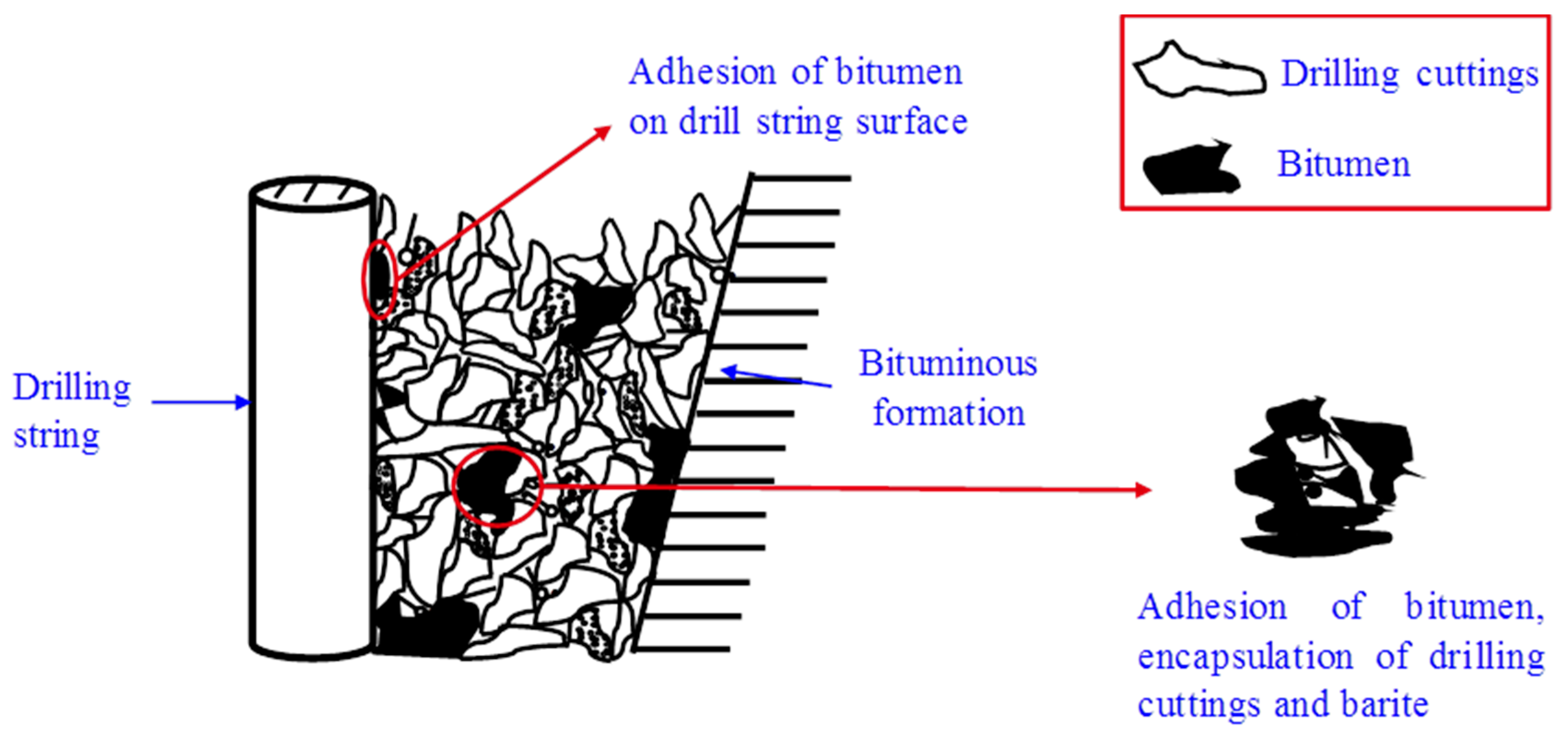
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Samples
2.2. Single Factor Experiment
2.3. Single Solvent Extraction and Composite Solvent Extraction
2.4. SARA Analysis
2.5. Viscosity Measurement and Element Content Analysis
3. Results and Discussion
3.1. Single Factor Experiment
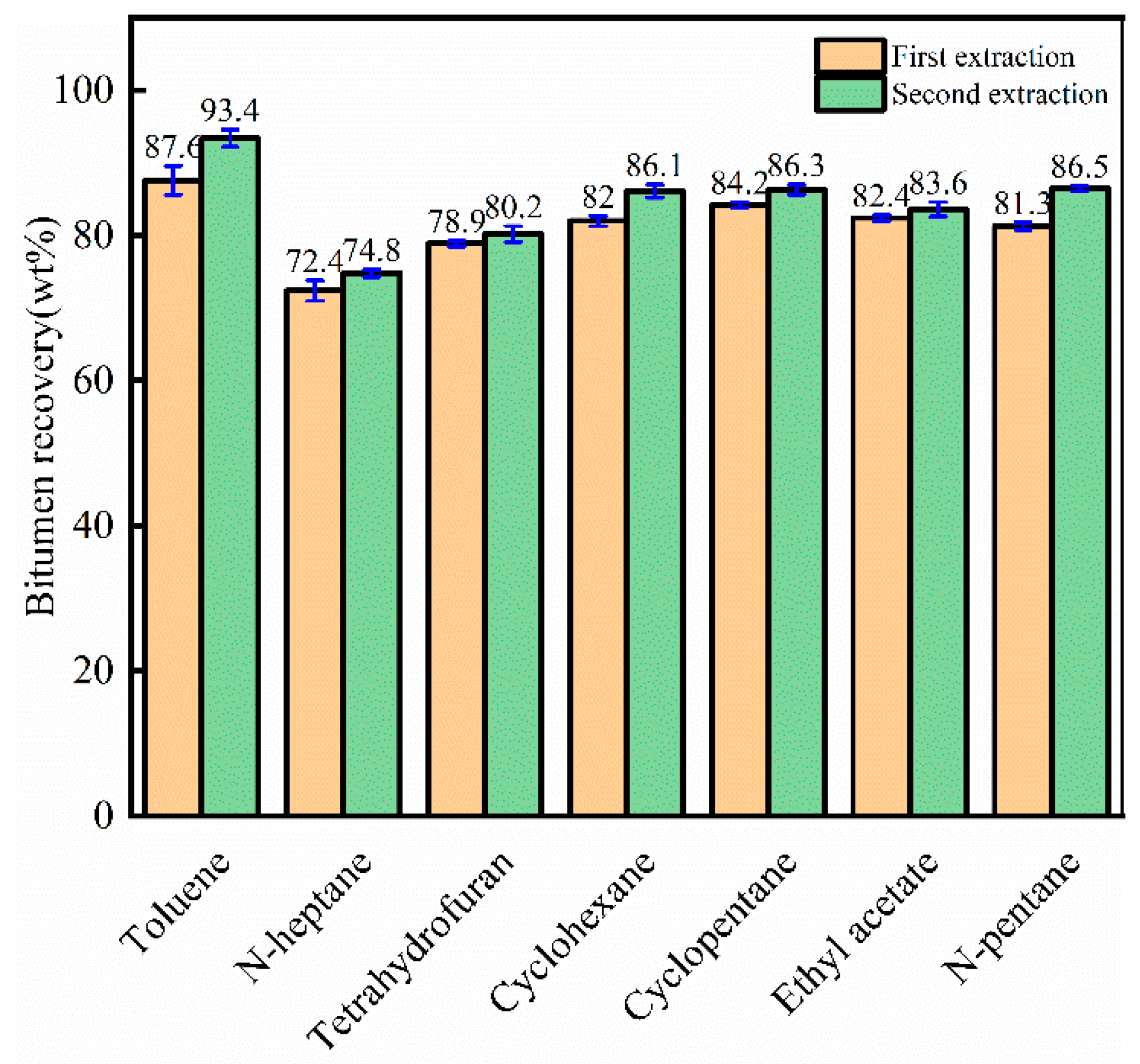
