Effect of Thermomechanical Treatments on the Phases, Microstructure, Microhardness and Young’s Modulus of Ti-25Ta-Zr Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
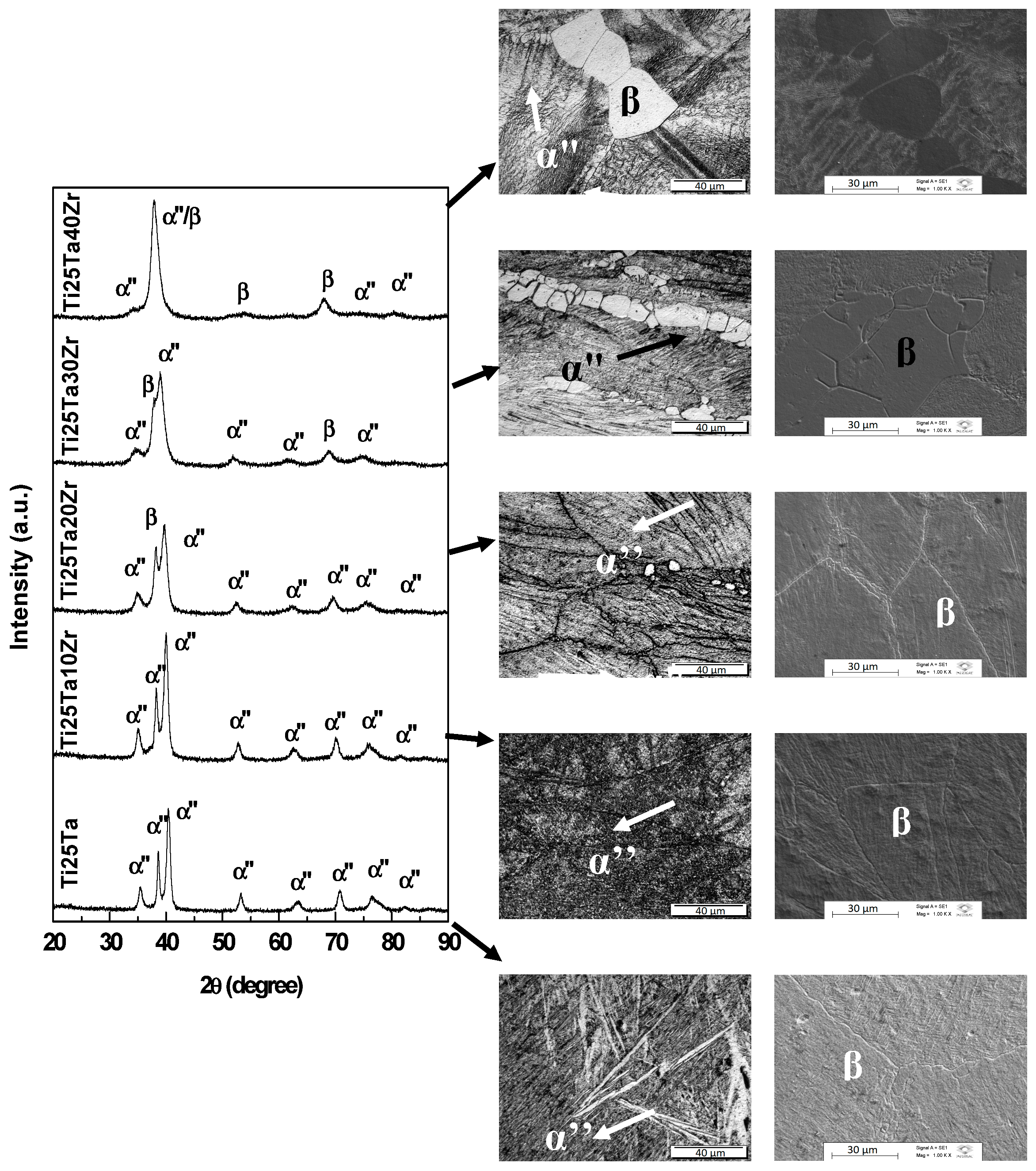
3.1. Phase Identification
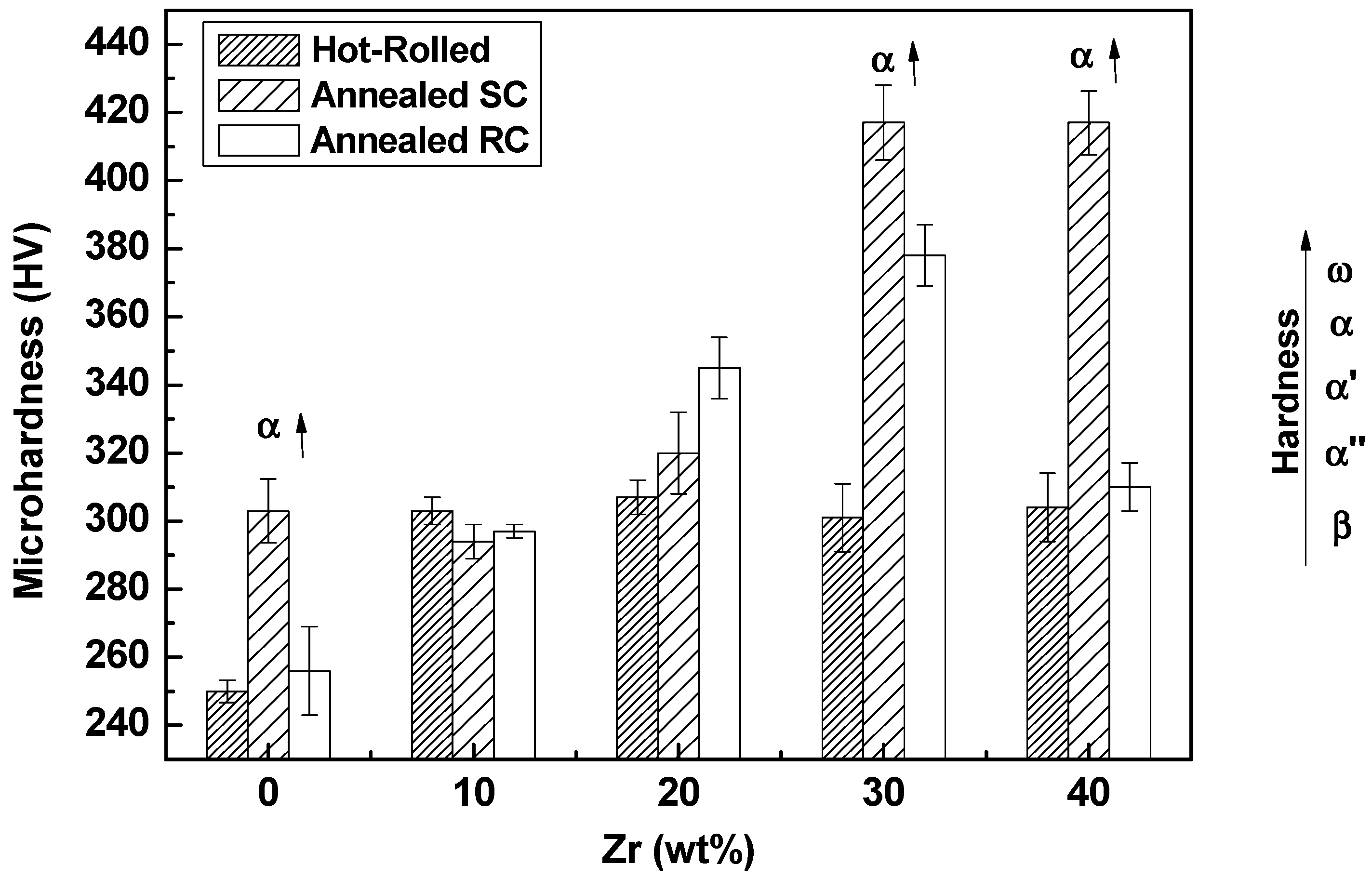
3.2. Mechanical Characterization
4. Discussion
5. Conclusions
- X-ray diffraction and microscopy reveal that the crystalline structure of the alloys was sensitive to the addition of zirconium and sensitive to thermomechanical treatments.
- The zirconium acted as a β-stabilizing element combined with tantalum in Ti-25Ta-xZr alloys.
- Alloys subjected to a slow-cooling annealing heat treatment have the α phase in the crystalline structure.
