Structure and Properties of Single-Layer MoS2 for Nano-Photoelectric Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of MoS2 Film
2.1.1. Treatment of the Sapphire Substrate
2.1.2. Growth of MoS2 by Vapor Deposition
2.2. Characterization and Test Methods
3. Results and Discussion
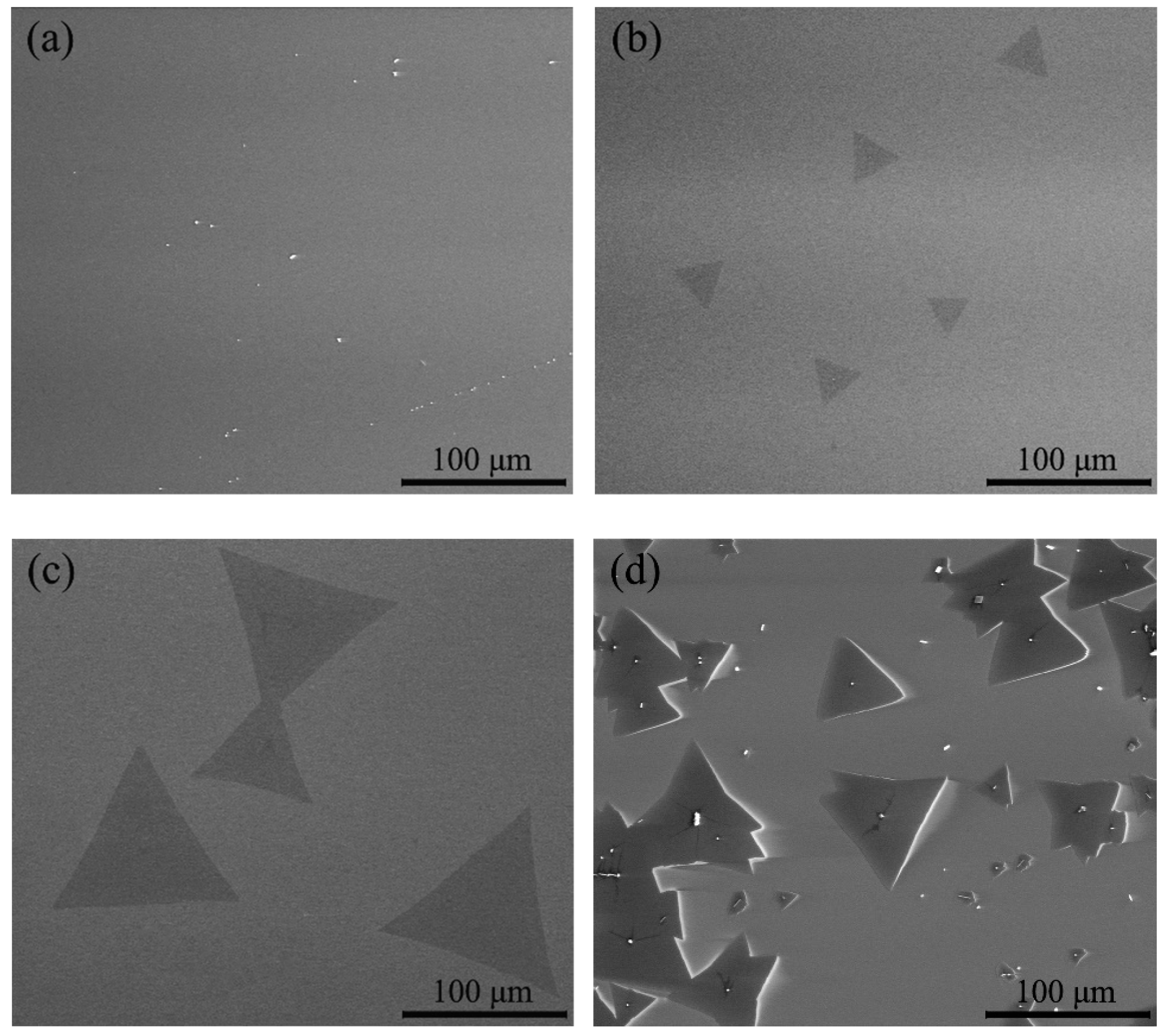
3.1. Effect of Heating Method on the Morphology and Layer Number of MoS2 Crystals
3.2. Effect of Temperature on the Morphology and Layer Number of MoS2 Crystals
3.3. Characterization of the MoS2 Structure
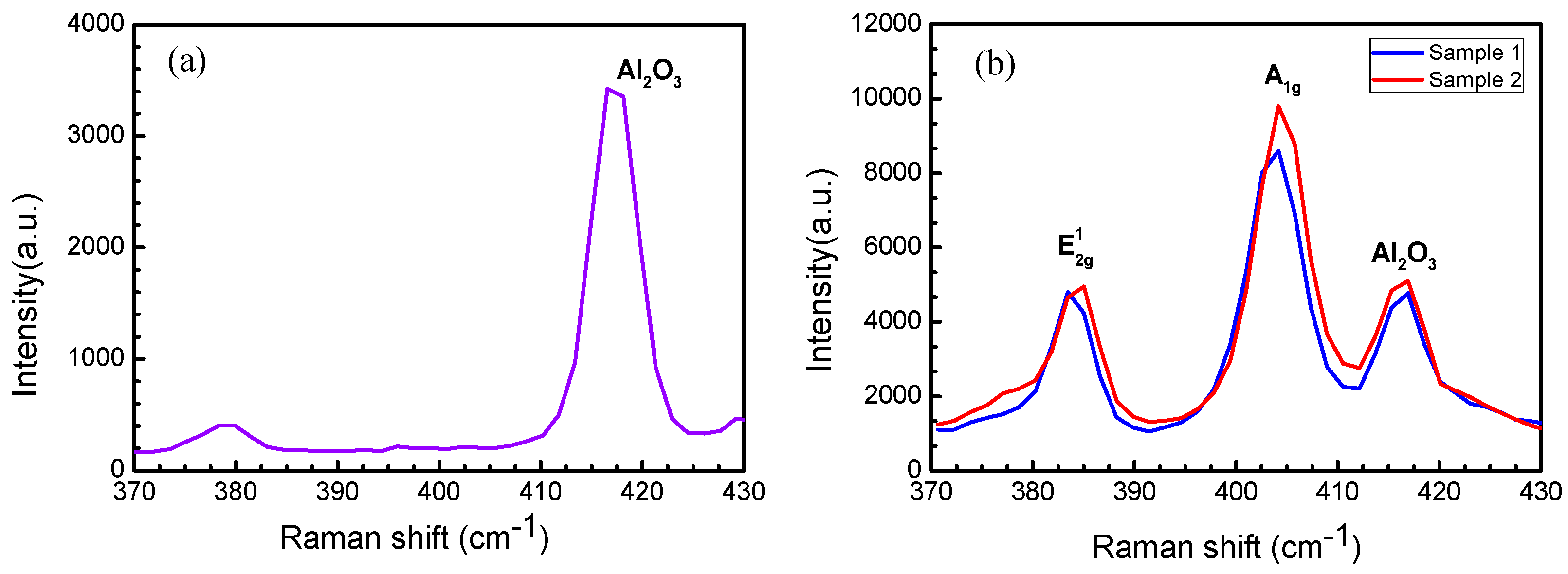
3.3.1. Characterization by Raman Spectroscopy
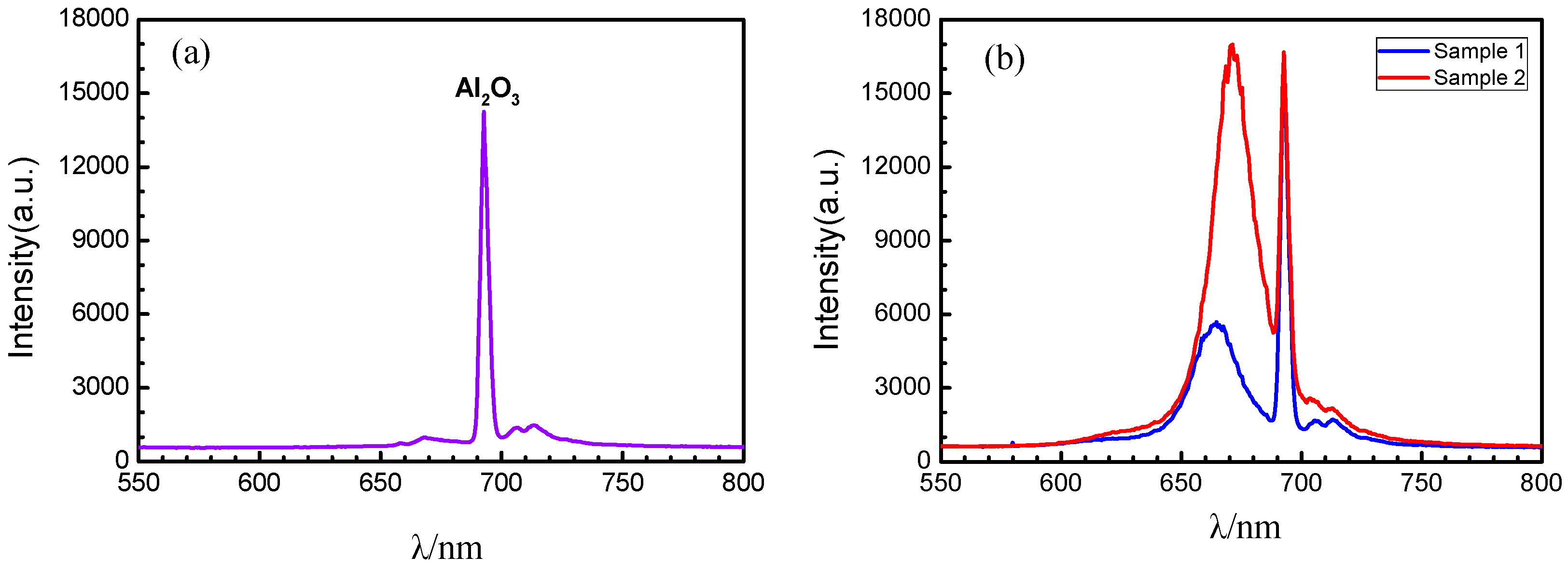
3.3.2. Characterization of Photoluminescence Spectra
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Discover of Graphene: Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Jiang, Z.M.; Zhang, Y.; Morozov, S.V.; Stormer, H.L.; Zeitler, U.; Maan, J.C.; Boebinger, G.S.; Kim, P.C.; Geim, A.K. Room Temperature Quantum Hall Effect in Graphene. Science 2007, 315, 1379. [Google Scholar] [CrossRef] [PubMed]
