Catalytic Soot Oxidation Activity of NiO–CeO2 Catalysts Prepared by a Coprecipitation Method: Influence of the Preparation pH on the Catalytic Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.2. Catalyst Characterization
2.3. Catalytic Activity Measurement
3. Results and Discussion
3.1. Catalyst Characterization
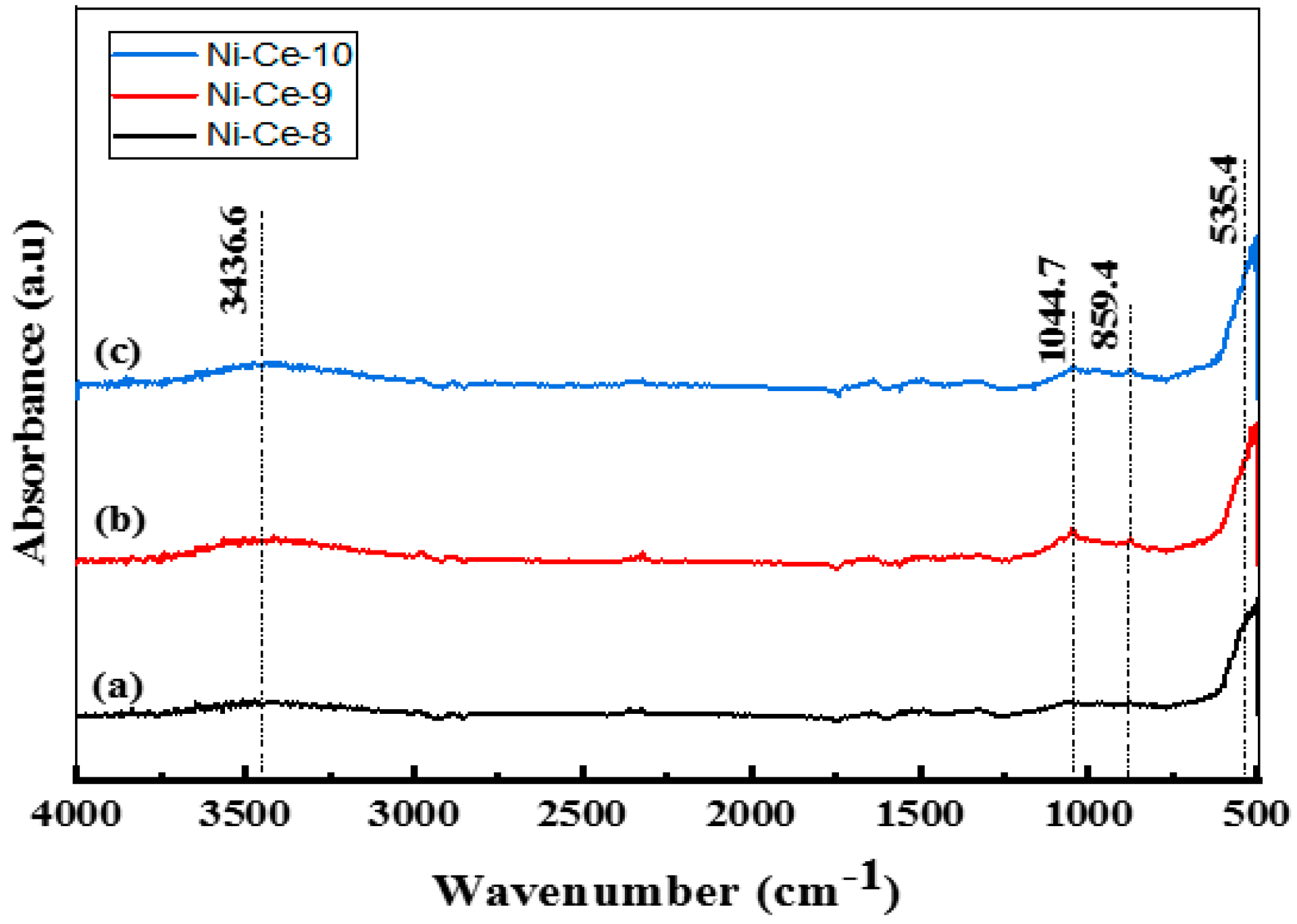
3.1.1. Thermogravimetric Analysis (TGA) and Fourier-Transform Infrared Spectroscopy Analysis (FTIR)
3.1.2. Elemental Composition (ICP)
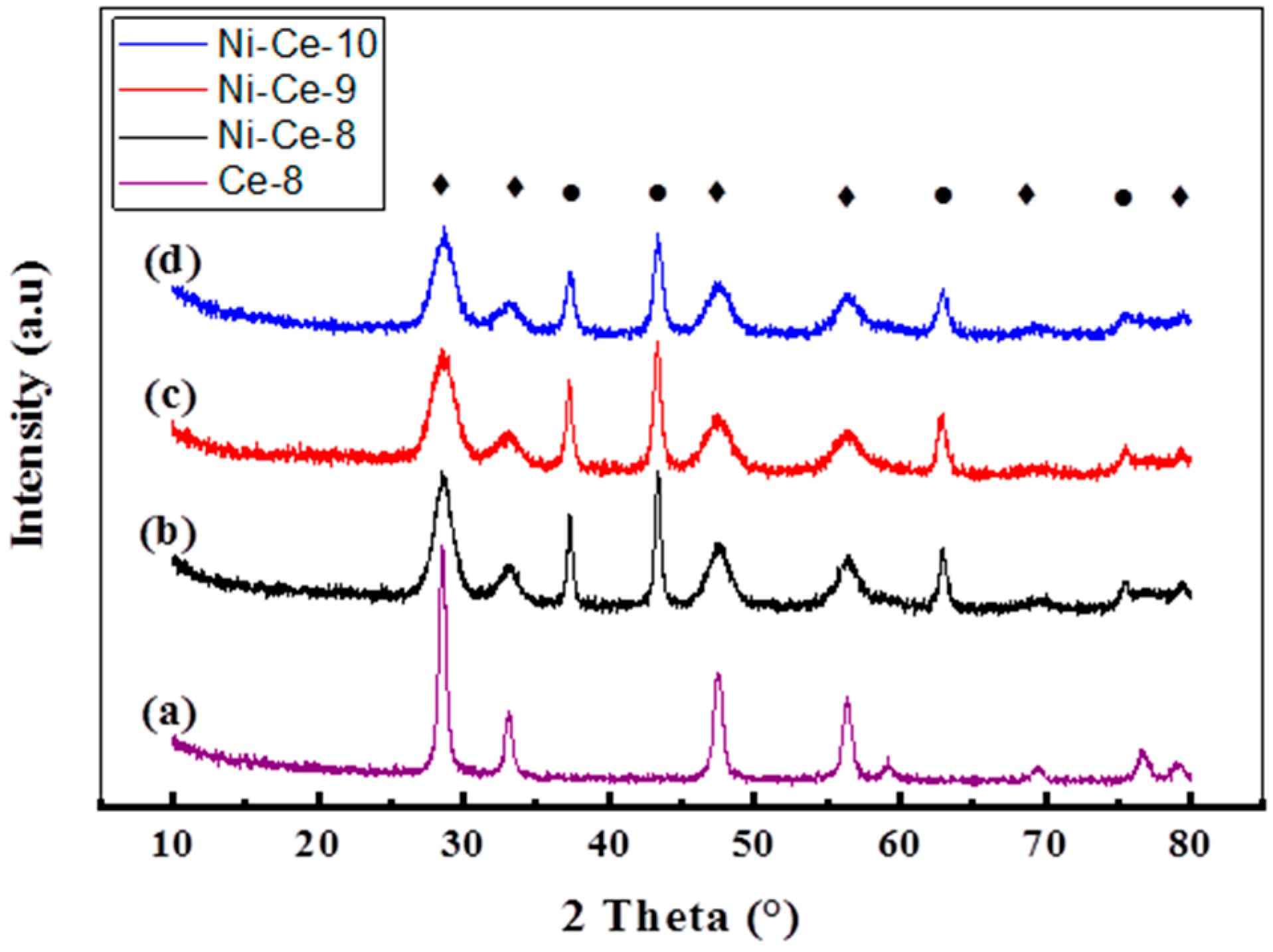
3.1.3. X-Ray Diffraction Analysis (XRD)
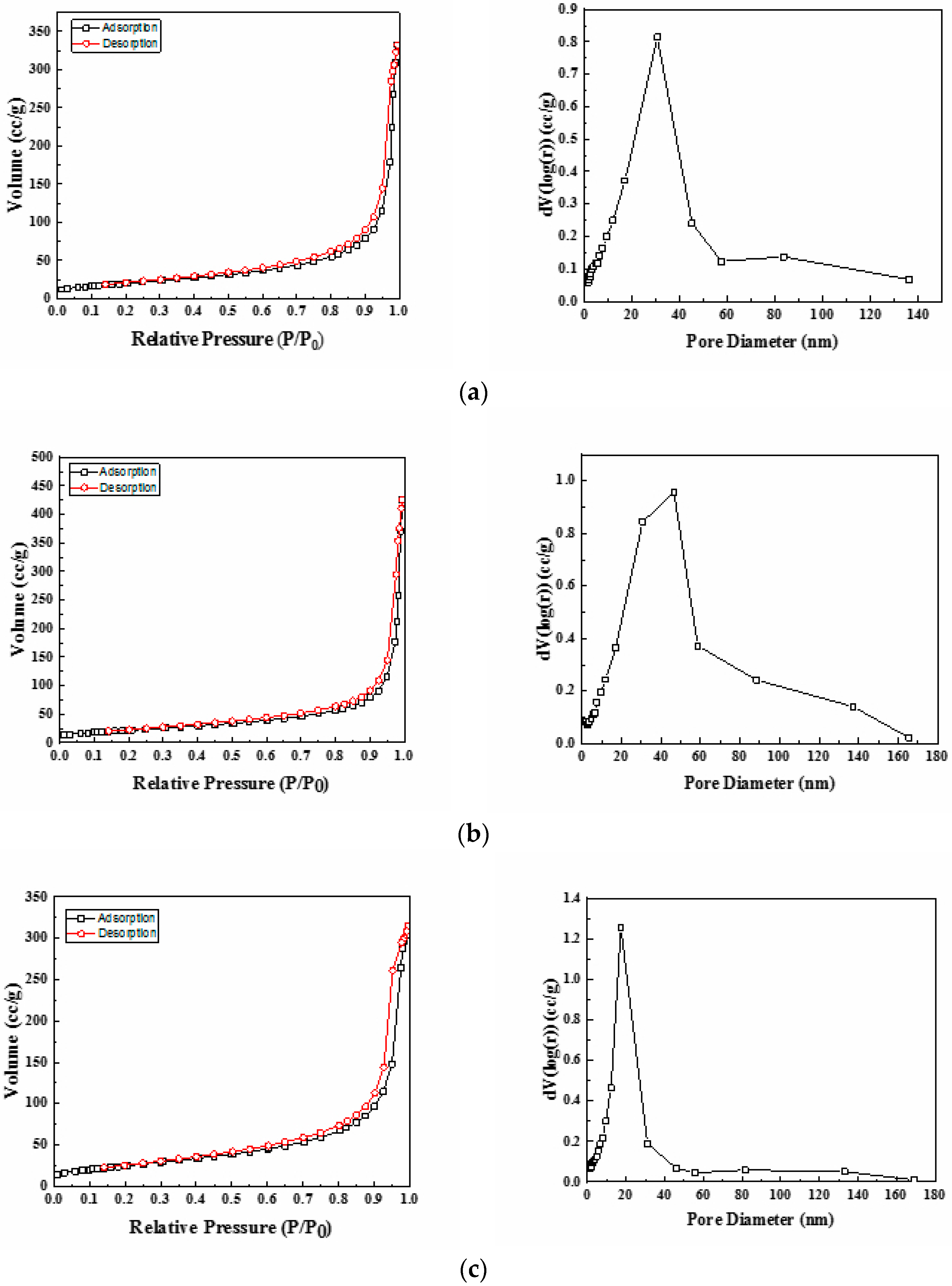
3.1.4. Surface Area BET Measurement
3.1.5. Temperature-Programmed Reduction (H2-TPR)
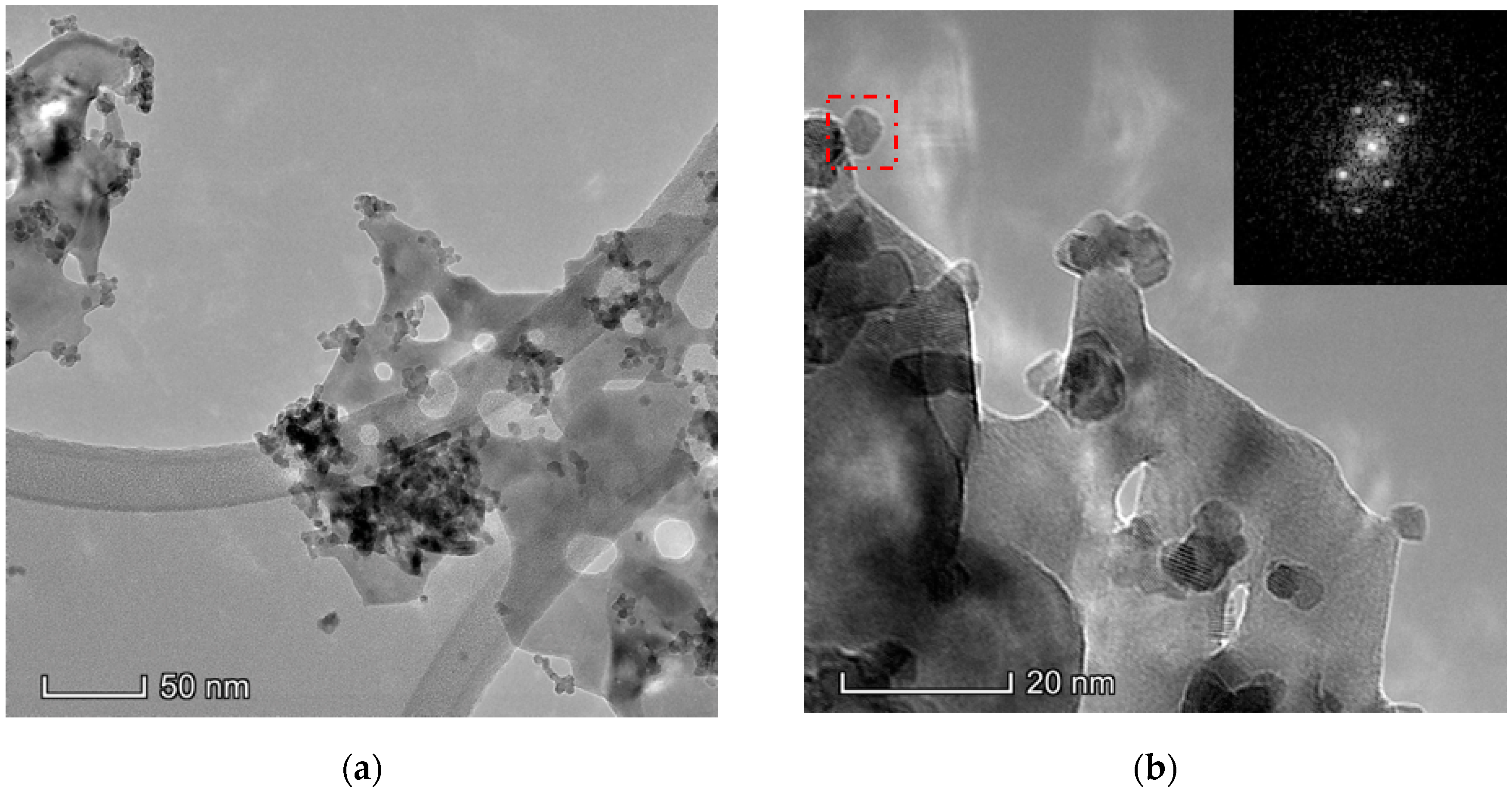
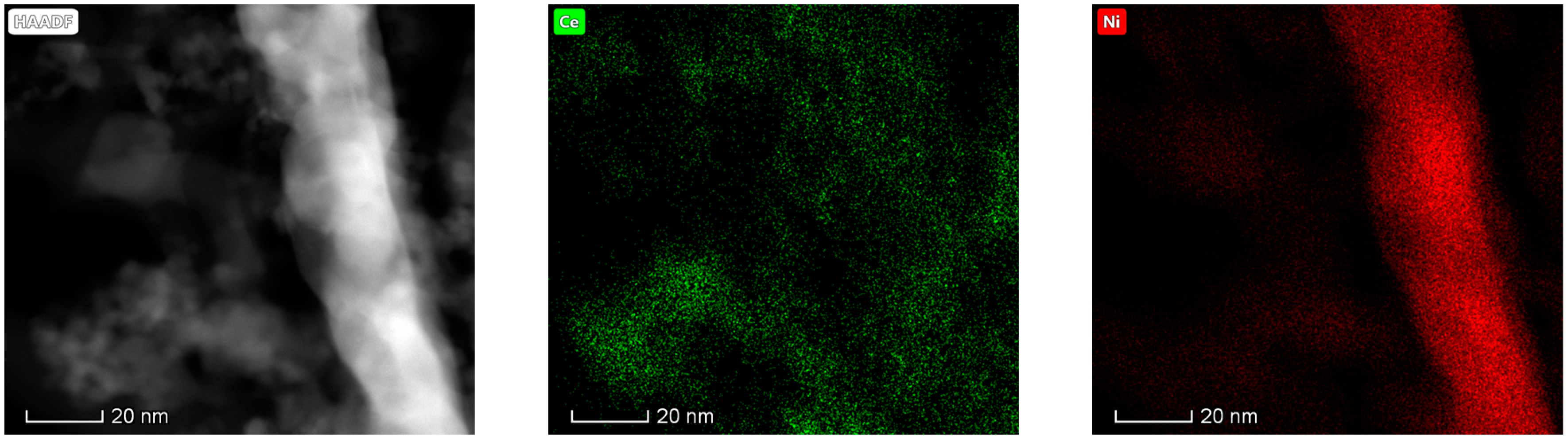
3.1.6. Electron Microscopy Analysis (HAADF/TEM-EDS)
3.2. Catalytic Activity Tests
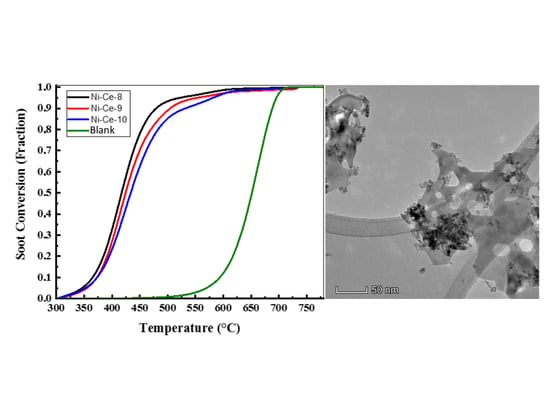
3.2.1. Soot Oxidation under Air
3.2.2. Apparent Activation Energy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bueno-López, A. Diesel soot combustion ceria catalysts. Appl. Catal. B Environ. 2014, 146, 1–11. [Google Scholar]
- Liu, J.; Zhao, Z.; Chen, Y.; Xu, C.; Duan, A.; Jiang, G. Different valent ions-doped cerium oxides and their catalytic performances for soot oxidation. Catal. Today 2011, 175, 117–123. [Google Scholar] [CrossRef]
- Zhang, W.; Niu, X.; Chen, L.; Yuan, F.; Zhu, Y. Soot Combustion over Nanostructured Ceria with Different Morphologies. Sci. Rep. 2016, 6, 29062. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Zhong, L.; Zhu, J.; Hong, J.; Li, J.; Li, H.; Zhu, Y. CO and soot oxidation over macroporous perovskite LaFeO3. Catal. Today 2015, 258, 660–667. [Google Scholar] [CrossRef]