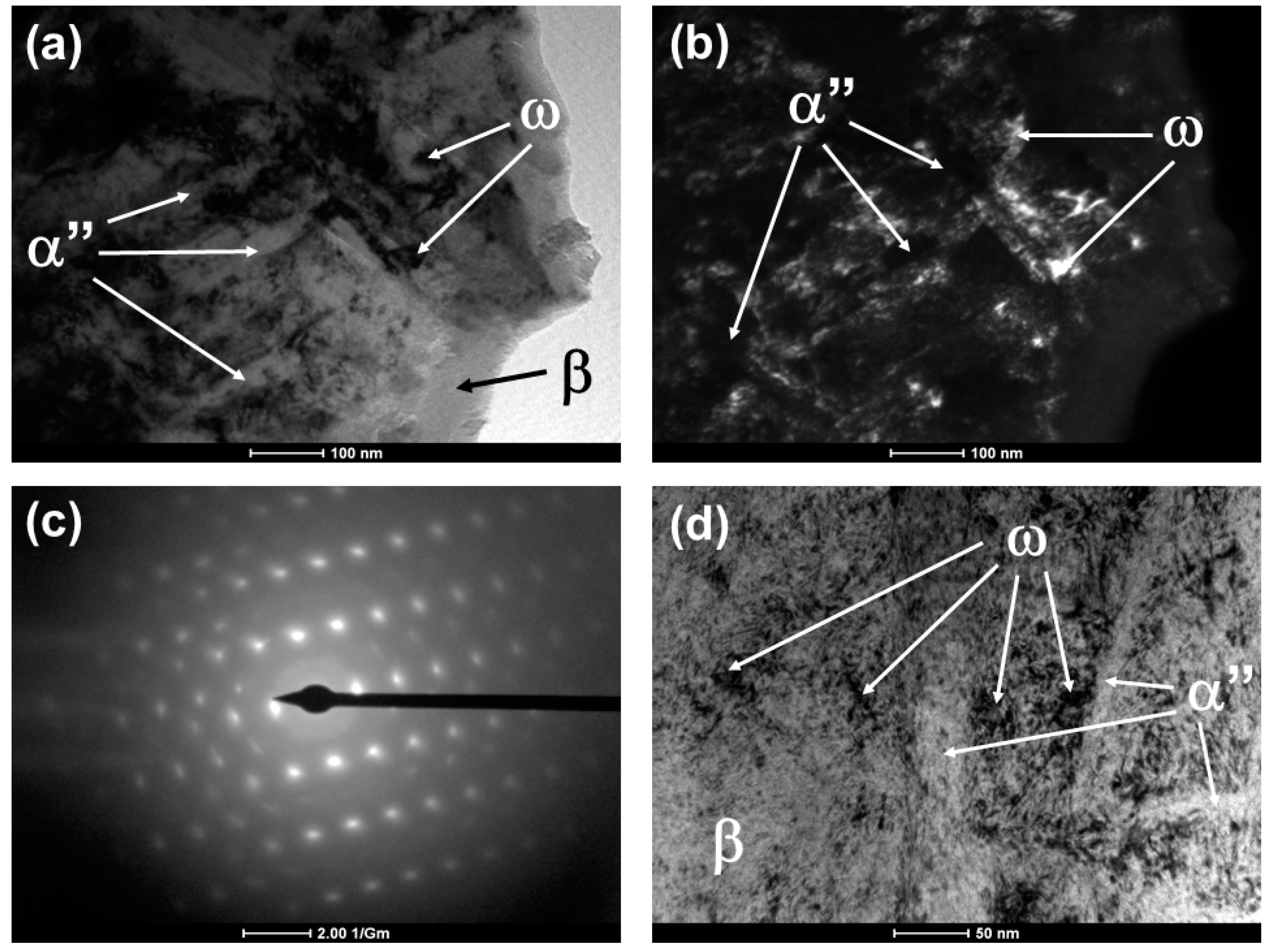
- The alloys subjected to a fast-cooling annealing heat treatment have the α” phase, can retain the β phase with high zirconium content and rapid cooling precipitates the omega phase, but high levels of zirconium suppress this phase.
- Hardness increased due to solid solution hardening and due to ω phase and α-phase precipitation in alloys subjected to slow-cooling annealing heat treatment.
- The Young’s modulus decreases with high levels of zirconium due to the stabilization of the β phase.