- Mayorov, A.S.; Gorbachev, R.V.; Morozov, S.V.; Britnell, L.; Jalil, R.; Ponomarenko, L.A.; Blake, P.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Micrometer-scale Ballistic Transport in Encapsulated Graphene at Room Temperature. Nano Lett. 2011, 11, 2396–2399. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.B.; Tian, C.S.; Girit, C.; Zettl, A.; Crommie, M.; Shen, Y.R. Gate-variable optical transitions in Grapheme. Science 2008, 320, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Huang, R. Nonlinear Mechanics of Single-atomic-layer Graphene Sheets. Int. J. Appl. Mech. 2009, 1, 443–467. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct Gap Semiconductor. J. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging Photoluminescence in Monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Yoon, Y.; Ganapathi, K.; Salahuddin, S. How Good Can Monolayer MoS2 Transistors Be? Nano Lett. 2011, 11, 3768–3773. [Google Scholar] [CrossRef]
- Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar]
- De, S.; Kunal, K.; Aluru, N.R. Nonlinear Intrinsic Dissipation in Single Layer MoS2 Resonators. Rsc Adv. 2017, 7, 6403–6410. [Google Scholar] [CrossRef]
- Tang, H.J.; Wang, J.G.; Yin, H.J.; Zhao, H.J.; Wang, D.; Tang, Z.Y. Growth of Polypyrrole Ultrathin Films on MoS2 Monolayers as High-Performance Supercapacitor Electrodes. Adv. Mater. 