Impact of Basalt Filler and Ceramizable Additives on the Toxicity of Gaseous Products Emitted from Thermal Decomposition of Silicone Rubber Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
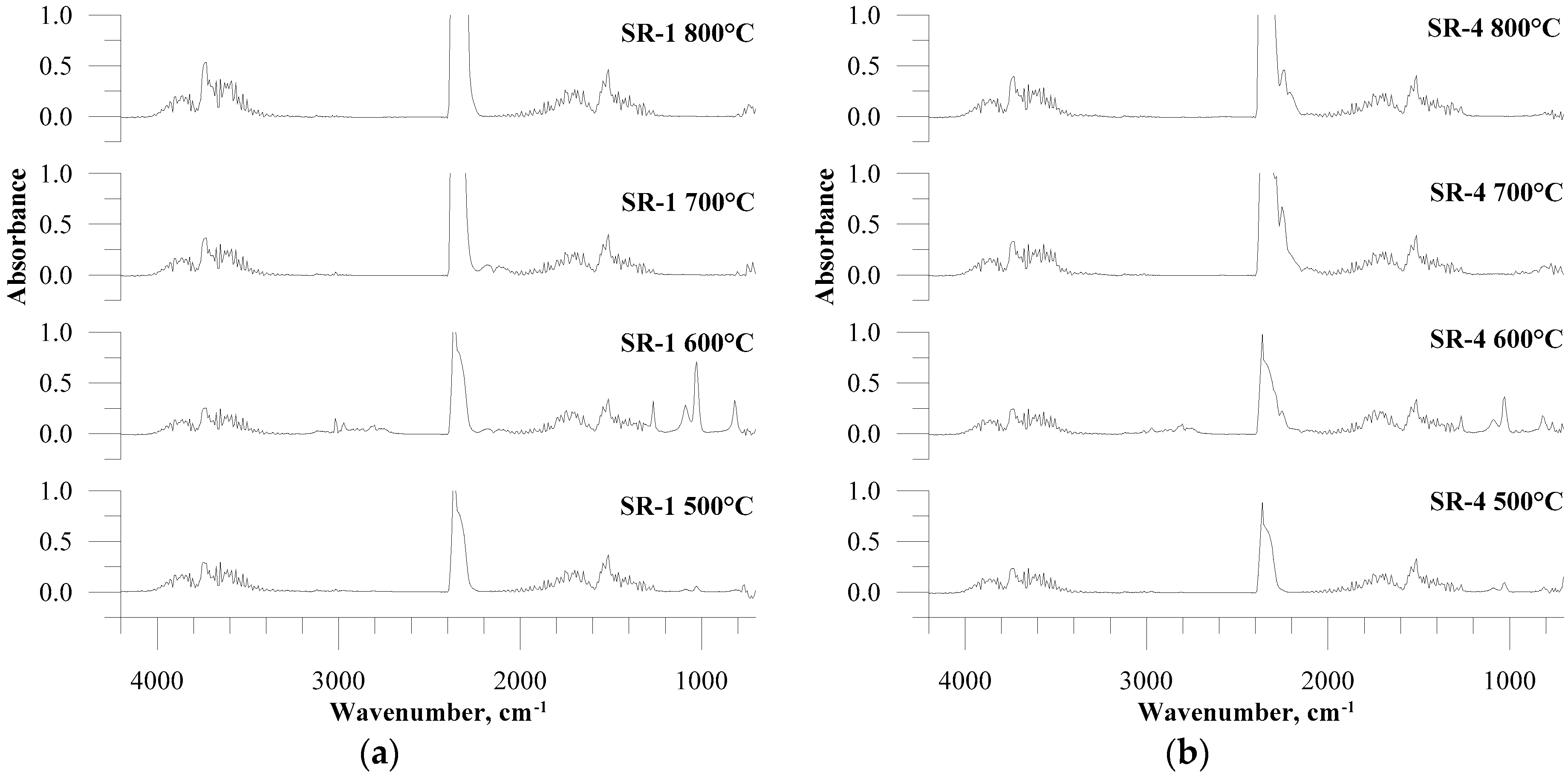
3.1. FTIR Spectra Analysis
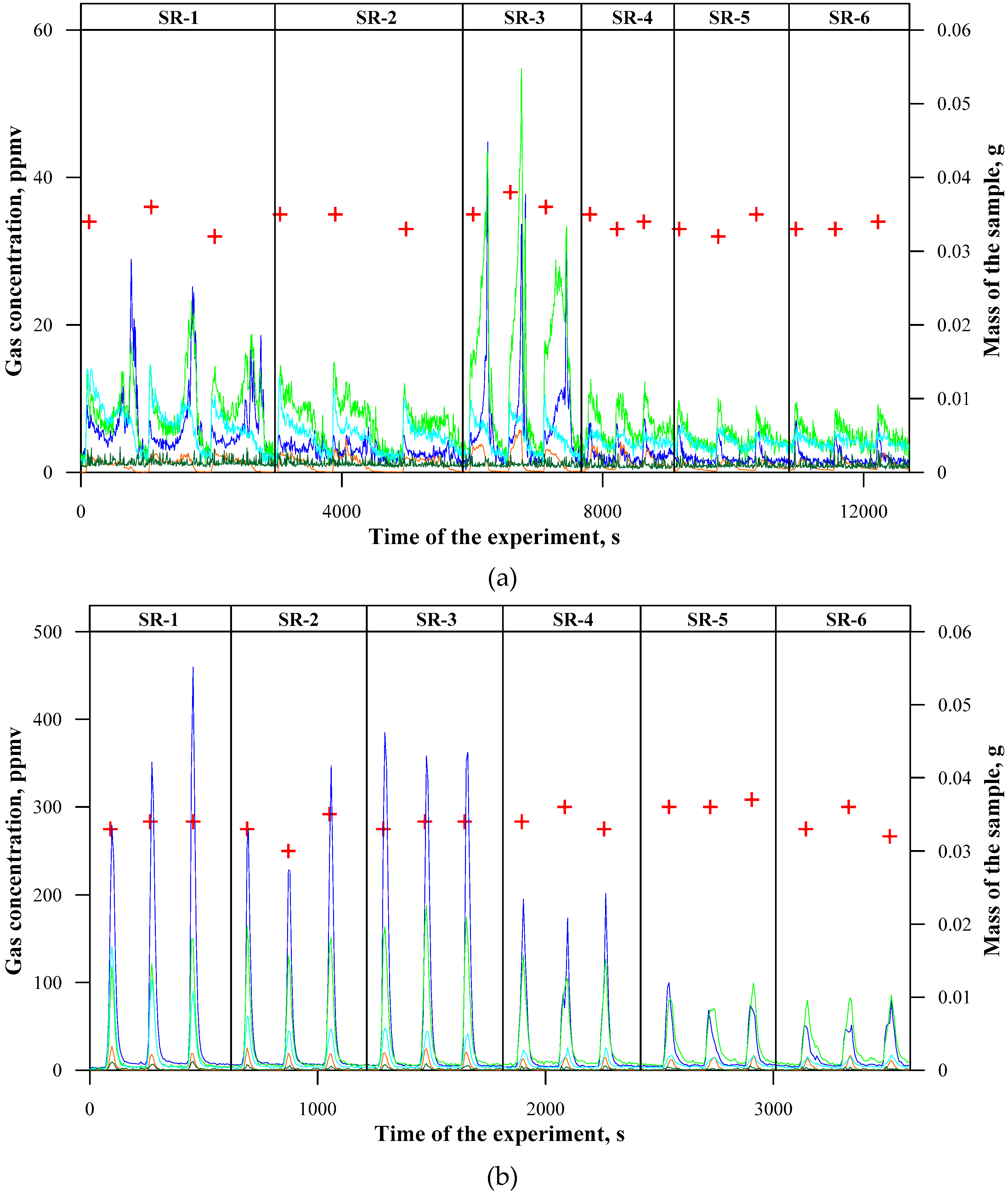
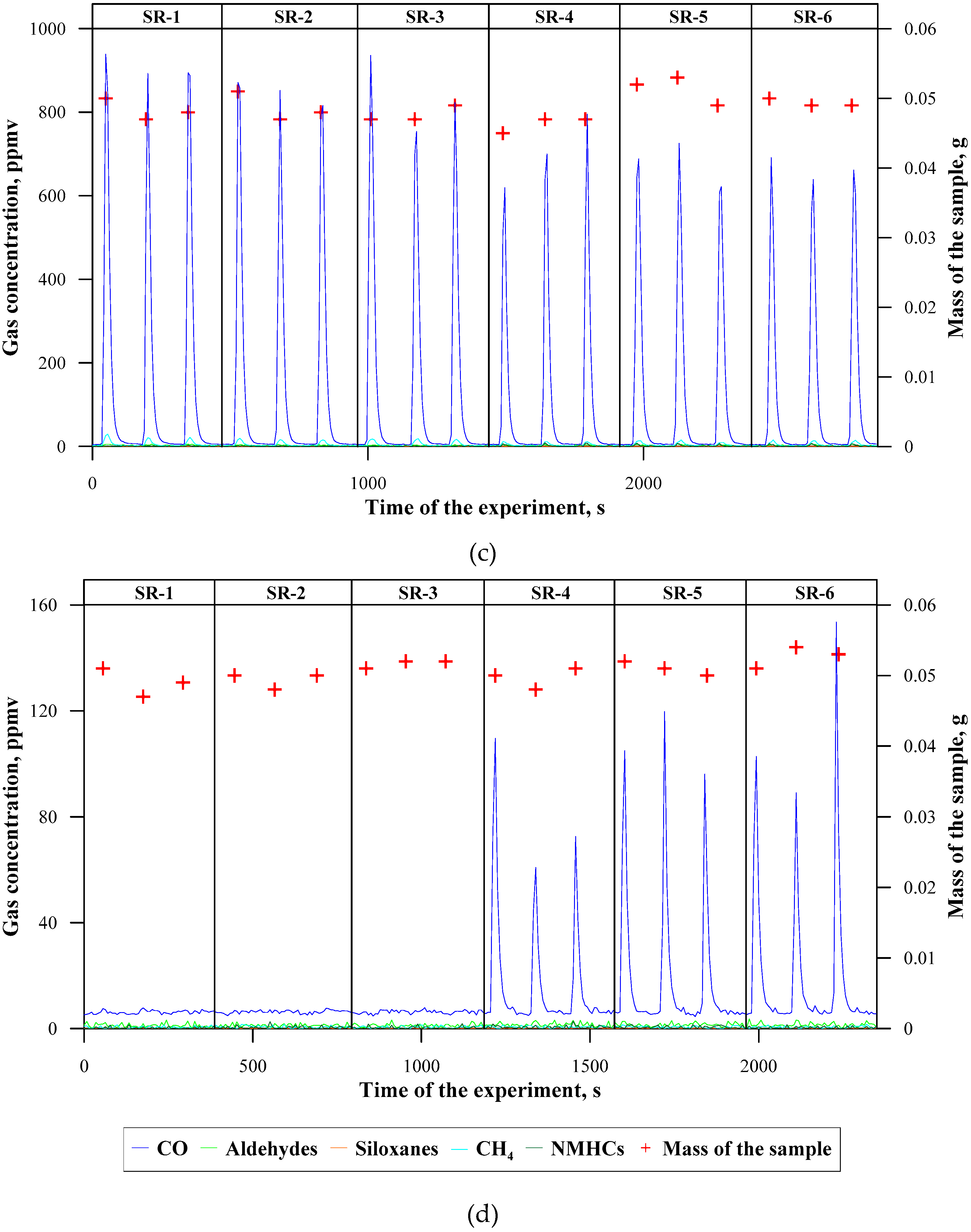
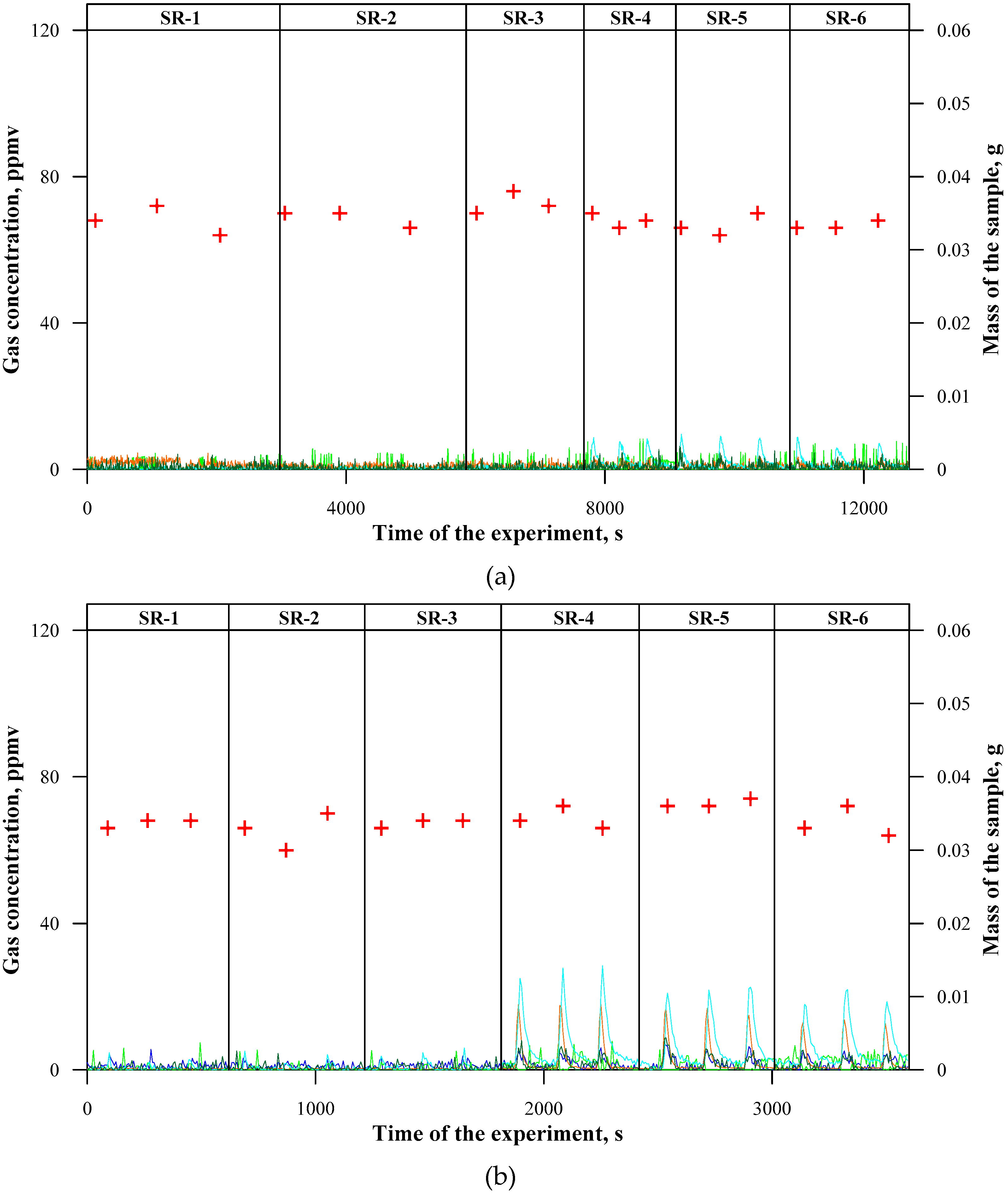
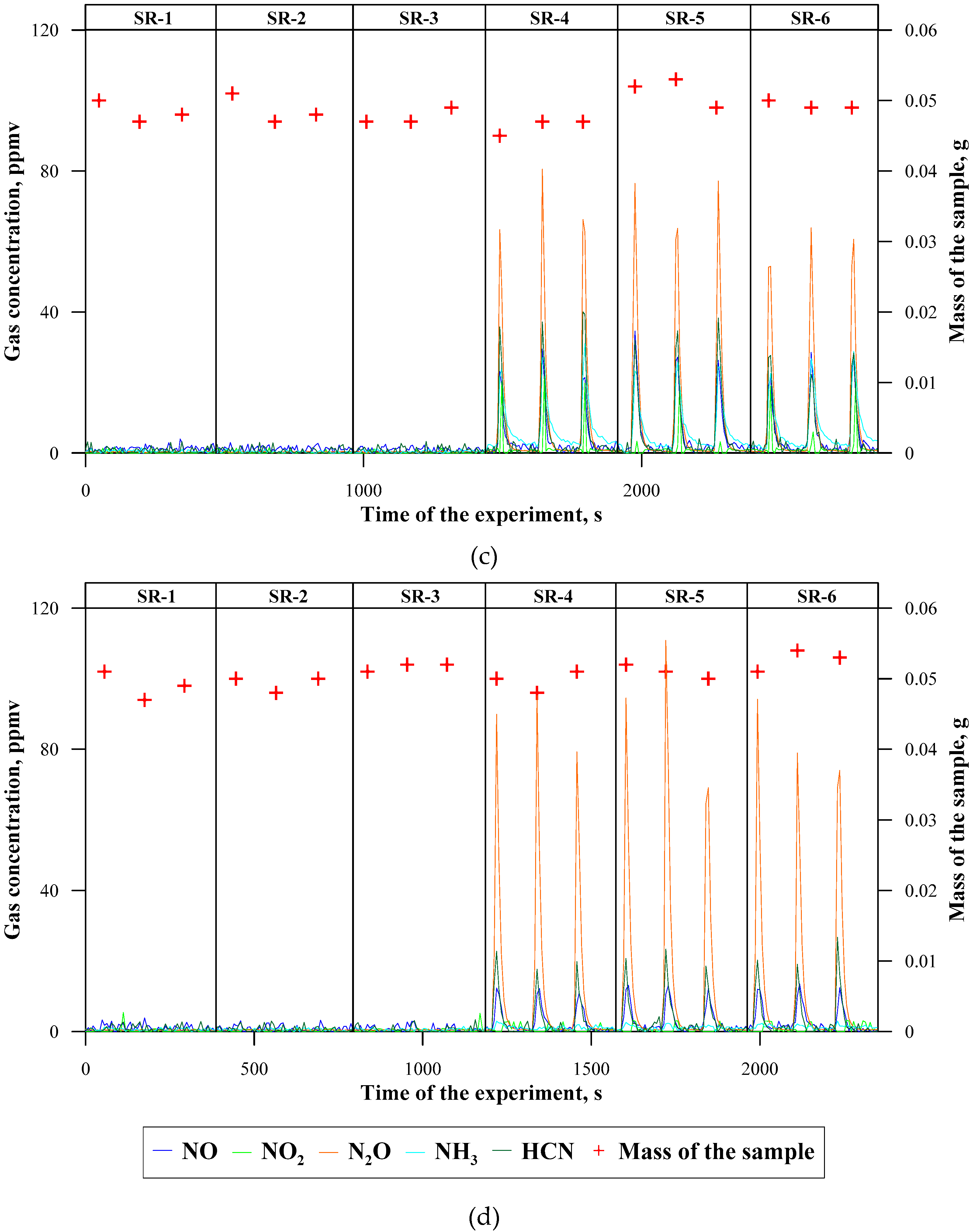
3.2. Concentrations of Emitted Gaseous Products
3.3. Balance of Thermal Degradation Process
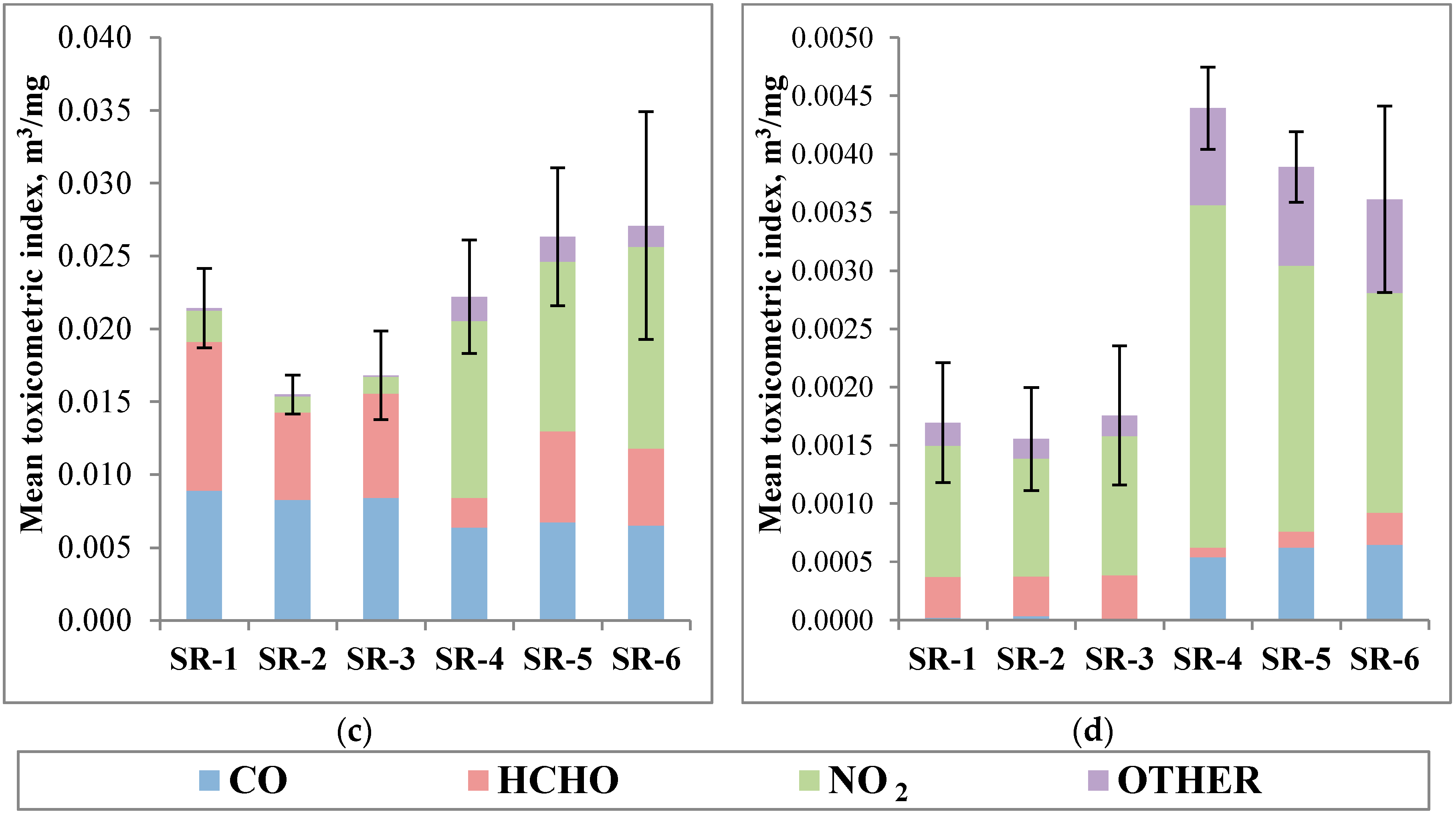
3.4. Toxicity Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stec, A.A. Fire toxicity—The elephant in the room? Fire Saf. J. 2017, 91, 79–90. [Google Scholar] [CrossRef]
- McKenna, S.T.; Birtles, R.; Dickens, K.; Walker, R.G.; Spearpoint, M.J.; Stec, A.A.; Hull, T.R. Flame retardants in UK furniture increase smoke toxicity more than they reduce fire growth rate. Chemosphere 2018, 196, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Giebułtowicz, J.; Rużycka, M.; Wroczyński, P.; Purser, D.A.; Stec, A.A. Analysis of fire deaths in Poland and influence of smoke toxicity. Forensic Sci. Int. 2017, 277, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Hartzell, G.E. Overview of combustion toxicology. Toxicology 1996, 115, 7–23. [Google Scholar] [CrossRef]
- Smith, S.M.; Stuhmiller, J.H.; Januszkiewicz, A.J. Evaluation of lethality estimates for combustion gases in military scenarios. Toxicology 1996, 115, 157–165. [Google Scholar] [CrossRef]