- The thermomechanical process and heat treatment with rapid cooling retain the β phase, improving mechanical biocompatibility with the decreasing of the elastic modulus values of the materials down to E = 60 GPa for Ti-25Ta-40Zr alloy.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meyers, M.A.; Chen, P.Y.; Lin, A.Y.M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef] [Green Version]
- Noumbissi, S.; Scarano, A.; Gupta, S. A Literature Review Study on Atomic Ions Dissolution of Titanium and Its Alloys in Implant Dentistry. Materials 2019, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where We Have Been and Where We Are Going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Biologically and Mechanically Biocompatible Titanium Alloys. Mater. Trans. 2008, 49, 2170–2178. [Google Scholar] [CrossRef] [Green Version]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T. Effects of Ta content on Young’s modulus and tensile properties of binary Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. A 2004, 371, 283–290. [Google Scholar] [CrossRef]
- Lee, Y.T.; Welsch, G. Young’s modulus and damping of Ti 6Al 4V alloy as a function of heat treatment and oxygen concentration. Mater. Sci. Eng. A 1990, 128, 77–89. [Google Scholar] [CrossRef]
- Froes, F.H. Titanium Alloys: Thermal Treatment and Thermomechanical Processing. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Buschow, K.H.J., Flemings, M.C., Kramer, J.E., Veyssiere, P., Cahn, W.R., Ilschner, B., Mahajan, S., Eds.; Elsevier: Oxford, UK, 2001; pp. 9369–9373. [Google Scholar]
- Weiss, I.; Semiatin, S.L. Thermomechanical processing of beta titanium alloys—An overview. Mater. Sci. Eng. A 1998, 243, 46–65. [Google Scholar] [CrossRef]
- Lütjering, G.; Williams, J.C.; Gysler, A. Microstructure and Mechanical Properties of Titanium Alloys. In Microstructure and Properties of Materials; World Scientific: Washington, DC, USA, 1998; pp. 1–77. [Google Scholar]
- Oldani, C.; Dominguez, A. Titanium as a Biomaterial for Implants. In Recent Advances in Arthroplasty; Fokter, S.K., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 149–162. [Google Scholar]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Kolli, R.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. BioMed Res. Int. 2016, 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of Ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Poh, C.K. Titanium Alloys in Orthopaedics, in Titanium Alloys—Advances in Properties Control; InTech: Rijeka, Croatia, 2013; pp. 1–20. [Google Scholar]
- Zhou, Y.-L.; Niinomi, M. Ti–25Ta Alloy with the Best Mechanical Compatibility in Ti–Ta Alloys for Biomedical Applications. Mater. Sci. Eng. C 2009, 29, 1061–1065. [Google Scholar] [CrossRef]
- Wang, K. The use of titanium for medical applications in the USA. Mater. Sci. Eng. A 1996, 213, 134–137. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Kuroda, P.A.B.; Grandini, C.R. Structure, Microstructure, and Selected Mechanical Properties of Ti-Zr-Mo Alloys for Biomedical Applications. Adv. Mater. Res. 2014, 922, 75–80. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Vicente, F.B.; Araújo, R.O.; Lourenço, M.L.; Kuroda, P.A.B.; Buzalaf, M.A.R.; Grandini, C.R. Effect of the substitutional elements on the microstructure of the Ti-15Mo-Zr and Ti-15Zr-Mo systems alloys. J. Mater. Res. Technol. 2015, 4, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Araújo, R.O.d.; Buzalaf, M.A.R.; Grandini, C.R. Preparation and Characterization of Ti-10Mo-xZr Alloys for Biomedical Applications. Mater. Sci. Forum 2016, 869, 940–945. