Behavior of Intermetallic Compounds of Al-Ti Composite Manufactured by Spark Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
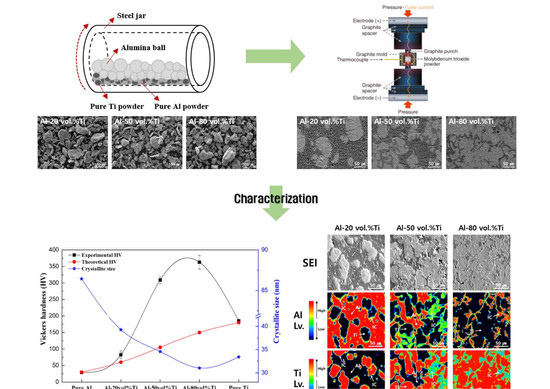
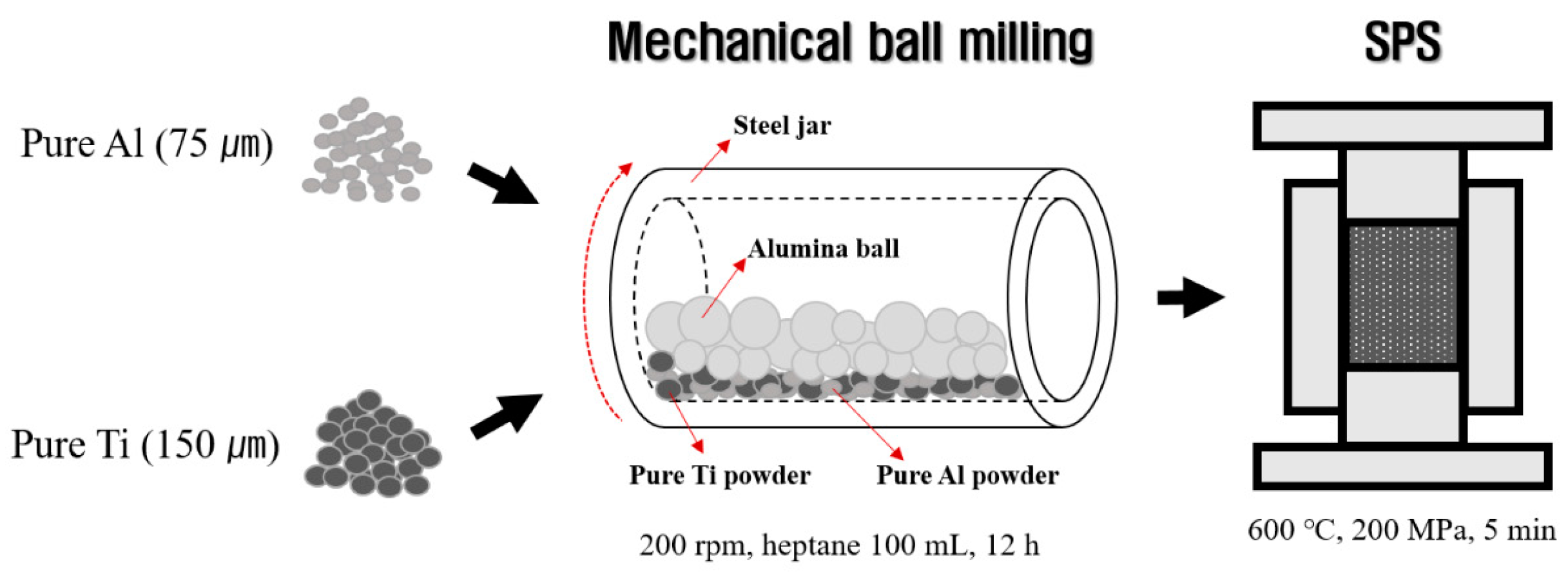
2.1. Fabrication of Al-Ti Composite Powders
2.2. Fabrication of Al-Ti Composites
2.3. Characterization of Al-Ti Composites
3. Results and Discussion
3.1. Morphologies of Al-Ti Composite Powders
3.2. Particle Size Analysis of Al-Ti Composite Powders
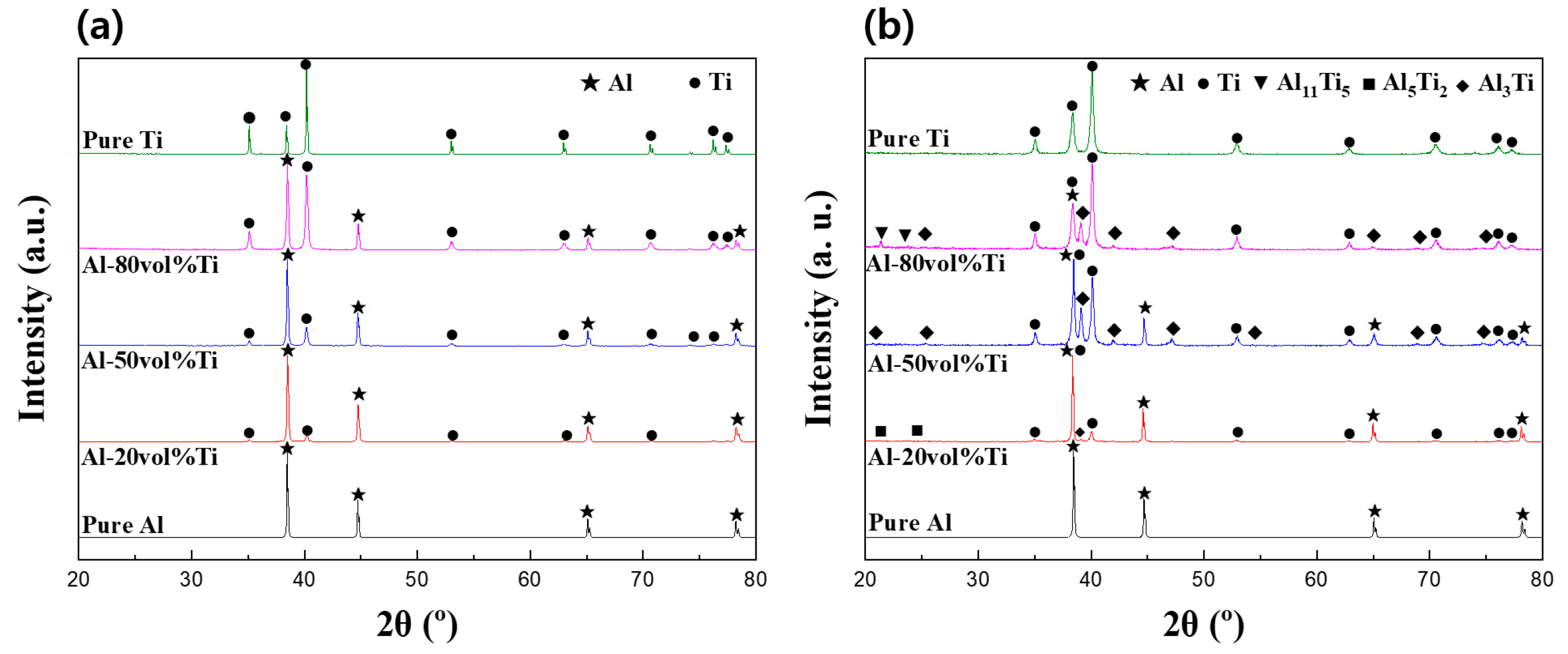
3.3. Phase Analysis of Al-Ti Composite Powders and Composites
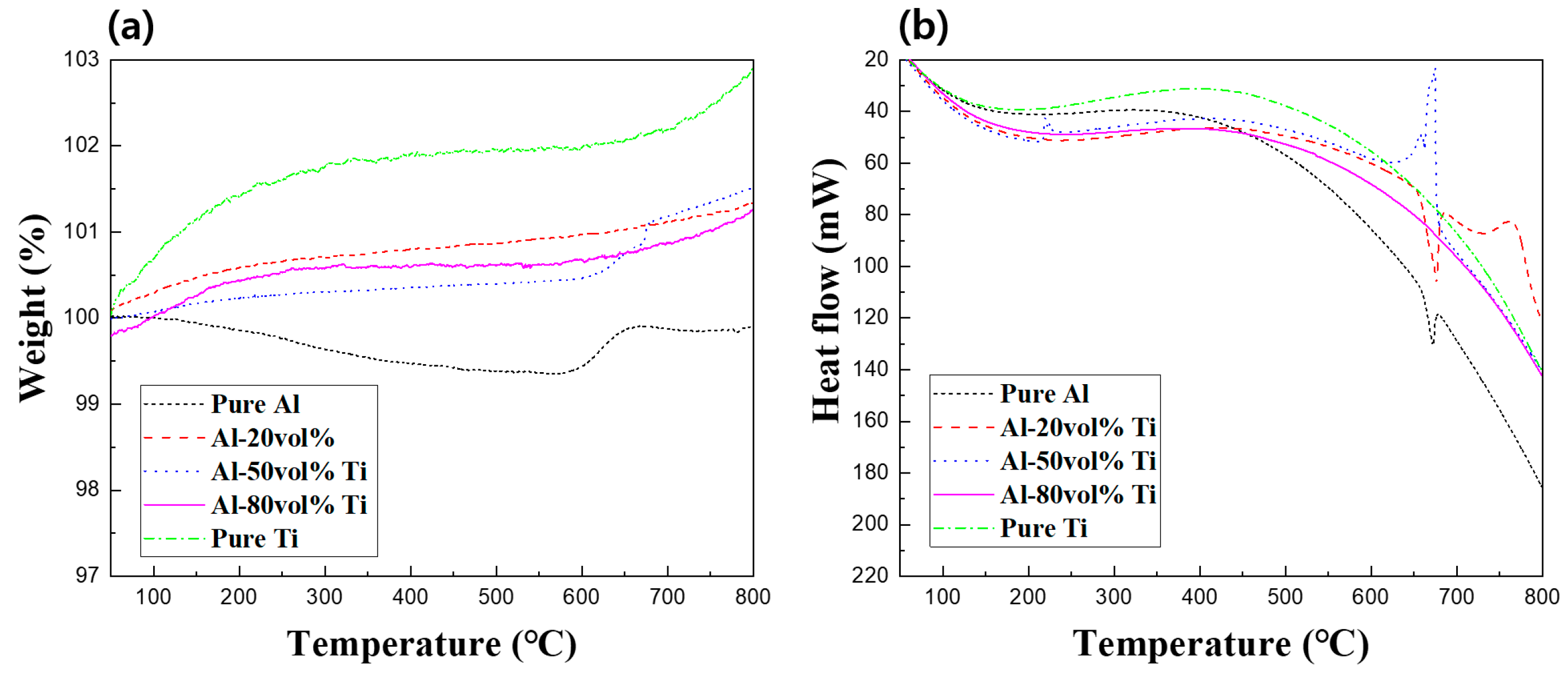
3.4. Thermal Analysis of Al-Ti Composite Powders and Composites
3.5. Microstructural Analysis of Al-Ti Composites
3.6. Vickers Hardness and Crystallite Size of Al-Ti Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vistasp, M.K.; Zhao, L. Use of composites for 21st century civil infrastructure. Comput. Methods Appl. Mech. Eng. 2000, 185, 433–454. [Google Scholar]
- Sahin, Y. Preparation and some properties of SiC particle reinforced aluminium alloy composites. Mater. Des. 2003, 24, 671–679. [Google Scholar] [CrossRef]