2015, 27, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.Y.; Li, H.; Li, H.; Jiang, L.; Shi, Y.M.; Sun, Y.H.; Lu, G.; Zhang, Q.; Chen, X.D.; Zhang, H. Single-layer MoS2 Phototransistors. Acs Nano 2012, 6, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sanchez, O.; Lembke, D.; Kayci, M.; Radenovic, A.; Kis, A. Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nanotechnol. 2013, 8, 497. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Yin, Z.Y.; Li, H.; Liu, J.Q.; Cao, X.H.; Zhang, Q.; Zhang, H. Layer Thinning and Etching of Mechanically Exfoliated MoS2 Nanosheets by Thermal Annealing in Air. Small 2013, 9, 3314–3319. [Google Scholar]
- Zeng, Z.Y.; Sun, T.; Zhu, J.X.; Huang, X.; Yin, Z.Y.; Lu, G.; Fan, Z.X.; Yan, Q.Y.; Hng, H.H.; Zhang, H. An Effective Method for the Fabrication of Few-layer-thick Inorganic Nanosheets. Angew. Chem. Int. Ed. Engl. 2012, 51, 9052–9056. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.S.; Yang, Z.; Zhang, C.; Liu, Z.Y.; Weng, W.T.; Phang, I.Y.; Zhang, Z.; Pan, J.S.; Liu, T.X. Exfoliated MoS2 Nanosheets as Efficient Catalysts for Electrochemical Hydrogen Evolution. Electrochim. Acta 2013, 109, 269–275. [Google Scholar] [CrossRef]