- Janowska, G.; Kucharska-Jastrząbek, A.; Rybiński, P.; Wesołek, D.; Wójcik, I. Flammability of diene rubbers. J. Therm. Anal. Calorim. 2010, 102, 1043–1049. [Google Scholar] [CrossRef]
- Rybiński, P.; Janowska, G.; Kucharska-Jastrzabek, A.; Pajak, A.; Wojcik, I.; Wesolek, D.; Bujnowicz, K. Flammability of vulcanizates of diene rubbers. J. Therm. Anal. Calorim. 2012, 107, 1219–1224. [Google Scholar] [CrossRef]
- Troitzsch, J. International Plastics Flammability Handbook, 2nd ed.; Hanser Publishers: Munich, Austria; Vienna, Germany; New York, NY, USA, 1990. [Google Scholar]
- Tsuchiya, Y.; Sumi, K. Evaluation of the toxicity of combustion products. J. Fire Flammabl. 1972, 3, 46–50. [Google Scholar]
- ISO 13344: 2015. Estimation of Lethal Toxic Potency of Fire Effluents; ISO: Geneva, Switzerlands, 2015. [Google Scholar]
- Huang, G.; Zou, Y.; Xiao, M.; Wang, S.; Luo, W.; Han, D.; Meng, Y. Thermal degradation of poly(Lactide-Co-Propylene carbonate) measured by TG/FTIR and Py-GC/MS. Polym. Degrad. Stab. 2015, 117, 16–21. [Google Scholar] [CrossRef]
- Luo, W.; Xiao, M.; Wang, S.; Ren, S.; Meng, Y. Thermal degradation behavior of Copoly(propylene carbonate ε-Caprolactone) investigated using TG/FTIR and Py-GC/MS methodologies. Polym. Test. 2017, 58, 13–20. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Zhao, W.; Zhou, J. TG–FTIR-MS study of pyrolysis products evolving from peat. J. Anal. Appl. Pyrolysis 2016, 117, 296–309. [Google Scholar] [CrossRef]
- Lin, Y.; Liao, Y.; Yu, Z.; Fang, S.; Ma, X. A study on Co-Pyrolysis of bagasse and sewage sludge using TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2017, 151, 190–198. [Google Scholar] [CrossRef]
