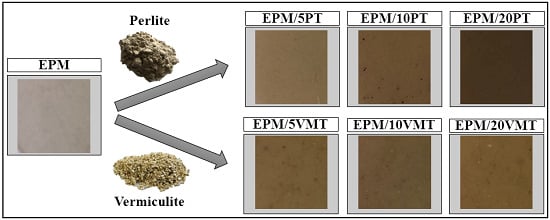
Characterization of Ethylene–propylene Composites Filled with Perlite and Vermiculite Minerals: Mechanical, Barrier, and Flammability Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation and Characterization Techniques
3. Results
3.1. Curing Measurements
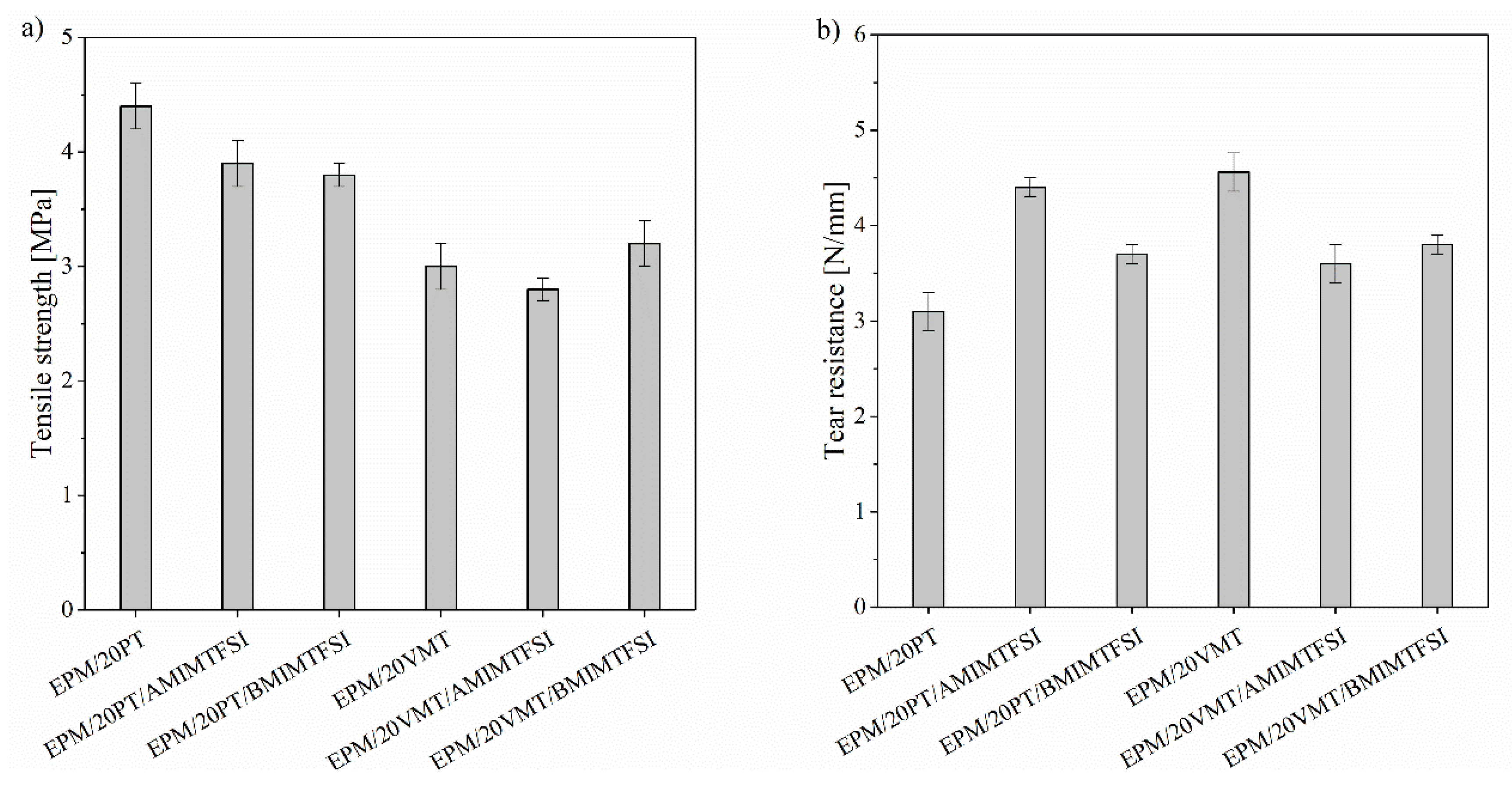
3.2. Mechanical Properties
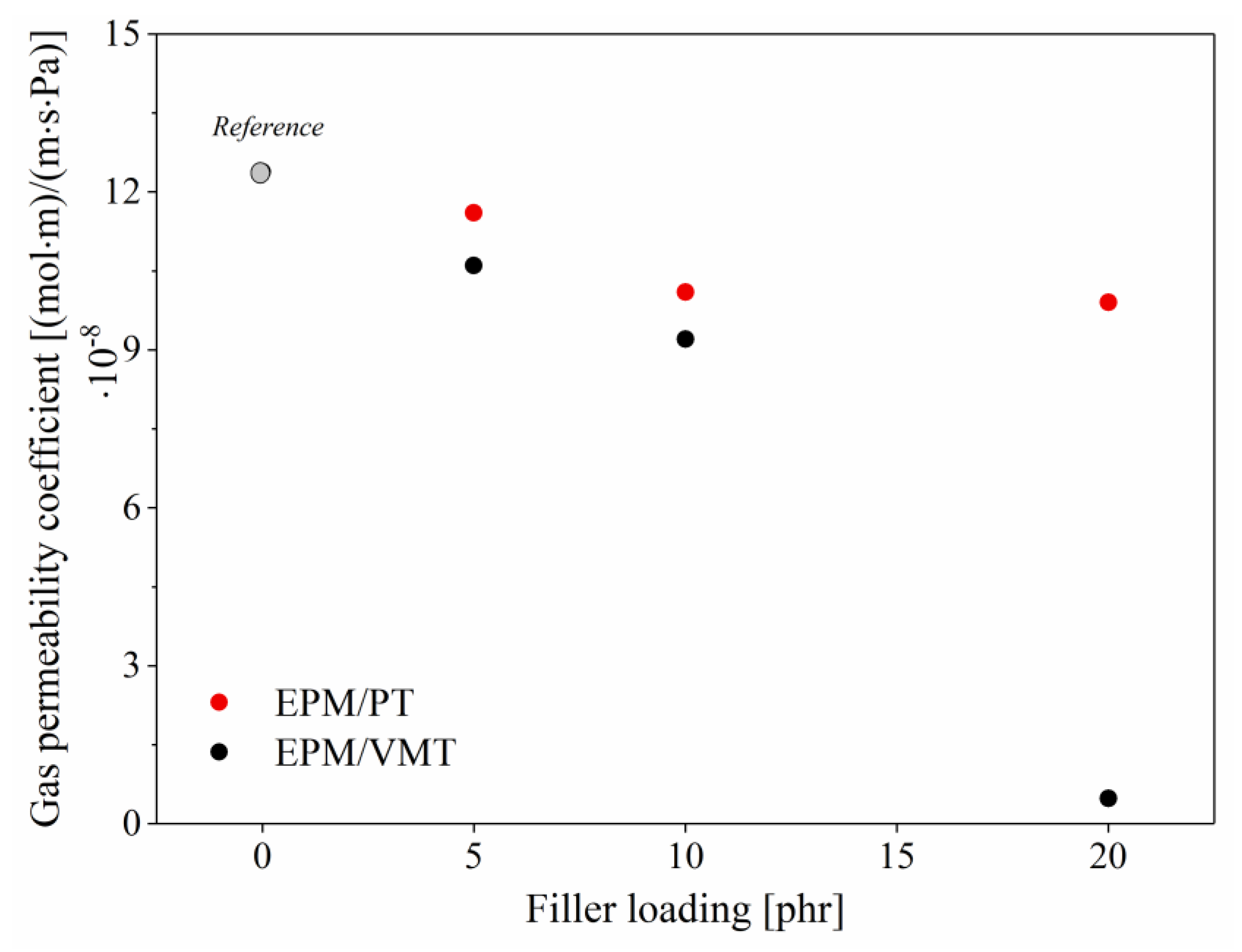
3.3. Barrier Performance
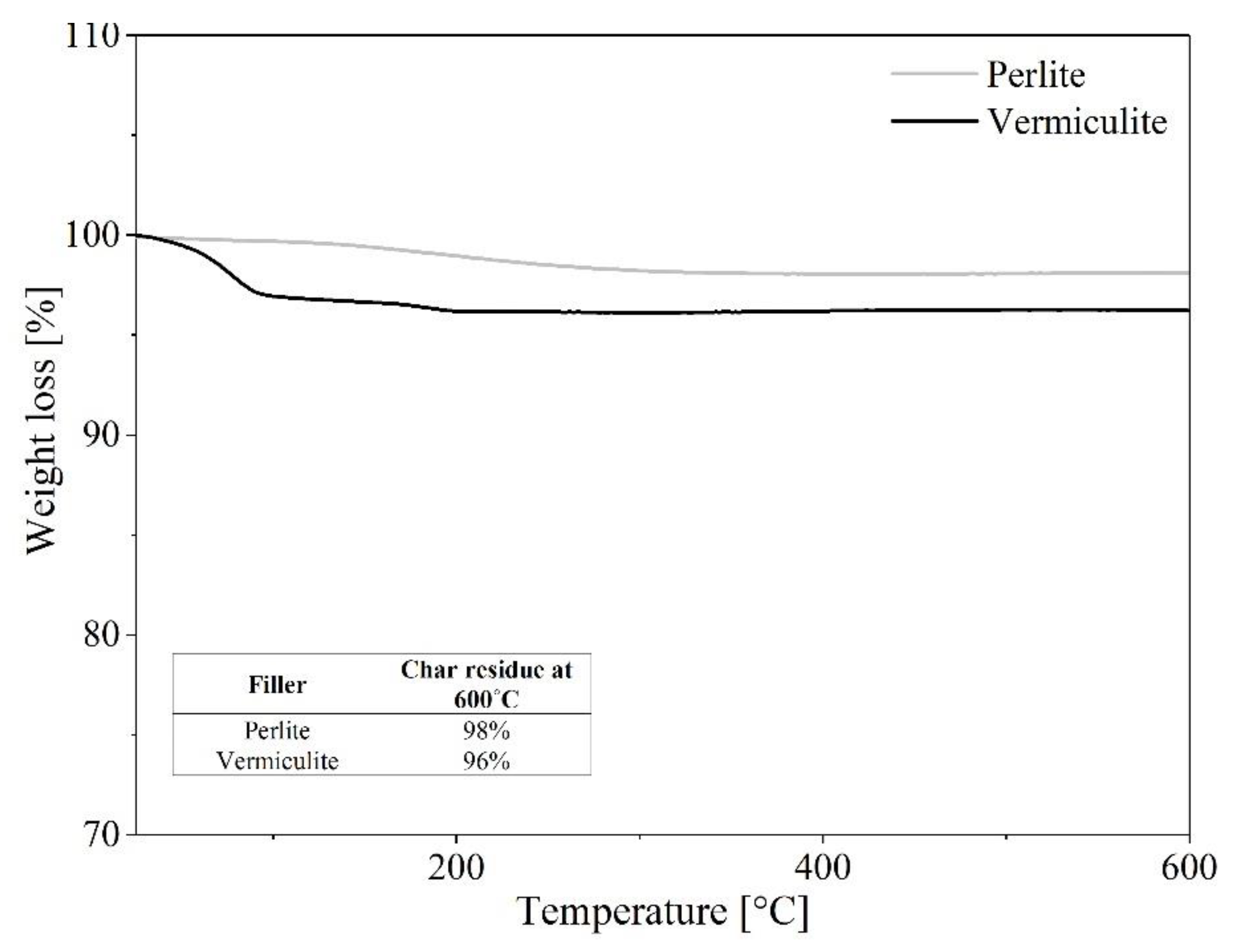
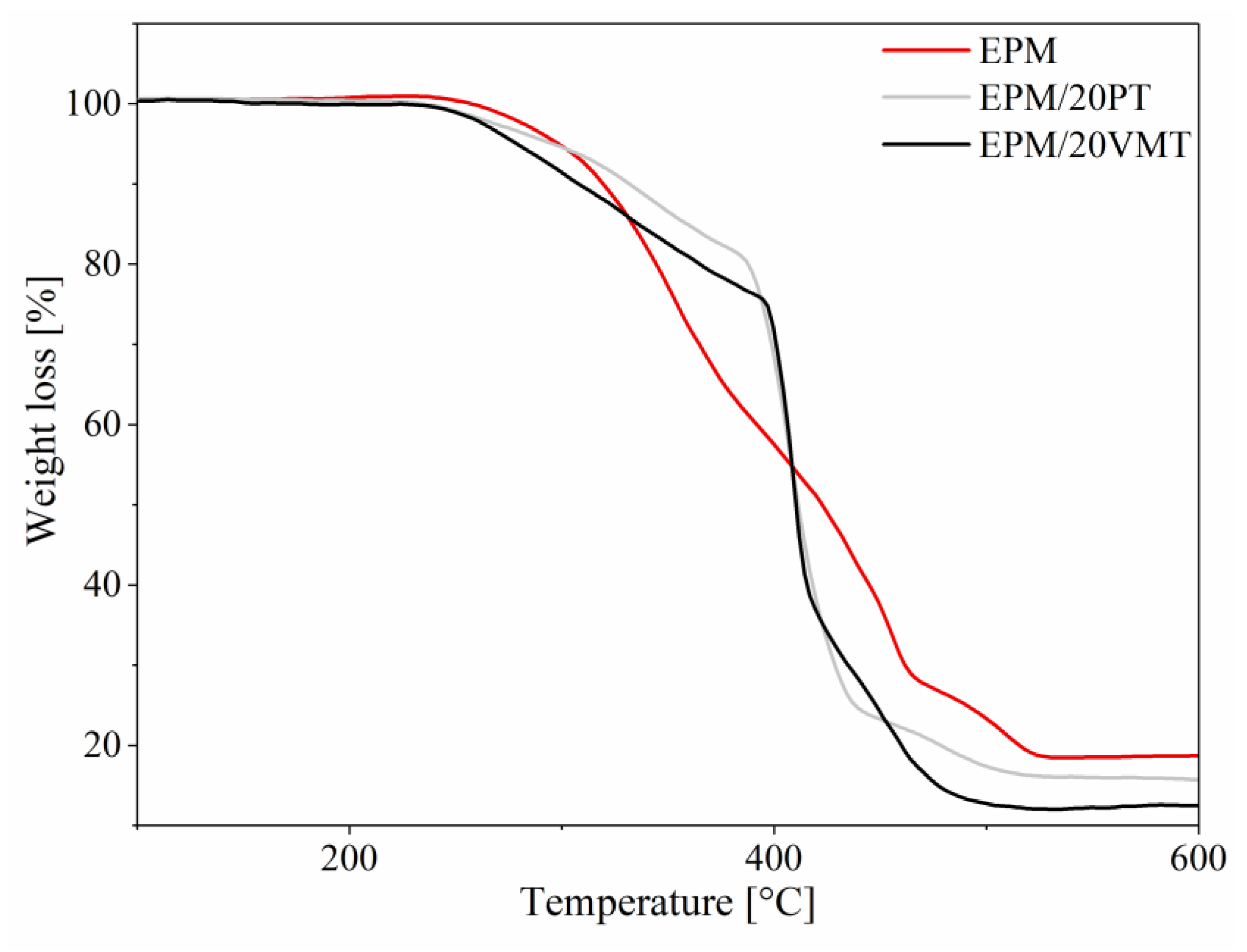
3.4. Thermal Stability
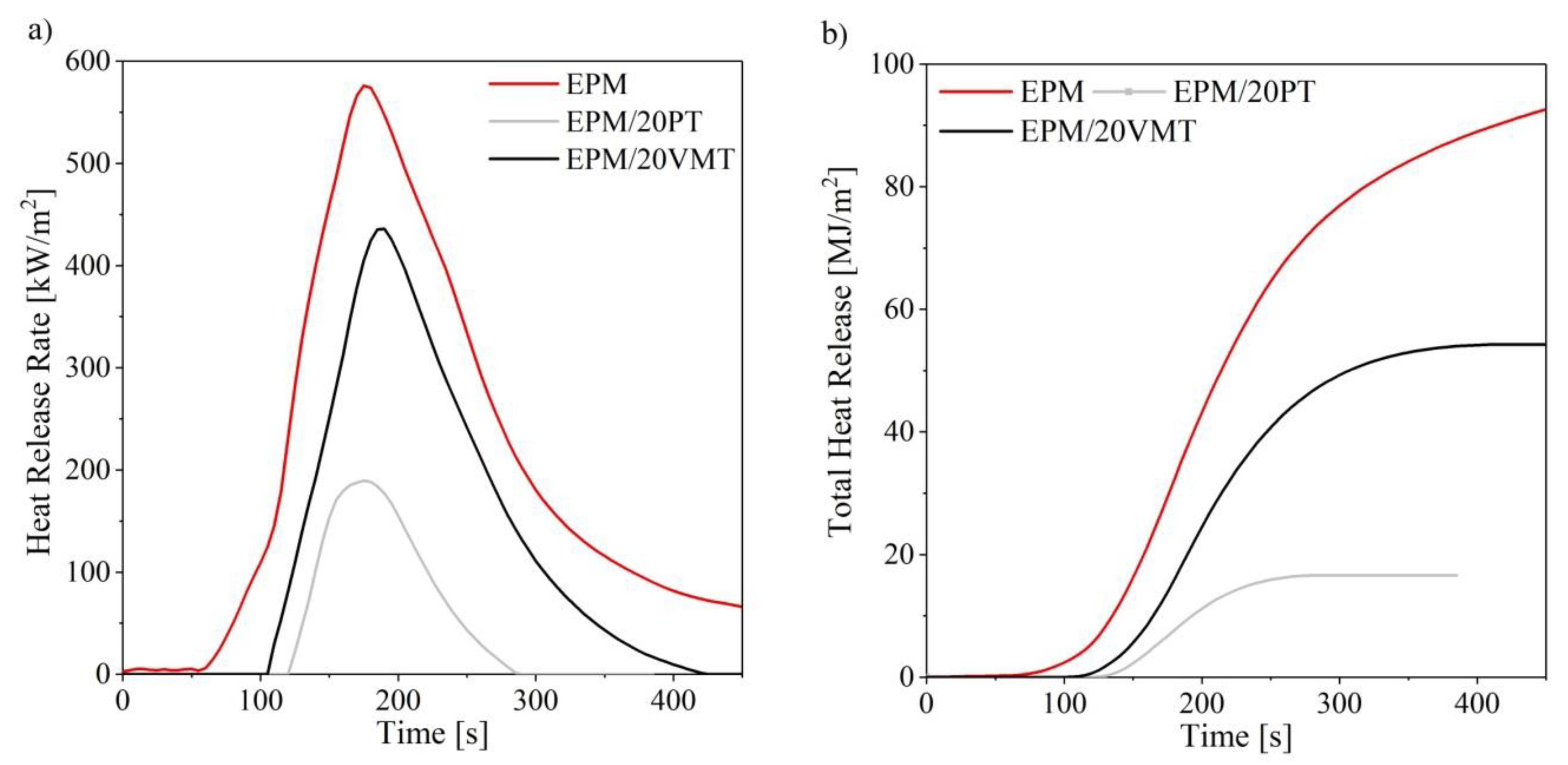
3.5. Flammability (Cone Calorimetry)
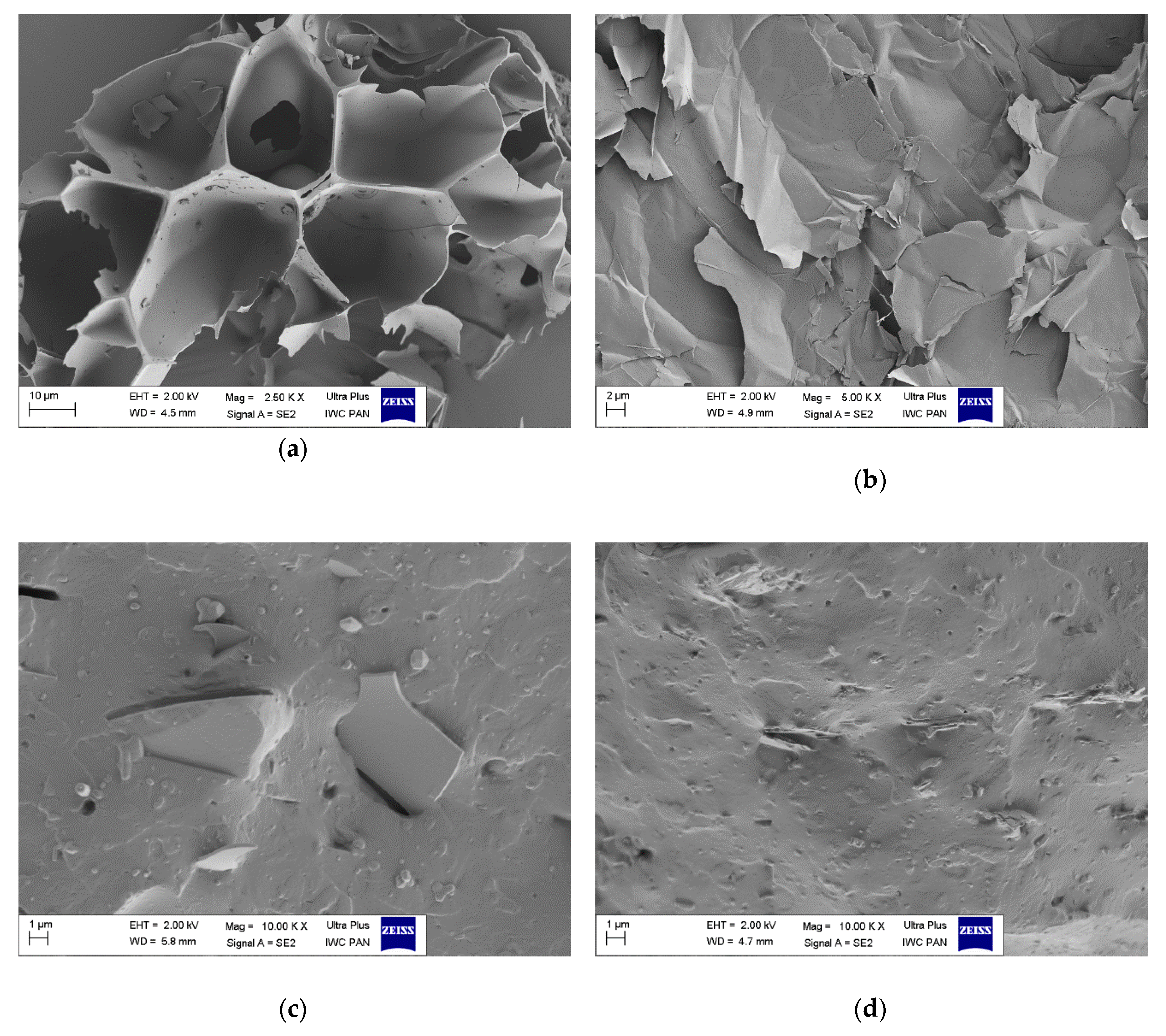
3.6. Morphology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hirano, K.; Asami, M. Phenolic resins—100 years of progress and their future. React. Funct. Polym. 2013, 73, 256–269. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Ahn, J.; Seo, M.; Nam, Y.S. Flame-retardant, flexible vermiculite–polymer hybrid film. RSC Adv. 2015, 5, 61768–61774. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Yang, H.; Lu, H.; Hu, Y. Synergistic effect of graphene on antidripping and fire resistance of intumescent FR poly (butylene succinate) composites. Ind. Eng. Chem. Res. 2011, 50, 5376–5383. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Tian, X.; Ding, X.; Xue, M.; Zhou, H.; Zheng, K. Mechanical and flame-retardant properties of styrene-ethylene-butylene-styrene/carbon nanotube composites containing bisphenol A bis (diphenyl phosphate). Compos. Sci. Technol. 2013, 82, 8–14. [Google Scholar] [CrossRef]
- Janas, D.; Rdest, M.; Koziol, K. Flame-retardant carbon nanotube films. Appl. Surf. Sci. 2017, 411, 177–181. [Google Scholar] [CrossRef]
