The Reaction Thermodynamics during Plating Al on Graphene Process
Abstract
:1. Introduction
2. Experiment and Simulation
2.1. Experiment
2.2. Computation Details
3. Results and Discussions
3.1. Preparation and Reaction Mechanism of Al-Coated Graphene
3.2. Reaction Thermodynamics during Plating Al on Graphene Process
3.2.1. Structure Optimization and Thermodynamic Properties of C2H5Br
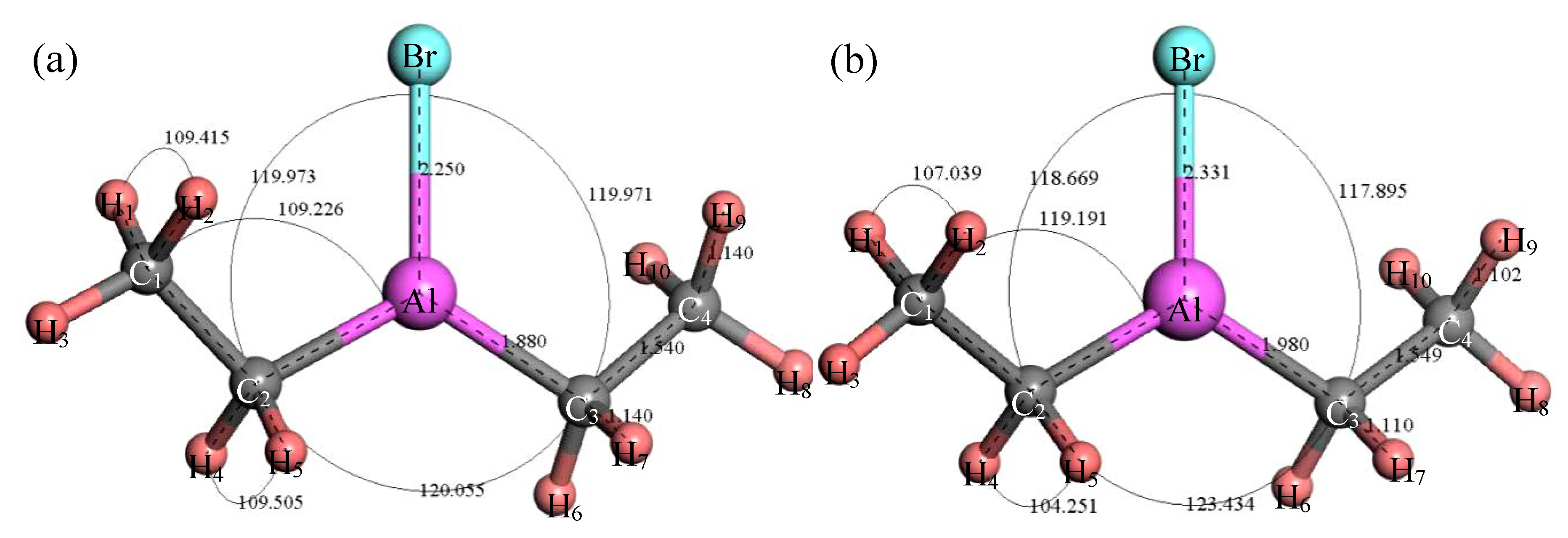
3.2.2. Structure Optimization and Thermodynamic Properties of (C2H5)2AlBr
3.2.3. Structure Optimization and Thermodynamic Properties of C2H5AlBr2
3.2.4. Structure Optimization and Thermodynamic Properties of (C2H5)3Al
3.2.5. Structure Optimization and Thermodynamic Properties of AlBr3
3.2.6. Structure Optimization and Thermodynamic Properties of C2H4
3.2.7. Structure Optimization and Thermodynamic Properties of H2
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boostani, A.F.; Yazdani, S.; Khosroshahi, R.A.; Jiang, Z.Y.; Wei, D. A novel graphene-stimulated semi-solid processing to fabricate advanced aluminium matrix nanocomposites. Mater. Sci. Eng. A 2018, 736, 316–322. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, F.; Xu, J.L.; Nian, Q.; Lin, D.; Chen, C.J.; Zhu, X.; Chen, Y.; Zhang, M. 3D printing graphene-aluminum nanocomposites. J. Alloys Compd. 2018, 746, 269–276. [Google Scholar] [CrossRef]
- Wen, Z.Q.; Zhao, Y.H.; Li, H.J.; Zhang, Y.M.; Wang, S.; Hou, H. Theoretical Calculations of the Ideal Strength of Ni, NiAl and Ni3Al in Tension and Shear. Sci. Adv. Mater. 2018, 10, 1420–1426. [Google Scholar] [CrossRef]
- Jia, J.G.; Liu, D.Q.; Gao, C.Q.; Ji, G.S.; Guo, T.M. Preparation and mechanical properties of short carbon fibers reinforced alpha-Al2O3-based composites. Ceram. Int. 2018, 44, 19345–19351. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhu, X.J.; Gao, J.H.; Zheng, Z.Z.; Wang, H.J. Milling Research and Tool Selection Design of SiC14Cu4Mg0.5Si based on Aluminium Matrix 2A14. J. Wuhan Univ. Technol. 2018, 31, 1377–1380. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, H.T.; Liu, H.; Wang, X.J.; Ma, Y.; Wang, N.; Umar, A.; Guo, Z.H. Determining Interfacial Shear Bond Strength in Thin Laminated Metal Composites. Sci. Adv. Mater. 2018, 10, 1543–1551. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Deng, S.J.; Liu, H.; Zhang, J.X.; Guo, Z.H.; Hou, H. First-principle investigation of pressure and temperature influence on structural, mechanical and thermodynamic properties of Ti(3)AC(2) (A = Al and Si). Comput. Mater. Sci. 2018, 154, 365–370. [Google Scholar] [CrossRef]