- Fang, S.; Yu, Z.; Ma, X.; Lin, Y.; Lin, Y.; Chen, L.; Fan, Y.; Liao, Y. Co-Pyrolysis characters between combustible solid waste and paper mill sludge by TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2017, 144, 114–122. [Google Scholar] [CrossRef]
- Rybiński, P.; Bradło, D.; Żukowski, W.; Syrek, B. Determination of toxic products emissions of polymers thermal decomposition using fluidised bed reactor and FTIR analysis. Polym. Test. 2019, 79, 9. [Google Scholar] [CrossRef]
- Blomqvist, P. Emissions from Fires Consequences for Human Safety and the Environment; Lund University: Lund, Sweden, 2005. [Google Scholar]
- Truchot, B.; Fouillen, F.; Collet, S. An experimental evaluation of the toxic gas emission in case of vehicle fires. In Proceedings of the 7th International Symposium on Tunnel Safety and Security (ISTSS), Montreal, QC, Canada, 16–18 March 2016; pp. 419–429. [Google Scholar]
- Fiore, V.; Scalici, T.; Di Bella, G.; Valenza, A. A review on basalt fibre and its composites. Compos. Part B Eng. 2015, 74, 74–94. [Google Scholar] [CrossRef]
- Imiela, M.; Anyszka, R.; Bieliński, D.M.; Lipińska, M.; Rybiński, P.; Syrek, B. Synergistic effect of mica, glass frit, and melamine cyanurate for improving fire resistance of styrene-butadiene rubber composites destined for ceramizable coatings. Coatings 2019, 9, 170. [Google Scholar] [CrossRef]