3.2. Single Solvent Extraction Experiment
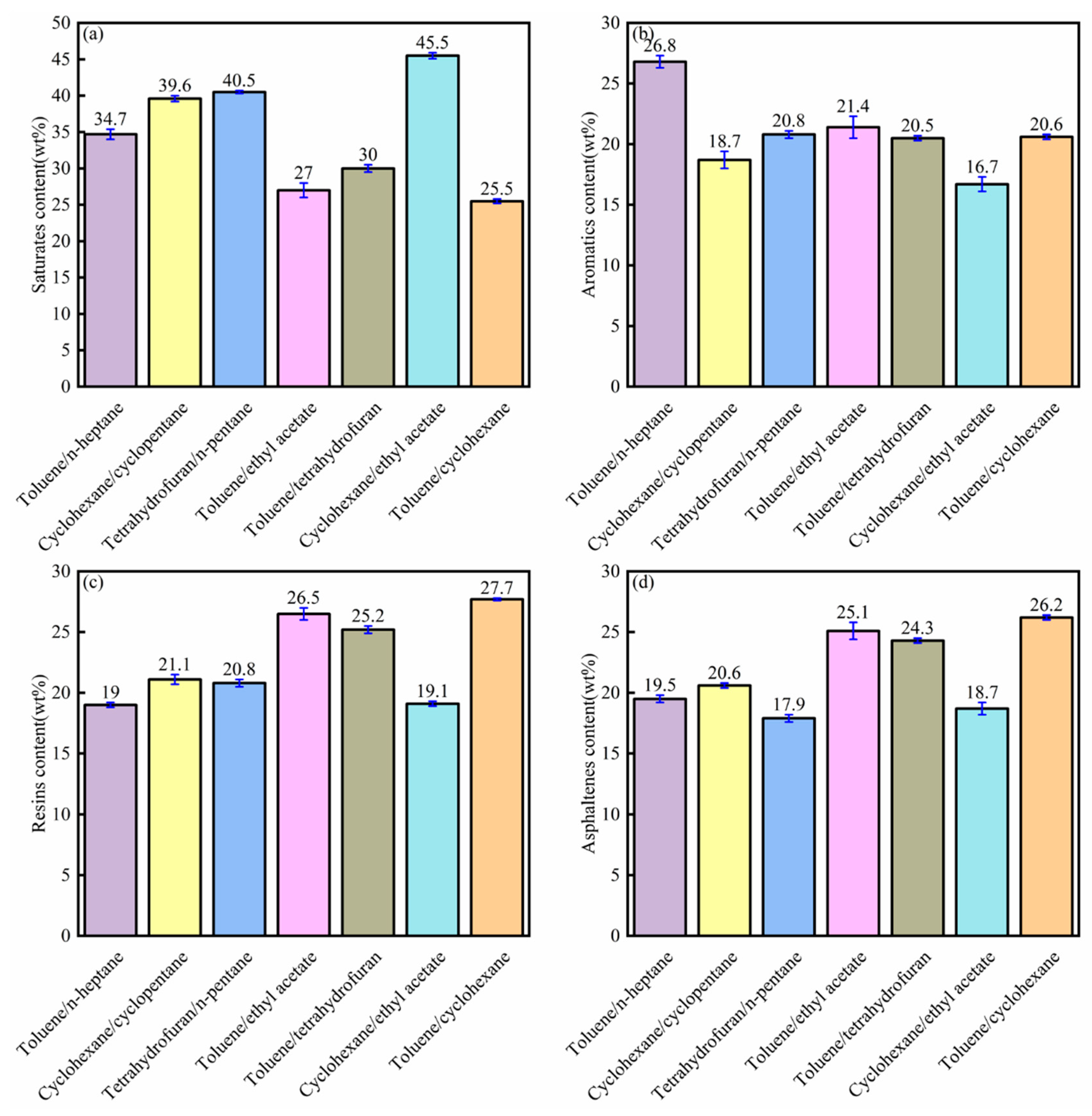
3.3. SARA Analysis of Bitumen from Single Solvent Extraction
3.4. Composite Solvent Extraction
3.5. SARA Analysis of Bitumen from Composite Solvent Extraction
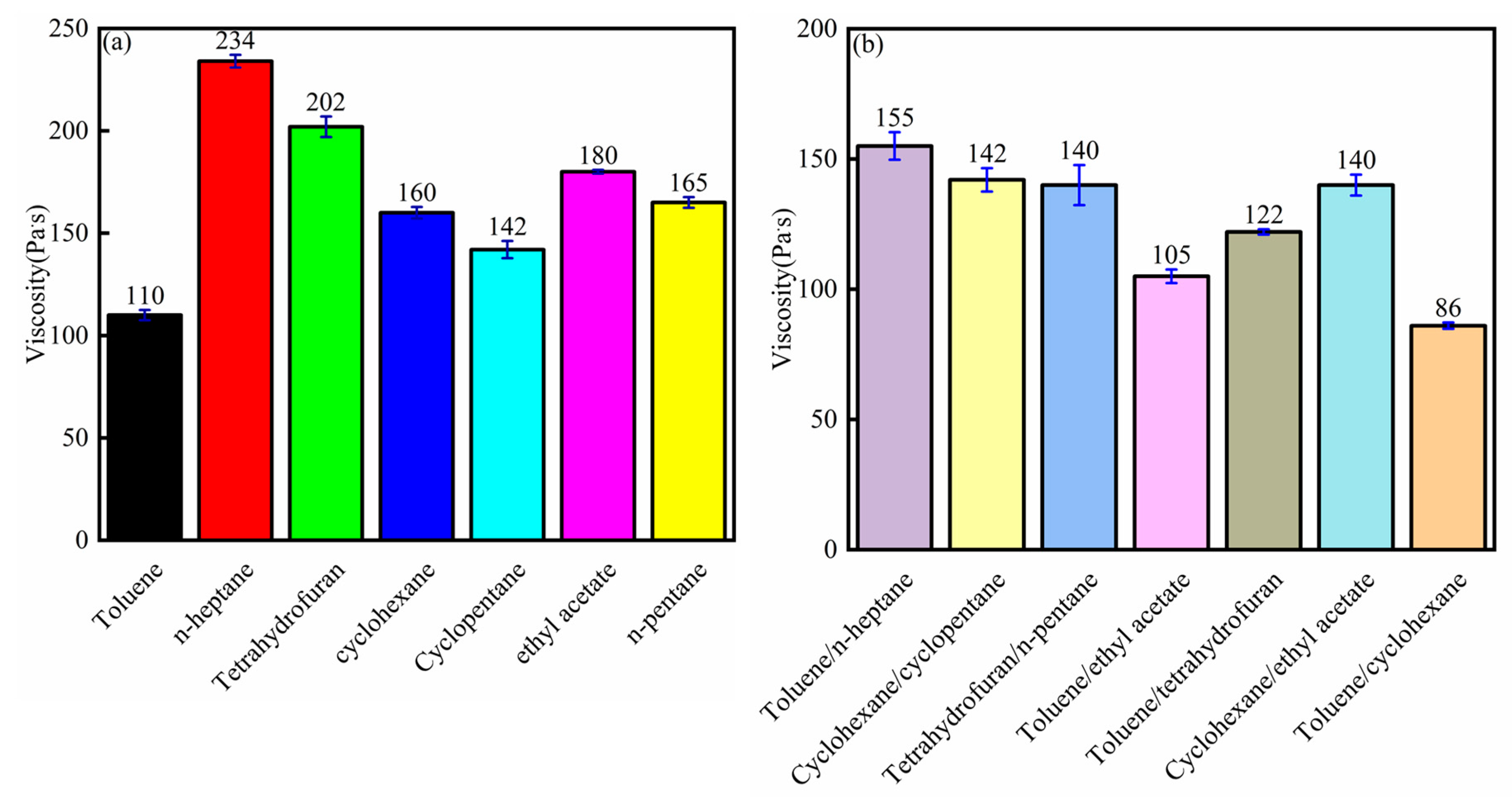
3.6. Viscosity Analysis
3.7. The Element Content Analysis of Bitumen
4. Conclusions
- (1)
- The optimal operation condition for bitumen recovery by toluene from crude bitumen samples was, as follows: the temperature was 40 °C, crude bitumen sample to solvent ratio was 1:10, stirring rate was 500 rpm, stirring time was 60 min, ultrasound time was 30 min.
- (2)
- The bitumen recovery increased significantly using the composite solvent compared to the single solvent. The highest bitumen recovery from crude bitumen samples was 98.9 wt%. SARA analysis indicated that the asphaltene content increased significantly from the single solvent to composite solvent. Toluene and cycloalkane showed the highest asphaltene content, while the n-alkanes showed the lowest asphaltene content. The composite solvent obtained the highest asphaltene content and bitumen recovery.