- Chen, B.; Liu, J. Contribution of hybrid fibers on the properties of the high-strength lightweight concrete having good workability. Cem. Concr. Res. 2005, 35, 913–917. [Google Scholar] [CrossRef]
- Lyu, M.Y.; Choi, T.G. Research trends in polymer materials for use in lightweight vehicles. Int. J. Precis. Eng. Manuf. 2015, 16, 213–220. [Google Scholar] [CrossRef]
- Kaczmar, J.W.; Pietrzak, K.; Włosiński, W. The production and application of metal matrix composite materials. J. Mater. Process. Technol. 2000, 106, 58–67. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Al-Qureshi, H.A. Automobile leaf springs from composite materials. J. Mater. Process. Technol. 2001, 118, 58–61. [Google Scholar] [CrossRef]
- Dimiduk, D.M. Gamma titanium aluminide alloys—an assessment within the competition of aerospace structural materials. Mater. Sci. Eng. A 1999, 263, 281–288. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—a materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Mohandas, T.; Banerjee, D.; Rao, V.V.K. Observations on impact toughness of electron beam welds of an α+ β titanium alloy. Mater. Sci. Eng. A 1998, 254, 147–154. [Google Scholar] [CrossRef]
- Ryu, J.R.; Moon, K.I.; Lee, K.S. Microstructure and mechanical properties of nanocrystalline Al–Ti alloys consolidated by plasma activated sintering. J. Alloy. Compd. 2000, 296, 157–165. [Google Scholar] [CrossRef]
- Nagae, T.; Saji, S.; Yanagimoto, T.; Nose, M.; Yokota, M. Preparation of functionally graded materials by pulse current pressure sintering of ball milled Al-50 at% Ti powder. Mater. Trans. JIM 2000, 41, 457–460. [Google Scholar] [CrossRef]
- Imayev, R.M.; Kaibyshev, O.A.; Salishchev, G.A. Mechanical behaviour of fine grained TiAl intermetallic compound—I. Superplasticity. Acta Metall. Mater. 1992, 40, 581–587. [Google Scholar] [CrossRef]
- Pope, D.P. High temperature ordered intermetallic alloys. MRS Proc. 1986, 81, 3. [Google Scholar] [CrossRef]
- Milman, Y.V.; Miracle, D.B.; Chugunova, S.I.; Voskoboinik, I.V.; Korzhova, N.P.; Legkaya, T.N.; Podrezov, Y.N. Mechanical behaviour of Al3Ti intermetallic and L12 phases on its basis. Intermetallics 2001, 9, 839–845. [Google Scholar] [CrossRef]
- Prescott, R.; Graham, M.J. The formation of aluminum oxide scales on high-temperature alloys. Oxid. Met. 1992, 38, 233–254. [Google Scholar] [CrossRef]
- Kofstad, P. High-temperature oxidation of titanium. J. Less-Common Met. 1967, 12, 449–464. [Google Scholar] [CrossRef]