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Buzalaf, M.A.R.; Grandini, C.R. Effect of molybdenum on structure, microstructure and mechanical properties of biomedical Ti-20Zr-Mo alloys. Mater. Sci. Eng. C 2016, 67, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, D.R.N.; Kuroda, P.A.B.; Lourenço, M.L.; Fernandes, C.J.C.; Buzalaf, M.A.R.; Zambuzzi, W.F.; Grandini, C.R. Development of Ti-15Zr-Mo alloys for applying as implantable biomedical devices. J. Alloys Compd. 2018, 749, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Correa, D.R.N.; Kuroda, P.A.B.; Lourenço, M.L.; Buzalaf, M.A.R.; Mendoza, M.E.; Archanjo, B.S.; Grandini, C.R. Microstructure and selected mechanical properties of aged Ti-15Zr-based alloys for biomedical applications. Mater. Sci. Eng. C 2018, 91, 762–771. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Hu, B.; Zhao, L.; Liu, S. Titanium alloy production technology, market prospects and industry development. Mater. Des. 2011, 32, 1684–1691. [Google Scholar] [CrossRef]
- Yan, L.; Yuan, Y.; Ouyang, L.; Li, H.; Mirzasadeghi, A.; Li, L. Improved mechanical properties of the new Ti-15Ta-xZr alloys fabricated by selective laser melting for biomedical application. J. Alloys Compd. 2016, 688, 156–162. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Ping, D.; Li, Y.; Lin, J.; Munir, K.S.; Yamabe-Mitarai, Y.; Wen, C. Extraordinary high strength Ti-Zr-Ta alloys through nanoscaled, dual-cubic spinodal reinforcement. Acta Biomater. 2017, 53, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Quadros, F.D.F.; Kuroda, P.A.B.; Sousa, K.D.S.J.; Donato, T.A.G.; Grandini, C.R. Preparation, structural and microstructural characterization of Ti-25Ta-10Zr alloy for biomedical applications. J.Mater. Res. Technol. 2019, 8, 4108–4114. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Buzalaf, M.A.R.; Grandini, C.R. Preparation, microstructural characterization, and selected mechanical properties of Ti-20Zr-2.5Mo and Ti-20Zr-7.5Mo used as biomaterial. Mater. Sci. Forum 2016, 869, 946–951. [Google Scholar] [CrossRef]
- Martins, J.R.S., Jr.; Araújo, R.O.; Donato, T.D.; Arana-Chavez, V.; Buzalaf, M.R.; Grandini, C.R. Influence of Oxygen Content and Microstructure on the Mechanical Properties and Biocompatibility of Ti–15 wt%Mo Alloy Used for Biomedical Applications. Materials 2014, 7, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, P.A.B.; Lourenço, M.L.; Correa, D.R.N.; Grandini, C.R. Thermomechanical treatments influence on the phase composition, microstructure, and selected mechanical properties of Ti–20Zr–Mo alloys system for biomedical applications. J. Alloys Compd. 2019, 812, 152108. [Google Scholar] [CrossRef]
- Luz, A.R.; Santos, L.S.; Lepienski, C.M.; Kuroda, P.B.; Kuromoto, N.K. Characterization of the morphology, structure and wettability of phase dependent lamellar and nanotube oxides on anodized Ti-10Nb alloy. Appl. Surf. Sci. 2018, 448, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Collings, E.W. The Physical Metallurgy of Titanium Alloys; ASM International: Cleveland, OH, USA, 1989. [Google Scholar]
- ASTM. E92-82—Standard Test Method for Vickers Hardness of Metallic Materials; ASTM International: Conshohocken, PA, USA, 2003. [Google Scholar]
- ASTM. E1876–01—Standard Test Method for Dynamic Young’s Modulus, Shear Modulus, and Poisson’s Ratio by Impulse Excitation of Vibration; ASTM International: Conshohocken, PA USA, 2002. [Google Scholar]
- Gray, G.T.; Morris, C.E.; Lawson, A.C. Omega Phase Formation in Titanium and Titanium Alloys; Los Alamos National Laboratory: Los Alamos, NM, USA, 1992; pp. 1–9. [Google Scholar]
- Tane, M.; Nakano, T.; Kuramoto, S.; Niinomi, M.; Takesue, N.; Nakajima, H. ω Transformation in cold-worked Ti–Nb–Ta–Zr–O alloys with low body-centered cubic phase stability and its correlation with their elastic properties. Acta Mater. 2013, 61, 139–150. [Google Scholar] [CrossRef]
- Davis, R.; Flower, H.M.; West, D.R.F. The decomposition of Ti-Mo alloy martensites by nucleation and growth and spinodal mechanisms. Acta Metall. 1979, 27, 1041–1052. [Google Scholar] [CrossRef]