- Lou, F.; Cheng, L.; Li, Q.; Wei, T.; Guan, X.; Guo, W. The combination of glass dust and glass fiber as fluxing agents for ceramifiable silicone rubber composites. RSC Adv. 2017, 7, 38805–38811. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ji, C.; Yan, Y.; Zhao, D.; Shi, L. Mechanical and ceramifiable properties of silicone rubber filled with different inorganic fillers. Polym. Degrad. Stab. 2015, 121, 149–156. [Google Scholar] [CrossRef]
- Anyszka, R.; Bieliński, D.M.; Pędzich, Z.; Parys, G.; Rybiński, P.; Zarzecka-Napierała, M.; Imiela, M.; Gozdek, T.; Siciński, M.; Okraska, M.; et al. Effect of mineral filler additives on flammability, processing and use of Silicone-Based ceramifiable composites. Polym. Bull. 2018, 75, 1731–1751. [Google Scholar] [CrossRef]
- Hu, S.; Chen, F.; Li, J.G.; Shen, Q.; Huang, Z.X.; Zhang, L.M. The ceramifying process and mechanical properties of silicone rubber/ammonium polyphosphate/aluminium hydroxide/mica composites. Polym. Degrad. Stab. 2016, 126, 196–203. [Google Scholar] [CrossRef]
- Imiela, M.; Anyszka, R.; Bieliński, D.M.; Pędzich, Z.; Zarzecka-Napierała, M.; Szumera, M. Effect of carbon fibers on thermal properties and mechanical strength of ceramizable composites based on silicone rubber. J. Therm. Anal. Calorim. 2016, 124, 197–203. [Google Scholar] [CrossRef]
- Rybiński, P.; Syrek, B.; Bradło, D.; Żukowski, W.; Anyszka, R.; Imiela, M. Influence of cenospheric fillers on the thermal properties, ceramisation and flammability of nitrile rubber composites. J. Compos. Mater. 2018, 52, 2815–2827. [Google Scholar] [CrossRef]