- Simonsen, S.B.; Dahl, S.; Johnson, E.; Helveg, S. Ceria-catalyzed Soot Oxidation studied by Environmental Transmission Electron Microscopy. J. Catal. 2008, 255, 1–5. [Google Scholar] [CrossRef]
- Cousin, R.; Capelle, S.; Courcot, D.; Aboukaı, A. Copper-vanadium-cerium oxide catalysts for carbon black oxidation. Appl. Catal. B Environ. 2007, 70, 247–253. [Google Scholar] [CrossRef]
- Neelapala, S.D.; Dasari, H. Catalytic soot oxidation activity of Cr-doped Ceria (Ce1−xCrxO2−δ) synthesized by sol-gel method with organic additives. Mater. Sci. Eng. Technol. 2018, 1, 155–159. [Google Scholar]
- Shimizu, K.; Kawachi, H.; Satsuma, A. Study of active sites and mechanism for soot oxidation by silver-loaded ceria catalyst. Appl. Catal. B Environ. 2010, 96, 169–175. [Google Scholar] [CrossRef]
- Obeid, E.; Lizarraga, L.; Tsampas, M.N.; Cordier, A.; Boréave, A.; Steil, M.C.; Blanchard, G.; Pajot, K.; Vernoux, P. Continuously regenerating Diesel Particulate Filters based on ionically conducting ceramics. J. Catal. 2014, 309, 87–96. [Google Scholar] [CrossRef]
- Aneggi, E.; De Leitenburg, C.; Trovarelli, A. On the role of lattice/surface oxygen in ceria—Zirconia catalysts for diesel soot combustion. Catal. Today 2012, 181, 108–115. [Google Scholar] [CrossRef]
- Oi-Uchisawa, J.; Wang, S.; Nanba, T.; Ohi, A.; Obuchi, A. Improvement of Pt catalyst for soot oxidation using mixed oxide as a support. Appl. Catal. B Environ. 2003, 44, 207–215. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Z.; Li, T.; Liu, J.; Duan, A.; Jiang, G. The novel catalysts of truncated polyhedron Pt nanoparticles supported on three-dimensionally ordered macroporous oxides (Mn, Fe, Co, Ni, Cu) with nanoporous walls for soot combustion. Appl. Catal. B Environ. 2014, 146, 57–70. [Google Scholar] [CrossRef]
- Nascimento, L.F.; Martins, R.F.; Silva, R.F.; Filho, P.C.D.S.; Serra, O.A. Ru-doped ceria-zirconia mixed oxides catalyze soot combustion. Reac. Kinet. Mech. Cat. 2014, 111, 149–165. [Google Scholar] [CrossRef]
