Residual Gas Adsorption and Desorption in the Field Emission of Titanium-Coated Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
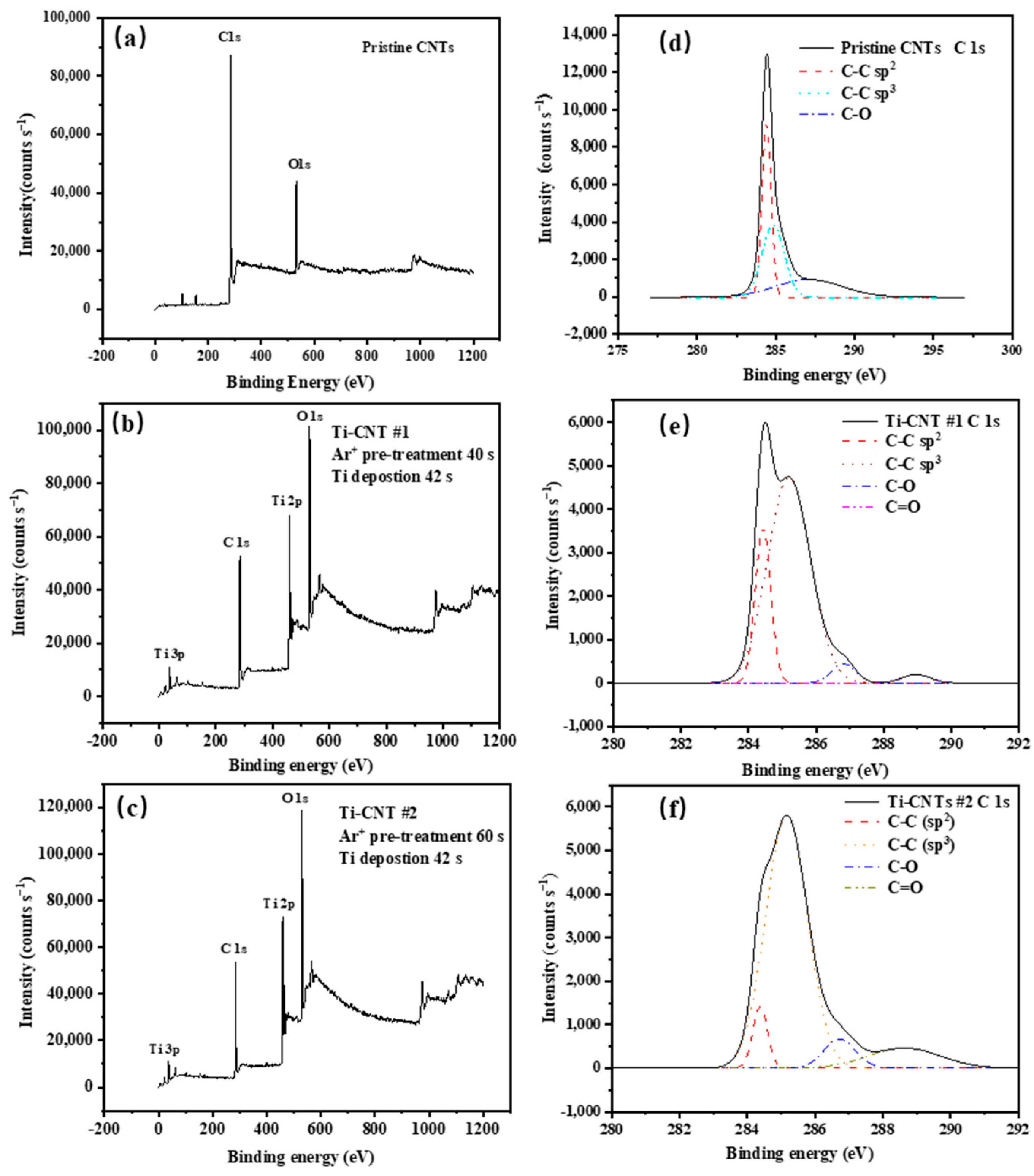
3.1. Characteristics of the CNT Samples
3.2. Gas Adsorption of Different CNTs at Room Temperature
3.3. Gas Desorption of CNTs under Field Emission
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Synthesis of Carbon Nanotubes. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Hafner, J.H.; Rinzler, A.G.; Colbert, D.T.; Smalley, R.E. Nanotubes as nanoprobes in scanning probe microscopy. Nature 1996, 384, 147–150. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Haben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386, 377–379. [Google Scholar] [CrossRef]
- di Bartolomeo, A.; Yang, Y.; Rinzan, M.B.M.; Boyd, A.K.; Barbara, P. Record Endurance for Single-Walled Carbon Nanotube–Based Memory Cell. Nanoscale Res. Lett. 2010, 5, 1852–1855. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Filatrella, G.; Sarno, M.; Di Bartolomeo, A. Multi-walled carbon nanotube films for the measurement of the alcoholic concentration. Micro Nano Lett. 2019, 14, 304–308. [Google Scholar] [CrossRef]
- Milne, W.I.; Teo, K.B.K.; Amaratunga, G.A.J.; Legagneux, P.; Gangloff, L.; Schnell, J.P.; Semet, V.; Binh, V.T.; Groening, O. Carbon nanotubes as field emission sources. J. Mater. Chem. 2004, 14, 933–943. [Google Scholar] [CrossRef]
- Liang, W.; Bockrath, M.; Bozovic, D.; Hafner, J.H.; Tinkham, M.; Park, H. Fabry—Perot interference in a nanotube electron waveguide. Nature 2001, 411, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Kane, C.L.; Dekker, C. High-Field Electrical Transport in Single-Wall Carbon Nanotubes. Phys. Rev. Lett. 2000, 84, 2941–2944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, W.; Zhang, X.; Lou, C.; Zhao, Z.; Wang, B. Very high field-emission current from a carbon-nanotube cathode with a pulse driving mode. IEEE Electron Device Lett. 2009, 30, 571–573. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Meng, P.; Yue, H.; Zheng, R.; Wu, X.; Cheng, G.-A. High current density and low emission field of carbon nanotube array microbundle. Appl. Phys. Lett. 2018, 112, 013101. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Cheng, Y.; Feng, Y.; Zhao, L.; Zhang, H.; Sun, J.; Dong, C. An overview of ionization gauges with carbon nanotube cathodes. J. Phys. D Appl. Phys. 2015, 48, 473001. [Google Scholar] [CrossRef]
- Wilfert, S.; Edelmann, C. Field emitter-based vacuum sensors. Vacuum 2012, 86, 556–571. [Google Scholar] [CrossRef]
- Rinzler, A.G.; Hafner, J.H.; Nikolaev, P.; Lou, L.; Kim, S.G.; Tomanek, D.; Nordlander, P.; Colbert, D.T.; Smalley, R.E. Unraveling nanotubes: Field emission from an atomic wire. Science 1995, 269, 1550–1553. [Google Scholar] [CrossRef]