- Becker, C.M.; Gabbardo, A.D.; Wypych, F.; Amico, S.C. Mechanical and flame retardant properties of epoxy/Mg-Al based LDH composites. Compos. Part A 2011, 42, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Olfs, H.W.; Torres-Dorante, L.O.; Eckelt, R.; Kosslick, H. Comparison of different synthesis routes for Mg-Al layered double hydroxides (LDH): Characterization of the structural phases and anion exchange properties. Appl. Clay Sci. 2009, 43, 459–464. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, Y.; Wang, S.; Gui, Z.; Chen, Z.; Fan, W. Intumescent flame retardant–montmorillonite synergism in polypropylene-layered silicate nanocomposites. Polym. Int. 2003, 52, 1396–1400. [Google Scholar] [CrossRef]
- Haurie, L.; Fernandez, A.I.; Velasco, J.I.; Chimenos, J.M.; Lopez Cuesta, J.-M.; Espiell, F. Thermal stability and flame retardancy of LDPE/EVA blends filled with synthetic hydromagnesite/aluminium hydroxide/montmorillonite and magnesium hydroxide/aluminium hydroxide/montmorillonite mixtures. Polym. Degrad. Stab. 2007, 92, 1082–1087. [Google Scholar] [CrossRef]
- Kader, M.A.; Kim, K.; Lee, Y.-S.; Nah, C. Preparation and properties of nitrile rubber/montmorillonite nanocomposites via latex blending. J. Mater. Sci. 2006, 41, 7341–7352. [Google Scholar] [CrossRef]
- Khobragade, P.S.; Hansora, D.P.; Naik, J.B.; Chatterjee, A. Flame retarding performance of elastomeric nanocomposites: A review. Polym. Degrad. Stab. 2016, 130, 192–244. [Google Scholar] [CrossRef]
- Talway, K.E. Chitosan phosphate: A new way for production of eco-friendly flame-retardant cotton textiles. J. Text. Inst. 2008, 99, 185–191. [Google Scholar]
- Jana, T.; Roy, B.C.; Maiti, S. Biodegradable film: 7. Modification of the biodegradable film for fire retardancy. Polym. Degrad. Stab. 2000, 69, 79–82. [Google Scholar] [CrossRef]