- Rybiński, P.; Syrek, B.; Żukowski, W.; Bradło, D.; Imiela, M.; Anyszka, R.; Blume, A.; Verbouwe, W. Impact of Basalt Filler on Thermal and Mechanical Properties, as Well as Fire Hazard, of Silicone Rubber Composites, Including Ceramizable Composites. Materials 2019, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Rybiński, P.; Żukowski, W.; Bradło, D. Effect of cenospheric fillers on the flammability and fire hazard of silicone rubber composites. J. Therm. Anal. Calorim. 2016, 125, 1373–1386. [Google Scholar] [CrossRef] [Green Version]
- Babrauskas, V.; Gann, R.G.; Levin, B.C.; Paabo, M.; Harris, R.H.; Peacock, R.D.; Yusa, S. A methodology for obtaining and using toxic potency data for fire hazard analysis. Fire Saf. J. 1998, 31, 345–358. [Google Scholar] [CrossRef]
- American Conference of Governmental Industrial Hygienists. TLVs and BEIs: Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices; ACGIH Worldwide: Cincinnati, OH, USA, 2017. [Google Scholar]


| Composite type | SR-1 | SR-2 | SR-3 | SR-4 | SR-5 | SR-6 |
|---|---|---|---|---|---|---|
| SR | 100 | 100 | 100 | 100 | 100 | 100 |
| DCP | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Silica | - | - | - | 15 | 15 | 15 |
| MC | - | - | - | 30 | 30 | 30 |
| CaCO3 | - | - | - | 15 | 15 | 15 |
| Glass frit | - | - | - | 30 | 30 | 30 |
| ZnB | - | - | - | 15 | 15 | 15 |
| BFL | - | 20 | - | - | 20 | - |
| BFS | - | - | 20 | - | - | 20 |
| %C | 34 | 28 | 28 | 22 | 20 | 20 |
| Temperature | 500 °C | 600 °C | 700 °C | 800 °C |
|---|---|---|---|---|
| SR-1 | 0.44 ± 0.02 | 0.57 ± 0.03 | 1.44 ± 0.03 | 1.81 ± 0.04 |
| SR-2 | 0.33 ± 0.02 | 0.47 ± 0.03 | 1.20 ± 0.09 | 1.52 ± 0.07 |