- Lee, Y.H.; Zhang, X.Q.; Zhang, W.; Chang, M.T.; Lin, C.T. Synthesis of large-area MoS2 atomic layers with chemical vapor deposition. Adv. Mater. 2012, 24, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.J.; Liu, Z.; Najmaei, S.; Ajayan, P.M.; Lou, J. Large-area Vapor-phase Growth and Characterization of MoS(2) Atomic Layers on a SiO(2) Substrate. Small 2012, 8, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Birdwell, A.G.; Amani, M.; Burke, R.A.; Ling, X.; Lee, Y.H.; Liang, X.L.; Peng, L.M.; Richter, C.A.; Kong, J.; et al. Broadband Optical Properties of Large-area Monolayer CVD Molybdenumdisulfide. Phys. Rev. B 2014, 90, 195434. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, J.K.; Chen, C.H.; Chang, Y.H.; Cheng, Y.J.; Li, L.J. High-Gain Phototransistors Based on a CVD MoS2 Monolayer. Adv. Mater. 2013, 25, 3456–3461. [Google Scholar] [CrossRef]
- Lee, J.; Wang, Z.; He, K.; Shan, J.; Feng, P.X.L. High frequency MoS2 nanomechanical resonators. Acs Nano 2013, 7, 6086–6091. [Google Scholar] [CrossRef]
- Verble, J.L.; Wieting, T.J. Lattice Mode Degeneracy in MoS2 and Other Layer Compounds. Phys. Rev. Lett. 1970, 25, 362–365. [Google Scholar] [CrossRef]