- Mishra, S.; Sonawane, S.H.; Singh, R.P.; Bendale, A.; Patil, K. Effect of nano-Mg(OH)2 on the mechanical and flame-retarding properties of polypropylene composites. J. Appl. Polym. Sci. 2004, 94, 116–122. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, Y.; Li, J.; Li, J.C. Synergistic effect of vermiculite on the intumescent flame retardance of polypropylene. J. Appl. Polym. Sci. 2010, 120, 1225–1233. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Z.; Zheng, M.; Sun, J. Thermal degradation and fire performance of plywood treated with expanded vermiculite. Fire Mater. 2016, 40, 427–433. [Google Scholar] [CrossRef]
- Park, Y.; Qian, Y.; Lindsay, C.; Nijs, C.; Camargo, R.; Stein, A.; Macosko, C. Polyol-assisted vermiculite dispersion in polyurethane nanocomposites. ACS Appl. Mater. Interfaces 2013, 5, 3054–3062. [Google Scholar] [CrossRef]
- Machado, L.C.R.; Lima, F.W.J.; Paniago, R.; Ardisson, J.D.; Sapag, K.; Lago, R.M. Polymer coated vermiculite–iron composites: Novel floatable magnetic adsorbents for water spilled contaminants. Appl. Clay Sci. 2006, 31, 207–215. [Google Scholar] [CrossRef]
- Suvorov, S.A.; Skurikhin, V.V. Vermiculite—A promising material for high-temperature heat insulators. Refract. Ind. Ceram. 2003, 44, 186–193. [Google Scholar] [CrossRef]
- Takahashi, S.; Goldberg, H.A.; Feeney, C.A.; Karim, D.P.; Farrell, M.; O’Leary, K.; Paul, D.R. Gas barrier properties of butyl rubber/vermiculite nanocomposite coatings. Polymer 2006, 47, 3083–3093. [Google Scholar] [CrossRef]
- Tjong, S.C.; Meng, Y.Z. Impact-modified polypropylene/vermiculite nanocomposites. J. Polym. Sci. Polym. Phys. 2003, 41, 2332–2341. [Google Scholar] [CrossRef]