- Jin, B.; Wei, Y.; Zhao, Z.; Liu, J.; Li, Y.; Li, R.; Duan, A.; Jiang, G. Three-dimensionally ordered macroporous CeO2/Al2O3-supported Au nanoparticle catalysts: Effects of CeO2 nanolayers on catalytic activity in soot oxidation. Chin. J. Catal. 2017, 38, 1629–1641. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Lan, J.; Xu, C.; Duan, A.; Jiang, G.; Wang, X. Catalytic Combustion of Soot over the Highly Active (La0.9K0.1CoO3) x/nmCeO2 Catalysts. J. Phys. Chem. C 2009, 113, 17114–17123. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Tsampas, M.N.; Zhao, C.; Boreave, A.; Bosselet, F.; Vernoux, P. La/Sr-based perovskites as soot oxidation catalysts for Gasoline Particulate Filters. Catal. Today 2015, 258, 525–534. [Google Scholar] [CrossRef]
- Weng, D.; Li, J.; Wu, X.; Si, Z. Modification of CeO2-ZrO2 catalyst by potassium for NOx-assisted soot oxidation. J. Environ. Sci. 2011, 23, 145–150. [Google Scholar] [CrossRef]
- López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J. Cu/Al2O3 catalysts for soot oxidation: Copper loading effect. Appl. Catal. B Environ. 2008, 84, 651–658. [Google Scholar]
- Guilhaume, N.; Bassou, B.; Bergeret, G.; Bianchi, D.; Bosselet, F.; Jouguet, B.; Mirodatos, C. In situ investigation of Diesel soot combustion over an AgMnOx catalyst. Appl. Catal. B Environ. 2012, 119, 287–296. [Google Scholar] [CrossRef]
- Bueno-López, A.; Moulijn, J.A.; Krishna, K.; Makkee, M. Potential rare earth modified CeO2 catalysts for soot oxidation I. Characterisation and catalytic activity with O2. Appl. Catal. B Environ. 2007, 75, 189–200. [Google Scholar]
- Rangaswamy, A.; Sudarsanam, P.; Reddy, B.M. Rare earth metal doped CeO2-based catalytic materials for diesel soot oxidation at lower temperatures. J. Rare Earths 2015, 33, 1162–1169. [Google Scholar] [CrossRef]