- Chen, G.; Chen, W.; Zhang, G.W.; Zheng, S.Q.; Zhang, Z.M. Microstructures and Mechanical Properties of Al-12Zn2.4Mg-1.2Cu Alloy under Different Deformation Ways. Rare Met. Mater. Eng. 2018, 45, 2237–2241. [Google Scholar]
- Gao, X.; Yue, H.Y.; Guo, E.J.; Zhang, H.; Lin, X.Y.; Yao, L.H.; Wang, B. Preparation and tensile properties of homogeneously dispersed graphene reinforced aluminum matrix composites. Mater. Des. 2016, 94, 54–60. [Google Scholar] [CrossRef]
- Zhao, M.; Xiong, D.B.; Tan, Z.Q.; Fan, G.L.; Guo, Q.; Guo, C.P.; Li, Z.Q.; Zhang, D. Lateral size effect of graphene on mechanical properties of aluminum matrix nanolaminated composites. Scr. Mater. 2017, 139, 44–48. [Google Scholar] [CrossRef]
- Bagheri, P.; Farivar, M.; Simchi, A. Graphene-mediated self-assembly of gold nanorods into long fibers with controllable optical properties. Mater. Lett. 2018, 224, 13–17. [Google Scholar] [CrossRef]
- Tsai, P.C.; Jeng, Y.R. Coalescence and epitaxial self-assembly of Cu nanoparticles on graphene surface: A molecular dynamics study. Comput. Mater. Sci. 2019, 156, 104–110. [Google Scholar] [CrossRef]
- Muszynski, R.; Seger, B.; Kamat, P.V. Decorating Graphene Sheets with Gold Nanoparticles. J. Phys. Chem. C 2008, 112, 5263–5266. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Tang, J.C.; Yu, F.X.; Ye, N. Preparation of graphene nanoplatelets reinforcing copper matrix composites by electrochemical deposition. J. Alloys Compd. 2018, 766, 266–273. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Yeom, M.S.; Shin, J.W.; Kim, H.; Cui, Y.; Kysar, J.W.; Hone, J.; Jung, Y.; Jeon, S.; Han, S.M. Strengthening effect of single-atomic-layer graphene in metal-graphene nanolayered composites. Nat. Commun. 2013, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, L.; Xu, M.T.; Xia, T.; Ruan, X.W.; Song, S.; Ma, H.Z. Preparation and mechanical property of electrodeposited Al-graphene composite coating. Mater. Des. 2016, 111, 522–527. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Li, L.; Bai, P.K.; Jin, Y.; Wu, L.Y.; Li, J.; Guan, R.G.; Qu, H.Q. The Heat Treatment Influence on the Microstructure and Hardness of TC4 Titanium Alloy Manufactured via Selective Laser Melting. Materials 2018, 11, 1318. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Li, L.; Tan, L.; Bai, P.K.; Li, J.; Wu, L.Y.; Liao, H.H.; Cheng, Y.H. Simulation of Stress Field during the Selective Laser Melting Process of the Nickel-Based Superalloy, GH4169. Materials 2018, 11, 1525. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Bai, P.K.; Guan, R.G.; Murugadoss, V.; Liu, H.; Wang, X.J.; Guo, Z. Microstructural evolution and mechanical strengthening mechanism of Mg-3Sn-1Mn-1La alloy after heat treatments. Mater. Sci. Eng. A 2018, 734, 200–209. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Guan, R.G.; Zhang, J.H.; Zhao, Z.Y.; Bai, P.K. Effects of process parameters of semisolid stirring on microstructure of Mg-3Sn-1Mn-3SiC (wt%) strip processed by rheo-rolling. Acta Metall. Sin. 2017, 30, 66–72. [Google Scholar] [CrossRef]