- Chen, S.; Wang, B.; Kang, J.; Chen, J.; Gai, J.; Yang, L.; Cao, Y. Synergistic effect of organic vermiculite on the flame retardancy and thermal stability of intumescent polypropylene composites. J. Macromol. Sci. Part B Phys. 2014, 52, 1212–1225. [Google Scholar] [CrossRef]
- Qian, Y.; Lindsay, C.; Macosko, C.; Stein, A. Synthesis and Properties of Vermiculite-Reinforced Polyurethane Nanocomposites. ACS Appl. Mater. Interfaces 2011, 3, 3709–3717. [Google Scholar] [CrossRef] [PubMed]
- Papa, E.; Medri, V.; Murri, A.N.; Laghi, L.; De Aloysio, G.; Bandini, S.; Landi, E. Characterization of alkali bonded expanded perlite. Constr. Build. Mater. 2018, 191, 1139–1147. [Google Scholar] [CrossRef]
- Celik, A.G.; Kilic, A.M.; Cakal, G.O. Expanded perlite aggregate characterization for use as a lightweight construction raw material. Physicochem. Probl. Miner. Process. 2013, 49, 689–700. [Google Scholar]
- Sahraeian, R.; Hashemi, S.A.; Esfandeh, M.; Ghasemi, I. Preparation of nanocomposites based on LDPE/Perlite: Mechanical and Morphological Studies. Polym. Polym. Compos. 2012, 20, 639–645. [Google Scholar] [CrossRef]
- Sahraeian, R.; Esfandeh, M.; Hashemi, S.A. Rheological, thermal and dynamic mechanic studies of LDPE/Perlite nanocomposites. Polym. Polym. Compos. 2013, 21, 243–249. [Google Scholar]
- Karaca, E.; Omeroglu, S.; Akcam, O. Investigation of the effects of perlite additive on some comfort and acoustical properties of polyester fabrics. J. Appl. Polym. Sci. 2016, 133, 43128. [Google Scholar] [CrossRef]
- Marzec, A.; Laskowska, A.; Boiteux, G.; Zaborski, M.; Gain, O.; Serghei, A. Study on weather aging of nitrile rubber composites containing imidazolium ionic liquids. Macromol. Symp. 2014, 342, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Laskowska, A.; Marzec, A.; Boiteux, G.; Zaborski, M.; Gain, O.; Serghei, A. Investigations of nitrile rubber composites containing imidazolium ionic liquids. Macromol. Symp. 2014, 341, 18–25. [Google Scholar] [CrossRef]