- Bueno-López, A.; Krishna, K.; Van Der Linden, B.; Mul, G.; Moulijn, J.; Makkee, M. On the mechanism of model diesel soot-O2 reaction catalysed A TAP study with isotopic O2. Catal. Today 2007, 121, 237–245. [Google Scholar] [CrossRef]
- Kustov, A.L.; Ricciardi, F.; Makkee, M. NOx Storage and High Temperature Soot Oxidation on Pt–Sr/ZrO2 Catalyst. Top. Catal. 2009, 52, 2058–2062. [Google Scholar] [CrossRef]
- Nascimento, L.F.; Martins, R.F.; Serra, O.A. Catalytic combustion of soot over Ru-doped mixed oxides catalysts. J. Rare Earths 2014, 32, 610–620. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Z.; Jiao, J.; Liu, J.; Duan, A.; Jiang, G. Preparation of ultrafine Ce-based oxide nanoparticles and their catalytic performances for diesel soot combustion. J. Rare Earths 2014, 32, 124–130. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.D.; Weng, D.; Ran, R. Ceria-based catalysts for soot oxidation: A review. J. Rare Earths 2015, 33, 567–590. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Liu, W.; Chen, W.; Ran, R.; Li, M.; Weng, D. Soot oxidation over CeO2 and Ag/CeO2: Factors determining the catalyst activity and stability during reaction. J. Catal. 2016, 337, 188–198. [Google Scholar] [CrossRef]
- Lin, F.; Delmelle, R.; Vinodkumar, T.; Reddy, B.M.; Wokaun, A.; Alxneit, I. Correlation between the structural characteristics, oxygen storage capacities and catalytic activities of dual-phase Zn-modified ceria nanocrystals. Catal. Sci. Technol. 2015, 5, 3556–3567. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Li, X.; Yao, Z.; Chen, Z.; Hong, M.; Zhu, R.; Liang, Y. Transition-Metal Doped Ceria Microspheres with Nanoporous Structures for CO Oxidation. Sci. Rep. 2016, 6, 23900. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Hu, W.; Zhang, N.; Li, Y.; Liao, Y. Facile synthesis of ceria–zirconia solid solutions with cubic–tetragonal interfaces and their enhanced catalytic performance in diesel soot oxidation. J. Catal. 2019, 377, 98–109. [Google Scholar] [CrossRef]
- Shan, W.; Ma, N.; Yang, J.; Dong, X.; Liu, C.; Wei, L. Catalytic oxidation of soot particulates over MnOx-CeO2 oxides prepared by complexation-combustion method. J. Nat. Gas. Chem. 2010, 19, 86–90. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Jampaiah, D.; Rao, K.N.; Reddy, B.M. Nanostructured Ce0.7Mn0.3O2-δ and Ce0.7Fe0.3O2-δ solid solutions for diesel soot oxidation. Appl. Catal. A Gener. 2014, 488, 1–10. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mamontov, G.V.; Zaikovskii, V.I.; La Parola, V.; Liotta, L.F.; Vodyankina, O.V. The role of metal–support interaction in Ag/CeO2 catalysts for CO and soot oxidation. Appl. Catal. B Environ. 2019, 260. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mikheeva, N.N.; Mamontov, G.V.; Salaev, M.A.; Liotta, L.F.; Vodyankina, O. Ag/CeO2 Composites for Catalytic Abatement of CO, Soot and VOCs. Catalysts 2018, 8, 285. [Google Scholar] [CrossRef]
- Neeft, J.P.A.; Makkee, M.; Moulijn, J.A. Metal oxides as catalysts for the oxidation of soot. Chem. Eng. J. 1996, 64, 295–302. [Google Scholar] [CrossRef]
- Araújo, A.J.M.; Silva, V.D.; Sousa, A.R.O.; Grilo, J.P.F.; Simões, T.A.; Macedo, D.A.; Nascimento, R.M.; Paskocimas, C.A. Battery-like behavior of Ni-ceria based systems: Synthesis, surface defects and electrochemical assessment. Ceram. Int. 2018, 45, 7157–7165. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, Y.J.; Koo, B.H.; Lee, C.G. Structural and magnetic properties of Ni doped CeO2 nanoparticles. J. Nanosci. Nanotechnol. 2010, 10, 7204–7207. [Google Scholar] [CrossRef]