- Cheng, P.; Zhao, Y.H.; Lu, R.P.; Hou, H. Effect of the morphology of long-period stacking ordered phase on mechanical properties and corrosion behavior of cast Mg-Zn-Y-Ti alloy. J. Alloys Compd. 2018, 764, 226–238. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Qu, S.J.; Feng, A.H.; Shen, J. Achieving grain refinement and enhanced mechanical properties in Ti-6Al-4V alloy produced by multidirectional isothermal forging. Mater. Sci. Eng. A 2017, 692, 127–138. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Qu, S.J.; Feng, A.H.; Shen, J.; Chen, D.L. Hot deformation behavior of Ti-6Al-4V alloy: Effect of initial microstructure. J. Alloys Compd. 2017, 718, 170–181. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Qu, S.J.; Feng, A.H.; Hu, X.; Shen, J. Microstructural mechanisms during multidirectional isothermal forging of as-cast Ti-6Al-4V alloy with an initial lamellar microstructure. J. Alloys Compd. 2019, 773, 277–287. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.Y.; Bai, P.K.; Qu, H.Q.; Liu, B.; Li, L.; Wu, L.Y.; Guan, R.G.; Liu, H.; Guo, Z.H. Microstructural evolution and mechanical properties of IN718 alloy fabricated by selective laser melting following different heat treatments. J. Alloys Compd. 2019, 772, 861–870. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Misra, R.D.K.; Bai, P.K.; Gao, J.F.; Li, Y.J.; Guan, R.G.; Guo, Z.H.; Liu, H. Novel process of coating Al on graphene involving organic aluminum accompanying microstructure evolution. Mater. Lett. 2018, 232, 202–205. [Google Scholar] [CrossRef]
- Lisovenko, A.S.; Morokuma, K.; Timoshkin, A.Y. Initial Gas Phase Reactions between Al(CH3)(3)/AIH(3) and Ammonia: Theoretical Study. J. Phys. Chem. A 2015, 119, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Wen, Z.Q.; Zhao, Y.H.; Fu, L.; Wang, N.; Han, P.D. First-principles investigations on structural, elastic, thermodynamic and electronic properties of Ni3X (X = Al, Ga and Ge) under pressure. Intermetallics 2014, 44, 110–115. [Google Scholar] [CrossRef]
- Yang, X.M.; Hou, H.; Zhao, Y.H.; Yang, L.; Han, P.D. First-principles investigation of the structural, electronic and elastic properties of MgxAl4−xSr (X = 0, 0.5, 1) phases. Comp. Mater. Sci. 2014, 84, 374–380. [Google Scholar] [CrossRef]