- De Heer, W.A.; Chatelain, A.; Ugarte, D. A Carbon Nanotube Field-Emission Electron Source. Science 1995, 270, 1179–1180. [Google Scholar] [CrossRef]
- Lee, K.J.; Hong, N.T.; Lee, S.; You, D.-W.; Jung, K.-W.; Yang, S.S. Simple fabrication of micro time-of-flight mass spectrometer using a carbon nanotube ionizer. Sens. Actuators B 2017, 243, 394–402. [Google Scholar] [CrossRef]
- Teo, K.B.; Minoux, E.; Hudanski, L.; Peauger, F.; Schnell, J.P.; Gangloff, L.; Legagneux, P.; Dieumegard, D.; Amaratunga, G.A.; Milne, W.I. Microwave devices: Carbon nanotubes as cold cathodes. Nature 2005, 437, 968. [Google Scholar] [CrossRef]
- Okawa, Y.; Kitamura, S.; Kawamoto, S.; Iseki, Y.; Hashimoto, K.; Noda, E. An experimental study on carbon nanotube cathodes for electrodynamic tether propulsion. Acta Astronaut. 2007, 61, 989–994. [Google Scholar] [CrossRef]
- Lei, W.; Zhu, Z.; Liu, C.; Zhang, X.; Wang, B.; Nathan, A. High-current field-emission of carbon nanotubes and its application as a fast-imaging X-ray source. Carbon 2015, 94, 687–693. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G.; Rossi, R.; Alvisi, M.; Rizzo, A.; Signore, M.A. Enhancement of sensitivity in gas chemiresistors based on carbon nanotube surface functionalized with noble metal (Au, Pt) nanoclusters. Appl. Phys. Lett. 2007, 90, 173123. [Google Scholar] [CrossRef]
- Kong, J.; Chapline, M.G.; Dai, H. Functionalized Carbon Nanotubes for Molecular Hydrogen Sensors. Adv. Mater. 2001, 13, 1384–1386. [Google Scholar] [CrossRef]
- Dean, K.A.; Chalamala, B.R. Field emission microscopy of carbon nanotube caps. J. Appl. Phys. 1999, 85, 3832–3836. [Google Scholar] [CrossRef]
- Dean, K.A.; von Allmen, P.; Chalamala, B.R. Three behavioral states observed in field emission from single-walled carbon nanotubes. J. Vac. Sci. Technol. B 1999, 17, 1959–1969. [Google Scholar] [CrossRef]
- Dong, C.; Luo, H.; Cai, J.; Wang, F.; Zhao, Y.; Li, D. Hydrogen sensing characteristics from carbon nanotube field emissions. Nanoscale 2016, 8, 5599–5604. [Google Scholar] [CrossRef] [PubMed]
- Grzebyk, T.; Górecka-Drzazga, A. Miniature ion-sorption vacuum pump with CNT field-emission electron source. J. Micromech. Microeng. 2013, 23, 015007. [Google Scholar] [CrossRef]
- Lotz, M.; Wilfert, S.; Kester, O. Development of a field emitter-based extractor gauge for the operation in cryogenic vacuum environments. In Proceedings of the IPAC2014, Dresden, Germany, 16–20 June 2014; pp. 2320–2322. [Google Scholar]
- Knápek, A. Methods of Preparation and Characterisation of Experimental Field-Emission Cathodes; Brno University of Technology: Brno, Czech Republic, 2013. [Google Scholar]