- Wu, G.; Wang, X.; Chen, B.; Li, J.; Zhao, N.; Wei, W.; Sun, Y. Fluorine-modified mesoporous Mg-Al mixed oxides: Mild and stable base catalysts for O-methylation of phenol with dimethyl carbonate. Appl. Catal. A Gene 2007, 329, 106–111. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Atzori, L.; Cutrufello, M.G.; Meloni, D.; Cannas, C.; Gazzoli, D.; Monaci, R.; Sini, M.F.; Rombi, E. Highly active NiO-CeO2 catalysts for synthetic natural gas production by CO2 methanation. Catal. Today 2018, 299, 183–192. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, T.W.; Lee, S.H.; Park, E.D. Effects of Na content in Na/Ni/SiO2 and Na/Ni/CeO2 catalysts for CO and CO2 methanation. Catal. Today 2018, 303, 159–167. [Google Scholar] [CrossRef]
- Xu, S.; Yan, X.; Wang, X. Catalytic performances of NiO–CeO2 for the reforming of methane with CO2 and O2. Fuel 2006, 85, 2243–2247. [Google Scholar] [CrossRef]
- Van Setten, B.A.A.L.; Makkee, M.; Moulijn, J.A. Science and technology of catalytic diesel particulate filters. Catal. Rev. Sci. Eng. 2007, 43, 489–564. [Google Scholar] [CrossRef]
- Stanmore, B.R.; Brilhac, J.F.; Gilot, P. The oxidation of soot: A review of experiments, mechanisms and models. Carbon 2001, 39, 2247–2268. [Google Scholar] [CrossRef]
- Bueno-López, A.; Krishna, K.; Makkee, M.; Moulijn, J.A. Enhanced soot oxidation by lattice oxygen via La3+ doped CeO2. J. Catal. 2005, 230, 237–248. [Google Scholar] [CrossRef]
- Guillén-hurtado, N.; Bueno-lópez, A.; García-garcía, A. Catalytic performances of ceria and ceria-zirconia materials for the combustion of diesel soot under NOx/O2 and O2. Importance of the cerium precursor salt. Appl. Catal. A Gene 2012, 437–438, 166–172. [Google Scholar]