- German, R.M. Powder Metallurgy and Particulate Materials Processing: The Processes, Materials, Products, Properties, and Applications; Metal Powder Industries Federation: Princeton, NJ, USA, 2005. [Google Scholar]
- Park, K.; Park, J.; Kwon, H. Fabrication and characterization of Al-SUS316L composite materials manufactured by the spark plasma sintering process. Mater. Sci. Eng. A 2017, 691, 8–15. [Google Scholar] [CrossRef]
- Shigematsu, I.; Nakamura, M.; Saitou, N.; Shimojima, K. Surface treatment of AZ91D magnesium alloy by aluminum diffusion coating. J. Mater. Sci. Lett. 2000, 19, 473–475. [Google Scholar] [CrossRef]
- Kwon, H.; Leparoux, M.; Kawasaki, A. Functionally graded dual-nanoparticulate-reinforced aluminium matrix bulk materials fabricated by spark plasma sintering. J. Mater. Sci. Technol. 2014, 30, 736–742. [Google Scholar] [CrossRef]
- Sheasby, P.G.; Wernick, S.; Pinner, R. Surface Treatment and Finishing of Aluminum and Its Alloys; Volumes 1 and 2 (5th Revised and Enlarged Edition); United States: New York, NY, USA, 1987. [Google Scholar]
- Anselmi-Tamburini, U.; Gennari, S.; Garay, J.E.; Munir, Z.A. Fundamental investigations on the spark plasma sintering/synthesis process: II. Modeling of current and temperature distributions. Mater. Sci. Eng. A 2005, 394, 139–148. [Google Scholar] [CrossRef]