| SR-3 | 0.34 ± 0.02 | 0.48 ± 0.02 | 1.18 ± 0.03 | 1.47 ± 0.10 |
| SR-4 | 0.32 ± 0.02 | 0.35 ± 0.02 | 0.76 ± 0.06 | 1.11 ± 0.08 |
| SR-5 | 0.31 ± 0.01 | 0.43 ± 0.01 | 0.73 ± 0.02 | 1.06 ± 0.05 |
| SR-6 | 0.33 ± 0.01 | 0.38 ± 0.01 | 0.61 ± 0.01 | 0.98 ± 0.07 |
| H2O | CO2 | CO | NOx | CH4 | NMHCs * | Aldehydes | Siloxanes | NH3+HCN | |
|---|---|---|---|---|---|---|---|---|---|
| 500 °C | |||||||||
| SR-1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 16.7 ± 5.4 | 0.9 ± 1.0 | 9.6 ± 5.2 | 2.4 ± 2.0 | 29.5 ± 4.1 | 39.0 ± 11.3 | 1.8 ± 0.3 |
| SR-2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 6.0 ± 1.9 | 1.1 ± 1.2 | 7.1 ± 1.8 | 2.3 ± 1.1 | 29.7 ± 5.8 | 52.3 ± 6.3 | 1.7 ± 0.2 |
| SR-3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 15.1 ± 1.7 | 0.9 ± 0.7 | 4.3 ± 0.9 | 1.1 ± 1.2 | 40.5 ± 6.5 | 37.2 ± 6.6 | 1.5 ± 0.9 |
| SR-4 | 0.0 ± 0.0 | 57.7 ± 2.4 | 2.3 ± 0.4 | 5.5 ± 1.6 | 0.9 ± 0.5 | 1.2 ± 1.0 | 7.2 ± 1.3 | 21.4 ± 2.9 | 1.2 ± 0.5 |
| SR-5 | 0.0 ± 0.0 | 61.5 ± 2.6 | 2.5 ± 0.3 | 4.3 ± 1.0 | 2.7 ± 2.5 | 1.4 ± 1.4 | 6.6 ± 1.5 | 16.8 ± 3.4 | 1.4 ± 0.7 |
| SR-6 | 0.0 ± 0.0 | 60.6 ± 6.1 | 2.0 ± 0.7 | 5.1 ± 0.8 | 1.6 ± 0.9 | 2.0 ± 1.6 | 7.1 ± 2.1 | 18.0 ± 5.5 | 1.2 ± 0.4 |
| 600 °C | |||||||||
| SR-1 | 0.0 ± 0.0 | 37.8 ± 4.3 | 28.4 ± 4.6 | 0.1 ± 0.1 | 5.6 ± 3.1 | 1.0 ± 0.8 | 11.7 ± 1.2 | 15.2 ± 5.9 | 0.3 ± 0.2 |
| SR-2 | 0.0 ± 0.0 | 33.7 ± 3.0 | 26.1 ± 4.6 | 0.1 ± 0.1 | 3.4 ± 0.7 | 0.8 ± 0.6 | 17.0 ± 1.1 | 18.4 ± 2.3 | 0.4 ± 0.2 |
| SR-3 | 0.0 ± 0.0 | 18.0 ± 2.0 | 36.0 ± 3.4 | 0.2 ± 0.1 | 3.1 ± 0.2 | 0.9 ± 0.8 | 20.8 ± 2.7 | 20.4 ± 3.5 | 0.6 ± 0.2 |