- dos Santos, R.B.; Rivelino, R.; de Brito Mota, F.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Dopant species with Al–Si and N–Si bonding in the MOCVD of AlN implementing trimethylaluminum, ammonia and silane. J. Phys. D Appl. Phys. 2015, 48, 295104. [Google Scholar] [CrossRef]
- Freitas, R.R.Q.; Gueorguiev, G.K.; de Brito Mota, F.; de Castilho, C.M.C.; Stafstrom, S.; Kakanakova-Georgieva, A. Reactivity of adducts relevant to the deposition of hexagonal BN from first-principles calculations. Chem. Phys. Lett. 2013, 583, 119–124. [Google Scholar] [CrossRef]
- Inagaki, Y.; Kozawa, T. Chemical Reaction Pathways for MOVPE Growth of Aluminum Nitride. ECS J. Solid State Sci. Technol. 2016, 5, 73–75. [Google Scholar] [CrossRef]
- Sangiovanni, D.G.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Ab initio molecular dynamics of atomic-scale surface reactions: Insights into metal organic chemical vapor deposition of AlN on grapheme. Phys. Chem. Chem. Phys. 2018, 20, 17751–17761. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, T.; Samanta, B.; Ansari, S.A.; Pal, S. Theoretical study of C–X [X = Cl, Br] bond activation on aluminum nanoclusters. Theor. Chem. Acc. 2016, 135, 234. [Google Scholar] [CrossRef]














| Substance | E (1Har/at) | H (kcal/mol) | G (kcal/mol) |
|---|---|---|---|
| C2H5Br | −423.7794203 | 43.533 | 23.127 |
| Al | −727.1831592 | 4.348 | −16.613 |
| (C2H5)2AlBr | −745.5311183 | 85.548 | 56.435 |
| C2H5AlBr2 | −1010.8496025 | 46.425 | 18.226 |
| 1ΔH (kcal/mol) | 2ΔG (kcal/mol) | 3ΔS (cal/mol·K) |
|---|---|---|
| −160.77 | −139.83 | −70.2 |
| Substance | E (Har/at) | H (kcal/mol) | G (kcal/mol) |
|---|---|---|---|
| (C2H5)3Al | −480.2022142 | 125.294 | 94.634 |
| AlBr3 | −1276.1579299 | 6.478 | −19.771 |
| ΔH (kcal/mol) | ΔG (kcal/mol) | ΔS (cal/mol·K) |
|---|---|---|
| 10.64 | 19.87 | 30.9 |
| Substance | E (Har/at) | H (kcal/mol) | G (kcal/mol) |
|---|---|---|---|
| C2H4 | −78.6243401 | 33.759 | 17.293 |
| H2 | −1.2899789 | 8.367 | −1.332 |
| ΔH (kcal/mol) | ΔG (kcal/mol) | ΔS (cal/mol·K) |
|---|---|---|
| −20.21 | −54.822 | 116.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Bai, P.; Li, L.; Li, J.; Wu, L.; Huo, P.; Tan, L. The Reaction Thermodynamics during Plating Al on Graphene Process. Materials 2019, 12, 330. https://doi.org/10.3390/ma12020330
Zhao Z, Bai P, Li L, Li J, Wu L, Huo P, Tan L. The Reaction Thermodynamics during Plating Al on Graphene Process. Materials. 2019; 12(2):330. https://doi.org/10.3390/ma12020330
Chicago/Turabian StyleZhao, Zhanyong, Peikang Bai, Liang Li, Jing Li, Liyun Wu, Pengcheng Huo, and Le Tan. 2019. "The Reaction Thermodynamics during Plating Al on Graphene Process" Materials 12, no. 2: 330. https://doi.org/10.3390/ma12020330