- Marzec, A.; Laskowska, A.; Boiteux, G.; Zaborski, M.; Gain, O.; Serghei, A. Properties of carboxylated nitrile rubber/hydrotalcite composites containing imidazolium ionic liquids. Macromol. Symp. 2014, 341, 7–17. [Google Scholar] [CrossRef]
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Rybiński, P.; Maniukiewicz, W.; Zaborski, M. Aluminum-magnesium hydroxycarbonate/azo dye hybrids as novel multifunctional colorants for elastomer composites. Polymers 2019, 11, 43. [Google Scholar] [CrossRef] [Green Version]
- Bokobza, L.; Rapoport, O. Reinforcement of natural rubber. J. Appl. Polym. Sci. 2002, 85, 2301–2316. [Google Scholar] [CrossRef]
- Pingot, M.; Szadkowski, B.; Zaborski, M. Experimental investigation on activity of cumene hydroperoxide and selected ionic liquids in butadiene rubber vulcanization. Adv. Polym. Technol. 2018, 37, 3432–3437. [Google Scholar] [CrossRef]
- Tjong, S.C.; Meng, Y.Z.; Hay, A.S. Novel preparation and properties of polypropylene−vermiculite Nanocomposites. Chem. Mater. 2002, 14, 44–51. [Google Scholar] [CrossRef]
- Laskowska, A.; Zaborski, M.; Boiteux, G.; Gain, O.; Marzec, A.; Maniukiewicz, W. Ionic elastomers based on carboxylated nitrile rubber (XNBR) and magnesium aluminum layered double hydroxide (hydrotalcite). Express Polym. Lett. 2014, 8, 374–386. [Google Scholar] [CrossRef]
- Gatos, K.; Karger-Kocsis, J. Effect of the aspect ratio of silicate platelets on the mechanical and barrier properties of hydrogenated acrylonitrile butadiene rubber (HNBR)/layered silicate nanocomposites. Eur. Polym. J. 2007, 43, 1097–1104. [Google Scholar] [CrossRef]