| SR-4 | 0.0 ± 0.0 | 23.2 ± 1.5 | 25.5 ± 3.4 | 5.9 ± 1.3 | 2.0 ± 0.2 | 1.0 ± 0.7 | 20.8 ± 1.7 | 17.6 ± 2.4 | 4.1 ± 1.2 |
| SR-5 | 0.0 ± 0.0 | 47.9 ± 0.9 | 11.2 ± 3.7 | 3.8 ± 0.9 | 1.5 ± 0.4 | 0.6 ± 0.7 | 15.2 ± 1.3 | 17.0 ± 4.0 | 2.8 ± 0.6 |
| SR-6 | 0.0 ± 0.0 | 44.6 ± 1.9 | 11.4 ± 3.4 | 4.5 ± 1.0 | 1.6 ± 0.4 | 0.8 ± 0.6 | 16.6 ± 1.7 | 17.6 ± 3.9 | 2.8 ± 0.2 |
| 700 °C | |||||||||
| SR-1 | 35.8 ± 3.1 | 46.0 ± 2.1 | 17.7 ± 1.8 | 0.1 ± 0.0 | 0.3 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 |
| SR-2 | 36.1 ± 1.6 | 43.5 ± 1.6 | 19.8 ± 1.7 | 0.0 ± 0.0 | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 |
| SR-3 | 39.5 ± 1.9 | 39.5 ± 5.3 | 20.4 ± 3.4 | 0.1 ± 0.1 | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.1 | 0.0 ± 0.0 |
| SR-4 | 18.6 ± 2.3 | 50.2 ± 1.6 | 24.0 ± 3.2 | 4.7 ± 0.6 | 0.3 ± 0.1 | 0.2 ± 0.2 | 0.1 ± 0.1 | 0.0 ± 0.0 | 2.0 ± 0.3 |
| SR-5 | 23.4 ± 2.7 | 42.2 ± 2.2 | 26.3 ± 1.1 | 5.2 ± 0.6 | 0.3 ± 0.1 | 0.2 ± 0.2 | 0.4 ± 0.2 | 0.0 ± 0.1 | 2.0 ± 0.3 |
| SR-6 | 14.6 ± 2.2 | 46.3 ± 4.4 | 30.2 ± 1.6 | 5.7 ± 0.8 | 0.4 ± 0.0 | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.1 ± 0.1 | 2.0 ± 0.4 |
| 800 °C | |||||||||
| SR-1 | 32.3 ± 3.4 | 67.5 ± 3.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| SR-2 | 32.8 ± 1.2 | 67.0 ± 1.2 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| SR-3 | 28.2 ± 0.9 | 71.6 ± 0.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| SR-4 | 24.1 ± 2.9 | 71.4 ± 1.9 | 1.4 ± 0.8 | 2.6 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.1 |