- Matsugi, K.; Kuramoto, H.; Hatayama, T.; Yanagisawa, O. Temperature distribution at steady state under constant current discharge in spark sintering process of Ti and Al2O3 powders. J. Mater. Process. Technol. 2003, 134, 225–232. [Google Scholar] [CrossRef]
- Marom, G.; Fischer, S.; Tuler, F.R.; Wagner, H.D. Hybrid effects in composites: Conditions for positive or negative effects versus rule-of-mixtures behaviour. J. Mater. Sci. 1978, 13, 1419–1426. [Google Scholar] [CrossRef]
- Hatch, J.E. Aluminum: Properties and Physical Metallurgy; American Society for Metals: Metals Park, OH, USA, 1984; Volume 143. [Google Scholar]
- Dunand, D.C. Processing of titanium foams. Adv. Eng. Mater. 2004, 6, 369–376. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Kim, H. On the rule of mixtures for the hardness of particle reinforced composites. Mater. Sci. Eng. A 2000, 289, 30–33. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Lasalmonie, A. Intermetallics: Why is it so difficult to introduce them in gas turbine engines? Intermetallics 2006, 14, 1123–1129. [Google Scholar] [CrossRef]
- Kwon, H.; Estili, M.; Takagi, K.; Miyazaki, T.; Kawasaki, A. Combination of hot extrusion and spark plasma sintering for producing carbon nanotube reinforced aluminum matrix composites. Carbon 2009, 47, 570–577. [Google Scholar] [CrossRef]
- Cho, S.; Kikuchi, K.; Miyazaki, T.; Kawasaki, A.; Arami, Y.; Silvain, J.F. Epitaxial growth of chromium carbide nanostructures on multiwalled carbon nanotubes (MWCNTs) in MWCNT–copper composites. Acta Mater. 2013, 61, 708–716. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. In Kolloidchemie Ein Lehrbuch; Springer: Berlin/Heidelberg, Germany, 1912; pp. 387–409. [Google Scholar]
- Lasalmonie, A.; Strudel, J.L. Influence of grain size on the mechanical behaviour of some high strength materials. J. Mater. Sci. 1986, 21, 1837–1852. [Google Scholar] [CrossRef]
- Park, K.; Park, J.; Kwon, H. Effect of intermetallic compound on the Al-Mg composite materials fabricated by mechanical ball milling and spark plasma sintering. J. Alloy. Compd. 2018, 739, 311–318. [Google Scholar] [CrossRef]




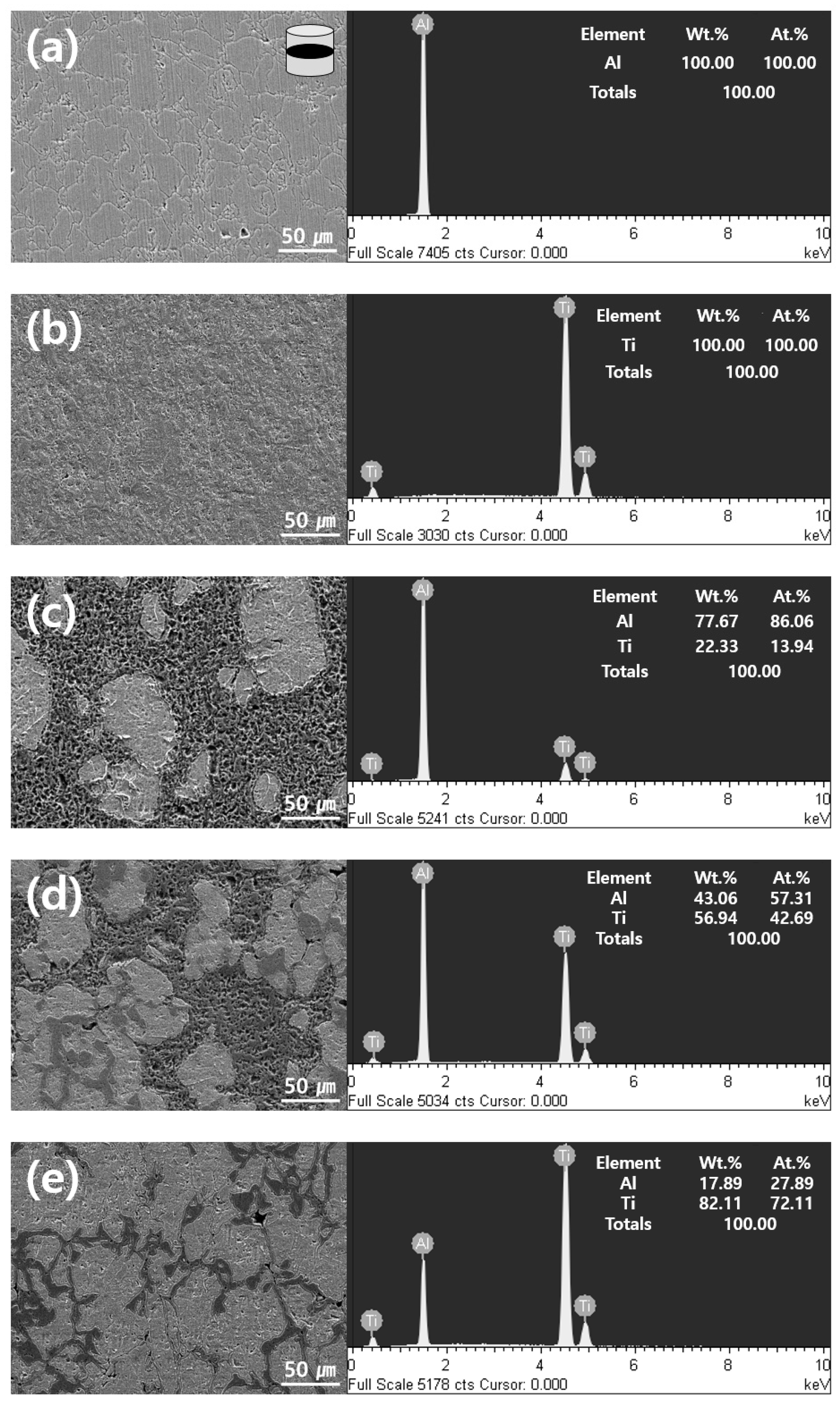

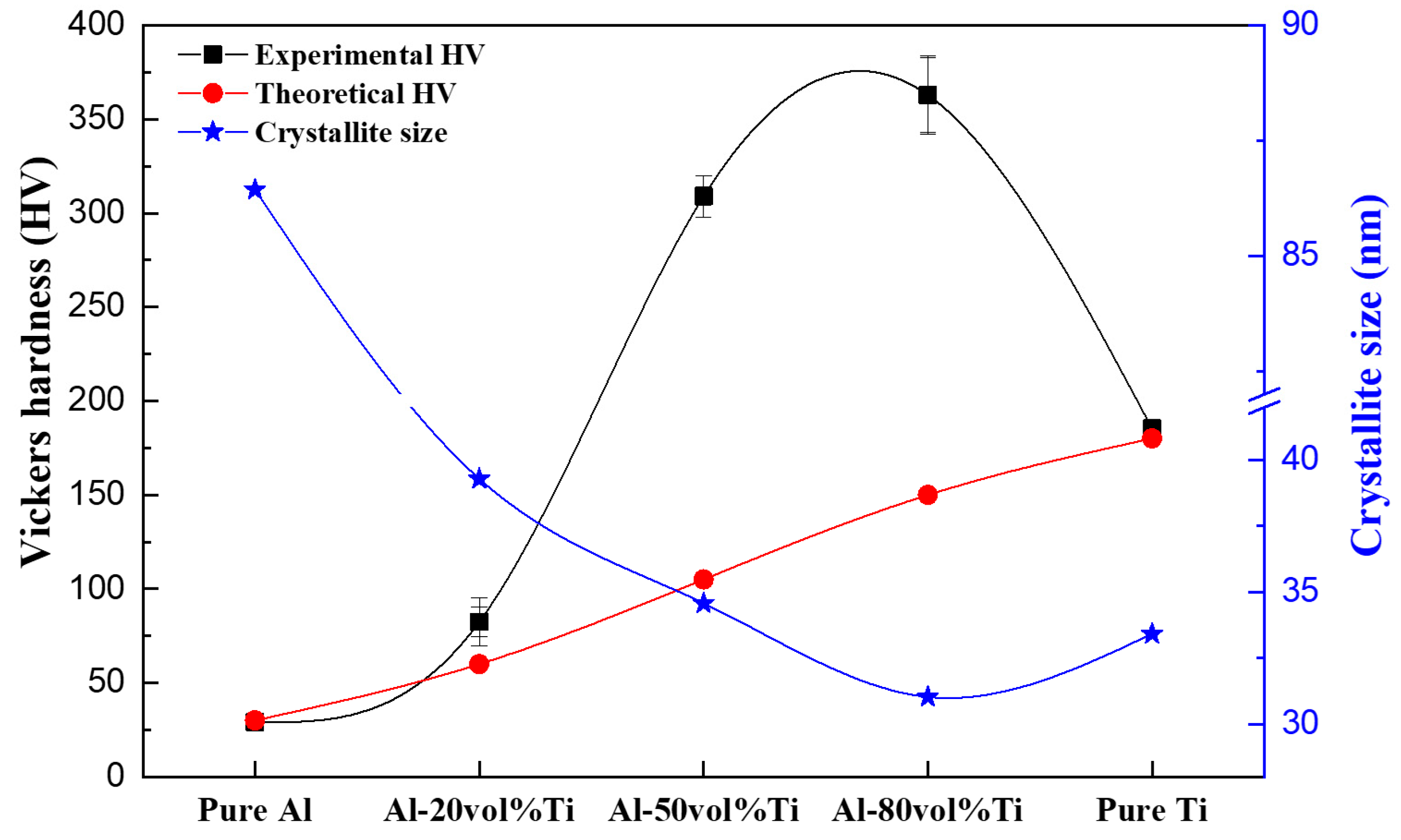
| Sample | Density | Vickers Hardness (HV) | Crystallite Size (nm) | Al3Ti Peak Intensity (%) | ||