- (3)
- The bitumen samples extracted from different solvents showed different viscosities. The bitumen viscosity influenced the bitumen recovery, and the lower the bitumen viscosity, the higher the bitumen recovery. The C/H ratio of the bitumen followed this rule.
Author Contributions
Funding
Conflicts of Interest
References
- Han, G.; Osmond, J.; Zambonini, M. A USD 100 million “Rock”: Bitumen in the deepwater Gulf of Mexico. SPE Drill. Completion 2010, 25, 290–299. [Google Scholar] [CrossRef]
- Arseniuk, S.E.; Becker, D.L.; Barrett, K.R.; Keller, D. Drilling Challenges in the bitumen saturated Grosmont formation. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 16–18 June 2009; Petroleum Society of Canada: Calgary, AB, Canada, 2009. [Google Scholar]
- Romo, L.; Shaughnessy, J.; Lisle, E. Challenges associated with Subsalt Tar in the Mad Dog Field. In Proceedings of the SPE Annual Technical Conference and Exhibition, Anaheim, CA, USA, 11–14 November 2007; Society of Petroleum Engineers: Richardson, TX, USA, 2007. [Google Scholar]
- Ezeakacha, C.P.; Salehi, S.; Hayatdavoudi, A. Experimental Study of Drilling Fluid’s Filtration and Mud Cake Evolution in Sandstone Formations. J. Energy Resour. Technol. 2017, 139, 022912. [Google Scholar] [CrossRef]
- Perez, G. Development of a Chemical Treatment for the Management of Wellbore Tar Adhesion. In Proceedings of the SPE International Thermal Operations and Heavy Oil Symposium, Calgary, AB, Canada, 1–3 November 2005; Society of Petroleum Engineers: Richardson, TX, USA, 2005. [Google Scholar]
- Yin, S.; Tian, T.; Wu, Z. Developmental characteristics and distribution law of fractures in a tight sandstone reservoir in a low-amplitude tectonic zone, eastern Ordos Basin, China. Geol. J. 2019, 54, 1–16. [Google Scholar] [CrossRef]
- Yin, S.; Zhao, J.; Wu, Z.; Ding, W. Strain energy density distribution of a tight gas sandstone reservoir in a low-amplitude tectonic zone and its effect on gas well productivity: A 3D FEM study. J. Pet. Sci. Eng. 2018, 170, 89–104. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Zhao, Z.; Liu, M.; Zhang, Q. Application of Dopamine Functional Materials in Water Pollution Control. Prog. Chem. 2019, 31, 571–579. [Google Scholar]
- He, L.; Lin, F.; Li, X.; Sui, H.; Xu, Z. Interfacial sciences in unconventional petroleum production: From fundamentals to applications. Chem. Soc. Rev. 2015, 44, 5446–5494. [Google Scholar] [CrossRef]
- Nie, F.; He, D.; Guan, J.; Li, X.; Hong, Y.; Wang, L.; Zheng, H.; Zhang, Q. Oil sand pyrolysis: Evolution of volatiles and contributions from mineral, bitumen, maltene, and SARA fractions. Fuel 2018, 224, 726–739. [Google Scholar] [CrossRef]
- Meng, M.; Qiu, Z.; Zhong, R.; Liu, Z.; Liu, Y.; Chen, P. Adsorption characteristics of supercritical CO2/CH4 on different types of coal and a machine learning approach. Chem. Eng. J. 2019, 368, 847–864. [Google Scholar] [CrossRef]
- Schramm, L.L.; Smith, R.G.; Stone, J.A. A surface-tension method for the determination of anionic surfactants in hot water processing of athabasca oil sands. Colloids Surf. 1984, 11, 247–263. [Google Scholar] [CrossRef]