- Subramaniam, K.; Das, A.; Heinrich, G. Development of conducting poly-chloroprene rubber using imidazolium based ionic liquid modified multi-walled carbon nanotubes. Compos. Sci. Technol. 2011, 71, 1441–1449. [Google Scholar] [CrossRef] [Green Version]
- Kalendova, A.; Merinska, D.; Gerard, J.F.; Slouf, M. Polymer/clay nanocomposites and their gas barrier properties. Polym. Compos. 2013, 34, 1418–1424. [Google Scholar] [CrossRef]
- Yano, K.; Usuki, A.; Okada, A.; Kurauchi, T.; Kamigaito, O. Synthesis and properties of polyimide–clay hybrid. J. Polym. Sci. Polym. Chem. 1993, 31, 2493–2498. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Zhang, Q.; Lu, Y. Gas barrier properties of natural rubber/kaolin composites prepared by melt blending. Appl. Clay Sci. 2010, 50, 255–259. [Google Scholar] [CrossRef]
- Folorunso, O.; Dodds, C.; Dimitrakis, G.; Kingman, S. Continuous energy efficient exfoliation of vermiculite through microwave heating. Int. J. Miner. Process. 2012, 114–117, 69–79. [Google Scholar] [CrossRef]
- Babrauskas, V.; Grayson, J.S. Heat Release in Fires; Elsevier Applied Science: London, UK, 1992; pp. 61–173. [Google Scholar]
- Grexa, O.; Lübke, H. Flammability parameters of wood tested on a cone calorimeter. Polym. Degrad. Stab. 2001, 74, 427–432. [Google Scholar] [CrossRef]
- Subramaniam, K.; Das, A.; Steinhauser, D.; Klüppel, M.; Heinrich, G. Effect of ionic liquid on dielectric, mechanical and dynamic mechanical properties of multi-walled carbon nanotubes/polychloroprene rubber composites. Eur. Polym. J. 2011, 47, 2234–2243. [Google Scholar] [CrossRef]


| Compound | Neat EPM | DCP | TTT | Perlite | Vermiculite | AMIMTFSI | BMIMTFSI |
|---|---|---|---|---|---|---|---|
| EPM | 100 | 1 | 0.25 | 0 | 0 | 0 | 0 |