- Wen, Z.; Duan, X.; Hu, M.; Cao, Y.; Ye, L.; Jiang, L.; Yuan, Y. Efficient low-temperature soot combustion by bimetallic Ag-Cu/SBA-15 catalysts. J. Environ. Sci. 2018, 64, 122–129. [Google Scholar] [CrossRef]
- Hu, Z.; Qiu, S.; You, Y.; Guo, Y.; Guo, Y.; Wang, L.; Zhan, W.; Lu, G. Hydrothermal synthesis of NiCeOx nanosheets and its application to the total oxidation of propane. Appl. Catal. B Environ. 2018, 225, 110–120. [Google Scholar] [CrossRef]
- Liu, Y.M.; Wang, L.C.; Chen, M.; Xu, J.; Cao, Y.; He, H.Y.; Fan, K.N. Highly Selective Ce–Ni–O Catalysts for Efficient Low Temperature Oxidative Dehydrogenation of Propane. Catal. Lett. 2009, 130, 350–354. [Google Scholar] [CrossRef]
- Neeft, J.P.A.; Nijhuis, T.X.; Smakman, E.; Makkee, M.; Moulijn, J.A. Kinetics of the oxidation of diesel soot. Fuel 1997, 76, 1129–1136. [Google Scholar] [CrossRef]





| Sample | Ni/Ce a | Weight (%) | Ni/Ce b | |
|---|---|---|---|---|
| Ce (wt %) | Ni (wt %) | |||
| Ni-Ce-8 | 1.25 | 32.3 ± 0.1 | 28.0 ± 0.2 | 0.87 |
| Ni-Ce-9 | 1.25 | 28.0 ± 0.3 | 28.0 ± 0.3 | 1 |
| Ni-Ce-10 | 1.25 | 32.4 ± 0.2 | 32.2 ± 0.2 | 0.99 |
| Sample | Crystallite | Position of the Most Intense Peak 2θ (°) | Average Crystallite Size (nm) | Lattice Parameter (a) NiO and CeO2 (Å) |
|---|---|---|---|---|
| Ce-8 | CeO2 | 28.56 | 12.6 | 5.410 |
| Ni-Ce-8 | NiO | 43.36 | 18.7 | 4.170 |
| CeO2 | 28.54 | 5.2 | 5.397 | |
| Ni-Ce-9 | NiO | 43.32 | 14.3 | 4.173 |
| CeO2 | 28.53 | 4.2 | 5.399 | |
| Ni-Ce-10 | NiO | 43.37 | 13.0 | 4.169 |
| CeO2 | 28.56 | 4.8 | 5.405 |
| Sample | SBET (m2/g) | VP (cm3/g) | DP (nm) |
|---|---|---|---|
| Ni-Ce-8 | 73 | 0.27 | 19.14 |
| Ni-Ce-9 | 80 | 0.27 | 56.99 |
| Ni-Ce-10 | 91 | 0.40 | 17.08 |
| Sample | Total H2 Consumption (mmol/g) (a) | Total H2 Consumption (mmol/g) (b) | ɛ (c) |
|---|---|---|---|
| Ce-8 | 2.9 | 1.8 | - |
| Ni-Ce-8 | 11.84 | 9.90 | 0.84 |