- Verble, J.L.; Wieting, T.J.; Reed, P.R. Rigid-layer lattice vibrations and van der waals bonding in hexagonal MoS2. Solid State Commun. 1972, 11, 941–944. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single- and few-layer MoS2. Acs Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; He, K.L.; Shan, J.; Heinz, T.F. Control of valley polarization in monolayer MoS2 by optical helicity. Nat. Nanotechnol. 2012, 7, 494–498. [Google Scholar] [CrossRef] [PubMed]

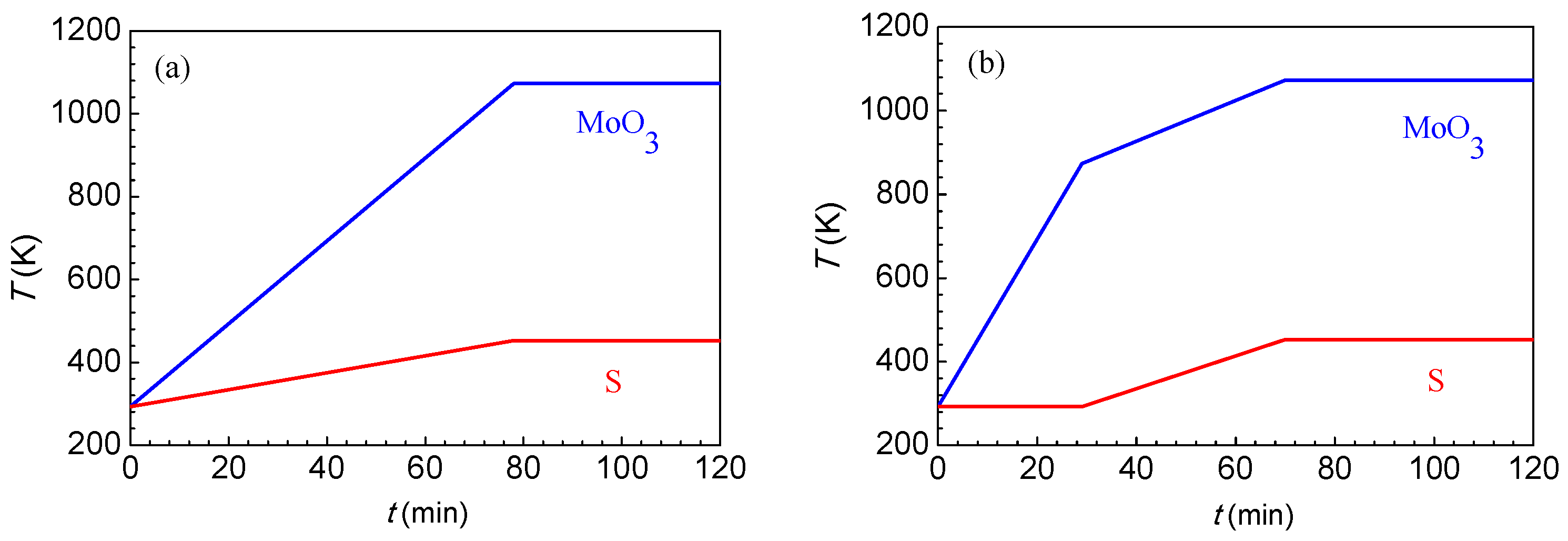



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, J.; Chang, H.; Xu, T. Structure and Properties of Single-Layer MoS2 for Nano-Photoelectric Devices. Materials 2019, 12, 198. https://doi.org/10.3390/ma12020198
Jian J, Chang H, Xu T. Structure and Properties of Single-Layer MoS2 for Nano-Photoelectric Devices. Materials. 2019; 12(2):198. https://doi.org/10.3390/ma12020198
Chicago/Turabian StyleJian, Jiaying, Honglong Chang, and Tao Xu. 2019. "Structure and Properties of Single-Layer MoS2 for Nano-Photoelectric Devices" Materials 12, no. 2: 198. https://doi.org/10.3390/ma12020198
APA StyleJian, J., Chang, H., & Xu, T. (2019). Structure and Properties of Single-Layer MoS2 for Nano-Photoelectric Devices. Materials, 12(2), 198. https://doi.org/10.3390/ma12020198