- Callister, W.D., Jr. Fundamentos da Ciência e Engenharia de Materiais, 2nd ed.; LTC: Rio de Janeiro, Brazil, 2006. [Google Scholar]
- Ho, W.F.; Ju, C.P.; Chern Lin, J.H. Structure and properties of cast binary Ti–Mo alloys. Biomaterials 1999, 20, 2115–2122. [Google Scholar] [CrossRef]
- Delvat, E.; Gordin, D.M.; Gloriant, T.; Duval, J.L.; Nagel, M.D. Microstructure, mechanical properties and cytocompatibility of stable beta Ti-Mo-Ta sintered alloys. J. Mech. Behav. Biomed. Mater. 2008, 1, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.L.; Tu, G.C.; Huang, C.A.; Liu, T.T. A study on the hardness variation of α- and β-pure titanium with different grain sizes. Mater. Sci. Eng. A 2005, 398, 93–98. [Google Scholar] [CrossRef]
- Sukedai, E.; Yukihiro, T.; Miyaji, D.; Matsumoto, H.; Nishizawa, H.; Hashimoto, H. Aging behavior of Ti–Mo alloys heavily compressed in ultra-high strain rate mode. Mater. Sci. Eng. A 2004, 387 (Suppl. C), 249–253. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; Wiley-VCH: New York, NY, USA, 2005. [Google Scholar]
- Zhou, Y.-L.; Niinomi, M. Microstructures and mechanical properties of Ti–50 mass% Ta alloy for biomedical applications. J. Alloys Compd. 2008, 466, 535–542. [Google Scholar] [CrossRef]
- Gabriel, S.B.; de Almeida, L.H.; Nunes, C.A.; Dille, J.; Soares, G.A. Maximisation of the ratio of microhardness to the Young’s modulus of Ti–12Mo–13Nb alloy through microstructure changes. Mater. Sci. Eng. C 2013, 33, 3319–3324. [Google Scholar] [CrossRef]
- Lee, C.M.; Ju, C.P.; Lin, J.H.C. Structure–property relationship of cast Ti–Nb alloys. J. Oral Rehabil. 2002, 29, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M.; Hendrickson, M.; Nandwana, P.; Alam, T.; Choudhuri, D.; Banerjee, R. Influence of oxygen on omega phase stability in the Ti-29Nb-13Ta-4.6Zr alloy. Scr. Mater. 2016, 123, 144–148. [Google Scholar] [CrossRef]
- Homma, T.; Arafah, A.; Haley, D.; Nakai, M.; Niinomi, M.; Moody, M.P. Effect of alloying elements on microstructural evolution in oxygen content controlled Ti-29Nb-13Ta-4.6Zr (wt%) alloys for biomedical applications during aging. Mater. Sci. Eng. A 2018, 709, 312–321. [Google Scholar] [CrossRef]
- Aleixo, G.T.; Lopes, E.S.; Contieri, R.; Cremasco, A.; Afonso, C.R.M.; Caram, R. Effects of Cooling Rate and Sn Addition on the Microstructure of Ti-Nb-Sn Alloys. Solid State Phenom. 2011, 172, 190–195. [Google Scholar] [CrossRef]
- Afonso, C.R.M.; Chaves, J.M.; Florêncio, O. Effect of Rapid Solidification on Microstructure and Elastic Modulus of β Ti–xNb–3Fe Alloys for Implant Applications. Adv. Eng. Mater. 2017, 19, 1600370. [Google Scholar] [CrossRef]
- Wu, D.; Isaksson, P.; Ferguson, S.J.; Persson, C. Young’s modulus of trabecular bone at the tissue level: A review. Acta Biomater. 2018, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuroda, P.A.B.; Quadros, F.d.F.; Araújo, R.O.d.; Afonso, C.R.M.; Grandini, C.R. Effect of Thermomechanical Treatments on the Phases, Microstructure, Microhardness and Young’s Modulus of Ti-25Ta-Zr Alloys. Materials 2019, 12, 3210. https://doi.org/10.3390/ma12193210
Kuroda PAB, Quadros FdF, Araújo ROd, Afonso CRM, Grandini CR. Effect of Thermomechanical Treatments on the Phases, Microstructure, Microhardness and Young’s Modulus of Ti-25Ta-Zr Alloys. Materials. 2019; 12(19):3210. https://doi.org/10.3390/ma12193210
Chicago/Turabian StyleKuroda, Pedro Akira Bazaglia, Fernanda de Freitas Quadros, Raul Oliveira de Araújo, Conrado Ramos Moreira Afonso, and Carlos Roberto Grandini. 2019. "Effect of Thermomechanical Treatments on the Phases, Microstructure, Microhardness and Young’s Modulus of Ti-25Ta-Zr Alloys" Materials 12, no. 19: 3210. https://doi.org/10.3390/ma12193210