| Ni-Ce-9 | 11.53 | 9.82 | 0.85 |
| Ni-Ce-10 | 13.28 | 11.03 | 0.83 |
| Contact Mode | Catalyst | Temperature (°C) | |||
|---|---|---|---|---|---|
| T10 a | T50 a | T90 a | Tf b | ||
| Uncatalyzed | Pure soot (Printex-U) | 593 | 652 | 688 | 705 |
| Loose | Ce-8 | 536 | 610 | 664 | 690 |
| Ni-Ce-8 | 507 | 589 | 624 | 646 | |
| Ni-Ce-9 | 511 | 592 | 629 | 651 | |
| Ni-Ce-10 | 513 | 594 | 630 | 655 | |
| Tight | Ce-8 | 415 | 468 | 537 | 617 |
| Ni-Ce-8 | 365 | 417 | 479 | 598 | |
| Ni-Ce-9 | 372 | 424 | 504 | 611 | |
| Ni-Ce-10 | 375 | 433 | 531 | 616 | |
| Catalyst | Apparent Activation Energy (kJ/mol) ± 5, at x% Conversion | ||||||
|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | |
| Ni-Ce-8 | 109 | 111 | 114 | 118 | 121 | 126 | 133 |
| Ni-Ce-9 | 139 | 144 | 140 | 139 | 130 | 141 | 142 |
| Ni-Ce-10 | 133 | 125 | 128 | 131 | 136 | 142 | 150 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendieb Aberkane, A.; Yeste, M.P.; Fayçal, D.; Goma, D.; Cauqui, M.Á. Catalytic Soot Oxidation Activity of NiO–CeO2 Catalysts Prepared by a Coprecipitation Method: Influence of the Preparation pH on the Catalytic Performance. Materials 2019, 12, 3436. https://doi.org/10.3390/ma12203436
Bendieb Aberkane A, Yeste MP, Fayçal D, Goma D, Cauqui MÁ. Catalytic Soot Oxidation Activity of NiO–CeO2 Catalysts Prepared by a Coprecipitation Method: Influence of the Preparation pH on the Catalytic Performance. Materials. 2019; 12(20):3436. https://doi.org/10.3390/ma12203436
Chicago/Turabian StyleBendieb Aberkane, Amar, María Pilar Yeste, Djazi Fayçal, Daniel Goma, and Miguel Ángel Cauqui. 2019. "Catalytic Soot Oxidation Activity of NiO–CeO2 Catalysts Prepared by a Coprecipitation Method: Influence of the Preparation pH on the Catalytic Performance" Materials 12, no. 20: 3436. https://doi.org/10.3390/ma12203436