- Sergeev, E.; Knápek, A.; Grmela, L.; Šikula, J. Noise diagnostic method of experimental cold field-emission cathodes. In Proceedings of the 2013 22nd International Conference IEEE Noise and Fluctuations (ICNF), Montpellier, France, 24–28 June 2013; pp. 1–4. [Google Scholar]
- Cho, Y.; Kim, C.; Moon, H.; Choi, Y.; Park, S.; Lee, C.-K.; Han, S. Electronic Structure Tailoring and Selective Adsorption Mechanism of Metal-coated Nanotubes. Nanoletters 2008, 8, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, D.; Sun, W.; Sun, J.; Li, G.; Zhang, H.; Xi, Z.; Pei, X.; Li, Y.; Cheng, Y. Synthesis and field electron emission properties of multi-walled carbon nanotube films directly grown on catalytic stainless steel substrate. Vacuum 2018, 149, 195–199. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Lun, N.; Wen, S. Synthesis of gold nano-catalysts supported on carbon nanotubes by using electroless plating technique. Mater. Chem. Phys. 2006, 97, 351–356. [Google Scholar] [CrossRef]
- Yamagiwa, K.; Ayato, Y.; Kuwano, J. Liquid-phase synthesis of highly aligned carbon nanotubes on preheated stainless steel substrates. Carbon 2016, 98, 225–231. [Google Scholar] [CrossRef]
- Latorre, N.; Cazana, F.; Sebastian, V.; Royo, C.; Romeo, E.; Centeno, M.A.; Monzón, A. Growth of carbonaceous nanomaterials over stainless steel foams effect of activation temperature. Catal. Today 2016, 273, 41–49. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, H.S.; Park, S.K.; Joo, J.; Lee, T.J.; Lee, C.J. Morphological change of multiwalled carbon nanotubes through high-energy (MeV) ion irradiation. J. Appl. Phys. 2005, 97, 026103. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Liu, P.; Zhang, L.; Liu, C.; Zhang, L.; Fan, S. The adsorption state and the evolution of field emission properties of graphene edges at different temperatures. RSC Adv. 2018, 8, 31830–31834. [Google Scholar] [CrossRef] [Green Version]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Charlier, J.-C. Defects in Carbon Nanotubes. Acc. Chem. Res. 2002, 35, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Bogle, K.A.; Bachhav, M.N.; Deo, M.S.; Valanoor, N.; Ogale, S.B. Enhanced nonvolatile resistive switching in dilutely cobalt doped TiO2. Appl. Phys. Lett. 2009, 95, 203502–203503. [Google Scholar] [CrossRef]
- Murray, P.T.; Back, T.C.; Cahay, M.M.; Fairchild, S.B.; Maruyama, B.; Lockwood, N.P.; Pasquali, M. Evidence for adsorbate-enhanced field emission from carbon nanotube fibers. Appl. Phys. Lett. 2013, 103, 053113. [Google Scholar] [CrossRef] [Green Version]
- Ago, H.; Kugler, T.; Cacialli, F.; Salaneck, W.R.; Shaffer, M.S.P.; Windle, A.H.; Friend, R.H. Work functions and surface functional groups of multiwall carbon nanotubes. J. Phys. Chem. B 1999, 103, 8116–8121. [Google Scholar] [CrossRef]