| EPM/5PT | 100 | 1 | 0.25 | 5 | 0 | 0 | 0 |
| EPM/10PT | 100 | 1 | 0.25 | 10 | 0 | 0 | 0 |
| EPM/20PT | 100 | 1 | 0.25 | 20 | 0 | 0 | 0 |
| EPM/20PT/AMIMTFSI | 100 | 1 | 0.25 | 20 | 0 | 2.5 | 0 |
| EPM/20PT/BMIMTFSI | 100 | 1 | 0.25 | 20 | 0 | 0 | 2.5 |
| EPM/5VMT | 100 | 1 | 0.25 | 0 | 5 | 0 | 0 |
| EPM/10VMT | 100 | 1 | 0.25 | 0 | 10 | 0 | 0 |
| EPM/20VMT | 100 | 1 | 0.25 | 0 | 20 | 0 | 0 |
| EPM/20VMT/AMIMTFSI | 100 | 1 | 0.25 | 0 | 20 | 2.5 | 0 |
| EPM/20VMT/BMIMTFSI | 100 | 1 | 0.25 | 0 | 20 | 0 | 2.5 |
| Compound | Mmin (dNm) | ΔM (dNm) | t02 (min) | t90 (min) | υe (mol/cm3) |
|---|---|---|---|---|---|
| EPM | 0.81 | 4.80 | 1.07 | 18.84 | 3.4 |
| EPM/5PT | 0.91 | 5.41 | 1.09 | 17.29 | 4.5 |
| EPM/10PT | 0.98 | 6.08 | 1.03 | 17.20 | 5.3 |
| EPM/20PT | 1.14 | 7.19 | 0.91 | 15.76 | 5.9 |
| EPM/20PT/AMIMTFSI | 0.96 | 5.37 | 0.83 | 14.16 | 5.0 |
| EPM/20PT/BMIMTFSI | 0.97 | 5.42 | 0.90 | 15.28 | 4.8 |
| EPM/5VMT | 0.87 | 4.83 | 1.16 | 14.76 | 3.5 |
| EPM/10VMT | 0.93 | 4.86 | 1.03 | 13.29 | 3.6 |
| EPM/20VMT | 0.99 | 4.45 | 1.06 | 12.76 | 2.8 |
| EPM/20VMT/AMIMTFSI | 0.96 | 5.90 | 1.01 | 11.57 | 4.3 |
| EPM/20VMT/BMIMTFSI | 0.96 | 5.62 | 1.02 | 11.58 | 4.9 |
| Compound | T5 (°C) | T50 (°C) | TRMAX1 (°C) | TRMAX2 (°C) | dm/dt1 (%/min) | dm/dt2 (%/min) | P600 (%) |
|---|---|---|---|---|---|---|---|
| EPM | 298 | 423 | 350 | 442 | 4.85 | 5.36 | 18.82 |
| EPM/5PT | 271 | 400 | 300 | 403 | 1.95 | 23.53 | 1.17 |
| EPM/10PT | 272 | 405 | 305 | 401 | 1.38 | 17.3 | 6.63 |
| EPM/20PT | 278 | 410 | 270 | 407 | 1.50 | 34.76 | 12.61 |
| EPM/5VMT | 288 | 405 | 292 | 398 | 1.33 | 30.34 | 10.42 |
| EPM/10VMT | 262 | 404 | 260 | 402 | 1.24 | 39.71 | 4.18 |
| EPM/20VMT | 296 | 410 | 261 | 403 | 0.93 | 20.16 | 15.65 |