- He, J.J.; Geng, A.S.; Wu, L.L. Effect of Lithology on the Efficiency of the Hot Water-Based Extraction for Oil Sand Bitumen: A Case Study on Oil Sands from Houba, Sichuan and Tumuji, Inner Mongolia. Bull. Mineral. Petrol. Geochem. 2015, 34, 379–386. [Google Scholar]
- Khraisha, Y.H. Study of extraction and pyrolysis of Jordan tar sand. Int. J. Energy Res. 1999, 23, 833–839. [Google Scholar] [CrossRef]
- Dai, Q.; Chung, K.H. Hot water extraction process mechanism using model oil sands. Fuel 1996, 75, 220–226. [Google Scholar] [CrossRef]
- Tong, W.; Chao, Z.; Zhao, R.; Zhu, C.; Yang, C.; Liu, C. Solvent Extraction of Bitumen from Oil Sands. Energy Fuels 2014, 28, 2297–2304. [Google Scholar]
- Lin, F.; Stoyanov, S.R.; Xu, Y. Development, Recent Advances in Nonaqueous Extraction of Bitumen from Mineable Oil Sands: A Review. Org. Process Res. Dev. 2017, 21, 492–510. [Google Scholar] [CrossRef]
- Ma, Y.; Li, S. The pyrolysis, extraction and kinetics of Buton oil sand bitumen. Fuel Process. Technol. 2012, 100, 11–15. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, M.; Zhang, Z.; Zhang, D. Pyrolysis of an Indonesian Oil Sand in a Thermogravimetric Analyser and a Fixed-Bed Reactor. J. Anal. Appl. Pyrolysis 2016, 117, 191–198. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, C.; Ge, J.; Guo, W. 1H NMR and 13C NMR Studies of Oil from Pyrolysis of Indonesian Oil Sands. Energy Fuels 2016, 30, 2478–2491. [Google Scholar]
- He, D.M.; Nie, F.; Guan, J.; Hu, H.Q.; Zhang, Q.M. Hot Water Extraction and Fixed Bed Pyrolysis for Bitumen Recovery of an Indonesian Oil Sand. Appl. Mech. Mater. 2014, 672, 624–627. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, D.; Chen, W.; Liu, B.; Zhang, X. A comprehensive review of emulsion and its field application for enhanced oil recovery. Energy Sci. Eng. 2019, 7, 1046–1058. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yin, D.; Cao, R.; Zhang, C. The mechanism for pore-throat scale emulsion displacing residual oil after water flooding. J. Pet. Sci. Eng. 2018, 163, 519–525. [Google Scholar] [CrossRef]
- Zhong, H.; Qiu, Z.; Chai, J.; Guo, B.; Dong, S.; Liu, J. A preliminary study of the preparation of shale stabilizer with oil sludge-from waste to resource. J. Pet. Sci. Eng. 2018, 161, 50–60. [Google Scholar] [CrossRef]
- Li, X.; Hou, J.; Sui, H.; Sun, L.; Xu, L. Switchable-Hydrophilicity Triethylamine: Formation and Synergistic Effects of Asphaltenes in Stabilizing Emulsions Droplets. Materials 2018, 11, 2431. [Google Scholar] [CrossRef]
- Clark, K.A.; Pasternack, D.S. Hot Water Seperation of Bitumen from Alberta Bituminous Sand. Ind. Eng. Chem. 1932, 24, 1410–1416. [Google Scholar] [CrossRef]
- Alvarez-Majmutov, A.; Gieleciak, R.; Chen, J. Modeling the molecular composition of vacuum residue from oil sand bitumen. Fuel 2019, 241, 744–752. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Vakhin, A.V.; Mukhamatdinov, I.I.; Onishchenko, Y.V.; Feoktistov, D.A. Effects of calcite and dolomite on conversion of heavy oil under subcritical condition. Pet. Sci. Technol. 2019, 37, 687–693. [Google Scholar] [CrossRef]
- Bazzaz, M.; Darabi, M.K.; Little, D.N. A straightforward procedure to characterize nonlinear viscoelastic response of asphalt concrete at high temperatures. Transp. Res. Rec. 2018, 2672, 481–492. [Google Scholar] [CrossRef]