|---|---|---|---|---|---|---|
| Theoretical Density (g cm−3) | Experimental Density (g cm−3) | Relative Density (%) | ||||
| Pure Al | 2.70 | 2.65 ± 0.1 | 98.4 ± 0.2 | 29 ± 3 | 86.44 ± 5% | - |
| Al-20 vol.% Ti | 3.06 | 3.07 ± 0.1 | 100.2 ± 0.1 | 82 ± 17 | 39.29 ± 5% | 25 |
| Al-50 vol.% Ti | 3.60 | 3.70 ± 0.1 | 102.8 ± 0.2 | 309 ± 13 | 34.58 ± 5% | 100 |
| Al-80 vol.% Ti | 4.15 | 4.19 ± 0.1 | 101.1 ± 0.1 | 363 ± 26 | 31.03 ± 5% | 71 |
| Pure Ti | 4.50 | 4.48 ± 0.1 | 99.3 ± 0.1 | 185 ± 5 | 33.42 ± 5% | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Kim, D.; Kim, K.; Cho, S.; Kwon, H. Behavior of Intermetallic Compounds of Al-Ti Composite Manufactured by Spark Plasma Sintering. Materials 2019, 12, 331. https://doi.org/10.3390/ma12020331
Park K, Kim D, Kim K, Cho S, Kwon H. Behavior of Intermetallic Compounds of Al-Ti Composite Manufactured by Spark Plasma Sintering. Materials. 2019; 12(2):331. https://doi.org/10.3390/ma12020331
Chicago/Turabian StylePark, Kwangjae, Dasom Kim, Kyungju Kim, Seungchan Cho, and Hansang Kwon. 2019. "Behavior of Intermetallic Compounds of Al-Ti Composite Manufactured by Spark Plasma Sintering" Materials 12, no. 2: 331. https://doi.org/10.3390/ma12020331