- Janas, D.; Koziol, K.K.K. The influence of metal nanoparticles on electrical properties of carbon nanotubes. Appl. Surf. Sci. 2016, 376, 74–78. [Google Scholar] [CrossRef]
- Brodie, I.; Spindt, C.A. Vacuum Microelectronics. Adv. Electron. Electron Phys. 1992, 83, 1–106. [Google Scholar]
- Forbes, R.G. Use of a Spreadsheet to Test for Lack of Field Emission Orthodoxy. In Proceedings of the 2014 Tenth International Vacuum Electron Sources Conference (IVESC), Saint-Petersburg, Russia, 30 June–4 July 2014. [Google Scholar]
- Forbes, R.G. Development of a simple quantitative test for lack of field emission orthodoxy. Proc. R. Soc. A 2013, 469, 20130271. [Google Scholar] [CrossRef]
- Evtukh, A.; Hartnagel, H.; Yilmazoglu, O.; Mimura, H.; Pavlidis, D. Vacuum Nanoelectronic Devices: Novel Electron Sources and Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 315–374. ISBN 9781119037989. [Google Scholar]
- Schottky, W. Small-Shot Effect and Flicker Effect. Phys. Rev. 1926, 28, 74. [Google Scholar] [CrossRef]
- Kleint, C. Experimente zum Funkelrauschen bei Feldemission und Vergleich mit theoretischen Vorstellungen. Ann. Phys. 1963, 10, 309–320. [Google Scholar] [CrossRef]
- Redhead, P.A. The first 50 years of electron stimulated desorption (1918–1968). Vacuum 1997, 48, 585–596. [Google Scholar] [CrossRef]
- Redhead, P.A. UHV and XHV pressure measurements. Vacuum 1993, 44, 559–564. [Google Scholar] [CrossRef]










| Pressure (time) | Ti-CNT#2 (Pa) | Pristine CNTs (Pa) | Emission Current (µA) |
|---|---|---|---|
| pu (t0) | 6.98 × 10−7 | 4.16 × 10−7 | 0 |
| p1 (t1) | 7.73 × 10−7 | 4.51 × 10−7 | 500 |
| p1′ (t1 + 10 min) | 6.96 × 10−7 | 4.55 × 10−7 | 500 |
| p2 (t2) | 6.92 × 10−7 | 4.27 × 10−7 | 200 |
| p2′ (t2 + 10 min) | 6.78 × 10−7 | 4.22 × 10−7 | 200 |
| Δp (p2′ − pu) | −2.00 × 10−8 | 6.00 × 10−9 | 200 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, D.; Wurz, P.; Cheng, Y.; Wang, Y.; Wang, C.; Sun, J.; Li, G.; Fausch, R.G. Residual Gas Adsorption and Desorption in the Field Emission of Titanium-Coated Carbon Nanotubes. Materials 2019, 12, 2937. https://doi.org/10.3390/ma12182937
Zhang H, Li D, Wurz P, Cheng Y, Wang Y, Wang C, Sun J, Li G, Fausch RG. Residual Gas Adsorption and Desorption in the Field Emission of Titanium-Coated Carbon Nanotubes. Materials. 2019; 12(18):2937. https://doi.org/10.3390/ma12182937
Chicago/Turabian StyleZhang, Huzhong, Detian Li, Peter Wurz, Yongjun Cheng, Yongjun Wang, Chengxiang Wang, Jian Sun, Gang Li, and Rico Georgio Fausch. 2019. "Residual Gas Adsorption and Desorption in the Field Emission of Titanium-Coated Carbon Nanotubes" Materials 12, no. 18: 2937. https://doi.org/10.3390/ma12182937