- Darabi, M.K.; Huang, C.-W.; Bazzaz, M.; Masad, E.A.; Little, D.N. Characterization and validation of the nonlinear viscoelastic-viscoplastic with hardening-relaxation constitutive relationship for asphalt mixtures. Constr. Build. Mater. 2019, 216, 648–660. [Google Scholar] [CrossRef]
- Li, X.; Lin, H.; Wu, G.; Sun, W.; Sui, H. Operational Parameters, Evaluation Methods, And Fundamental Mechanisms: Aspects of Nonaqueous Extraction of Bitumen from Oil Sands. Energy Fuels 2012, 26, 3553–3563. [Google Scholar] [CrossRef]





| Variable Factors | 1 | 2 | 3 | 4 | Other Factors |
|---|---|---|---|---|---|
| Temperature (°C) | 25 | 40 | 55 | 70 | Condition a |
| Oil sands to solvent ratio | 1:1 | 1:5 | 1:10 | 1:20 | Condition b |
| Stirring rate (rpm) | 100 | 300 | 500 | 700 | Condition c |
| Stirring time (min) | 20 | 40 | 60 | 80 | Condition d |
| Ultrasound time (min) | 0 | 30 | 60 | 120 | Condition e |
| Condition | Temperature (°C) | Oil Sands to Solvent Ratio | Stirring Rate (rpm) | Stirring Time (min) | Ultrasound Time (min) |
|---|---|---|---|---|---|
| a | Variable | 1:10 | 500 | 60 | 30 |
| b | 40 | Variable | 500 | 60 | 30 |
| c | 40 | 1:10 | Variable | 60 | 30 |
| d | 40 | 1:10 | 500 | Variable | 30 |
| e | 40 | 1:10 | 500 | 60 | Variable |
| Bitumen from Single Solvent Extraction | C | H | O | N | S |
|---|---|---|---|---|---|
| toluene | 83.358 | 9.650 | 0.072 | 0.490 | 6.430 |
| n-heptane | 79.652 | 12.152 | 2.766 | 0.347 | 5.083 |
| tetrahydrofuran | 81.642 | 11.048 | 2.623 | 0.322 | 4.365 |
| cyclohexane | 82.658 | 10.346 | 0.028 | 0.432 | 6.536 |
| cyclopentane | 82.356 | 10.586 | 1.481 | 0.440 | 5.137 |
| ethyl acetate | 81.568 | 11.036 | 2.874 | 0.354 | 4.168 |
| n-pentane | 82.265 | 10.952 | 1.057 | 0.406 | 5.320 |
| Bitumen from Composite Solvent Extraction | C | H | O | N | S |
|---|---|---|---|---|---|
| toluene/n-heptane | 83.532 | 10.564 | 0.442 | 0.379 | 5.083 |
| cyclohexane/cyclopentane | 83.048 | 10.298 | 1.509 | 0.265 | 4.889 |
| tetrahydrofuran/n-pentane | 82.653 | 11.892 | 0.662 | 0.336 | 4.458 |
| toluene/ethyl acetate | 84.685 | 8.780 | 0.455 | 0.405 | 5.675 |
| toluene/tetrahydrofuran | 84.068 | 9.365 | 0.094 | 0.376 | 6.097 |
| cyclohexane/ethyl acetate | 82.964 | 11.068 | 0.021 | 0.983 | 4.964 |
| toluene/cyclohexane | 85.026 | 8.460 | 0.803 | 0.344 | 5.367 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Qiu, Z.; Zhong, H.; Nie, Z.; Li, J.; Huang, W.; Zhao, X. Bitumen Recovery from Crude Bitumen Samples from Halfaya Oilfield by Single and Composite Solvents—Process, Parameters, and Mechanism. Materials 2019, 12, 2656. https://doi.org/10.3390/ma12172656
Liu Y, Qiu Z, Zhong H, Nie Z, Li J, Huang W, Zhao X. Bitumen Recovery from Crude Bitumen Samples from Halfaya Oilfield by Single and Composite Solvents—Process, Parameters, and Mechanism. Materials. 2019; 12(17):2656. https://doi.org/10.3390/ma12172656
Chicago/Turabian StyleLiu, Yunfeng, Zhengsong Qiu, Hanyi Zhong, Zhen Nie, Jia Li, Weian Huang, and Xin Zhao. 2019. "Bitumen Recovery from Crude Bitumen Samples from Halfaya Oilfield by Single and Composite Solvents—Process, Parameters, and Mechanism" Materials 12, no. 17: 2656. https://doi.org/10.3390/ma12172656