| SR-5 | 27.8 ± 1.4 | 67.0 ± 1.3 | 1.7 ± 0.2 | 2.9 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.1 ± 0.2 | 0.0 ± 0.0 | 0.4 ± 0.0 |
| SR-6 | 21.9 ± 1.5 | 72.8 ± 2.2 | 1.9 ± 0.7 | 2.8 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.4 ± 0.1 |
| Temperature | 500 °C | 600 °C | 700 °C | 800 °C |
|---|---|---|---|---|
| SR-1 | 55.1 ± 1.4 | 62.8 ± 4.1 | 88.0 ± 6.3 | 100.0 ± 5.4 |
| SR-2 | 46.9 ± 2.7 | 61.3 ± 4.6 | 88.2 ± 3.9 | 99.7 ± 3.2 |
| SR-3 | 47.2 ± 1.3 | 66.8 ± 3.2 | 83.3 ± 1.8 | 102.6 ± 5.9 |
| SR-4 | 43.2 ± 3.1 | 54.7 ± 4.1 | 87.6 ± 8.6 | 105.2 ± 6.4 |
| SR-5 | 46.6 ± 1.5 | 68.4 ± 1.4 | 89.1 ± 1.7 | 103.9 ± 5.8 |
| SR-6 | 49.7 ± 1.5 | 61.8 ± 2.3 | 83.4 ± 1.9 | 104.9 ± 5.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybiński, P.; Syrek, B.; Żukowski, W.; Bradło, D. Impact of Basalt Filler and Ceramizable Additives on the Toxicity of Gaseous Products Emitted from Thermal Decomposition of Silicone Rubber Composites. Materials 2019, 12, 3478. https://doi.org/10.3390/ma12213478
Rybiński P, Syrek B, Żukowski W, Bradło D. Impact of Basalt Filler and Ceramizable Additives on the Toxicity of Gaseous Products Emitted from Thermal Decomposition of Silicone Rubber Composites. Materials. 2019; 12(21):3478. https://doi.org/10.3390/ma12213478
Chicago/Turabian StyleRybiński, Przemysław, Bartłomiej Syrek, Witold Żukowski, and Dariusz Bradło. 2019. "Impact of Basalt Filler and Ceramizable Additives on the Toxicity of Gaseous Products Emitted from Thermal Decomposition of Silicone Rubber Composites" Materials 12, no. 21: 3478. https://doi.org/10.3390/ma12213478