| Compound | TTI (s) | HRR (kW/m2) | HRRmax (kW/m2) | THR (MJ/m2) | EHC (MJ/kg) | EHCmax (MJ/kg) | MLR (g/m2∙s) |
|---|---|---|---|---|---|---|---|
| EPM | 70 | 202.7 | 576.0 | 92.9 | 51.0 | 79.0 | 0.206 |
| EPM/5PT | 68 | 204.1 | 445.9 | 57.7 | 31.8 | 77.5 | 0.257 |
| EPM/10PT | 70 | 195.5 | 390.5 | 49.8 | 38.1 | 79.2 | 0.212 |
| EPM/20PT | 90 | 66.34 | 189.7 | 16.6 | 10.4 | 64.2 | 0.224 |
| EPM/20PT/AMIMTFSI | 120 | 61.23 | 179.3 | 17.0 | 8.9 | 62.1 | 0.200 |
| EPM/20PT/BMIMTFSI | 110 | 63.52 | 170.2 | 16.2 | 9.3 | 60.2 | 0.265 |
| EPM/5VMT | 70 | 185.3 | 560.1 | 70.1 | 46.6 | 72.1 | 0.211 |
| EPM/10VMT | 73 | 171.8 | 529.6 | 47.9 | 75.8 | 78.2 | 0.014 |
| EPM/20VMT | 80 | 177.3 | 435.8 | 53.9 | 29.8 | 77.2 | 0.023 |
| EPM/20VMT/AMIMTFSI | 105 | 145.7 | 401.6 | 51.5 | 26.9 | 74.2 | 0.245 |
| EPM/20VMT/BMIMTFSI | 75 | 120.5 | 380.5 | 49.7 | 21.3 | 69.5 | 0.213 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szadkowski, B.; Marzec, A.; Rybiński, P.; Żukowski, W.; Zaborski, M. Characterization of Ethylene–propylene Composites Filled with Perlite and Vermiculite Minerals: Mechanical, Barrier, and Flammability Properties. Materials 2020, 13, 585. https://doi.org/10.3390/ma13030585
Szadkowski B, Marzec A, Rybiński P, Żukowski W, Zaborski M. Characterization of Ethylene–propylene Composites Filled with Perlite and Vermiculite Minerals: Mechanical, Barrier, and Flammability Properties. Materials. 2020; 13(3):585. https://doi.org/10.3390/ma13030585
Chicago/Turabian StyleSzadkowski, Bolesław, Anna Marzec, Przemysław Rybiński, Witold Żukowski, and Marian Zaborski. 2020. "Characterization of Ethylene–propylene Composites Filled with Perlite and Vermiculite Minerals: Mechanical, Barrier, and Flammability Properties" Materials 13, no. 3: 585. https://doi.org/10.3390/ma13030585