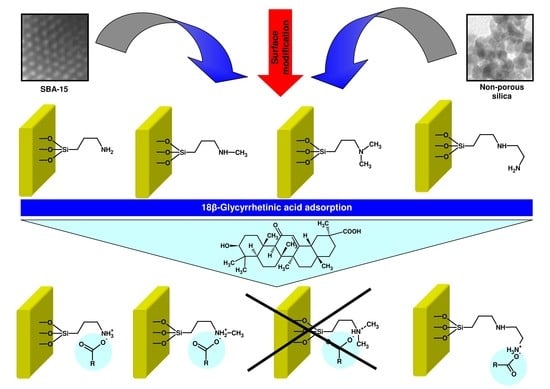
The Effect of SBA-15 Surface Modification on the Process of 18β-Glycyrrhetinic Acid Adsorption: Modeling of Experimental Adsorption Isotherm Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of SBA-15 Silica
2.3. Modification of Siliceous Adsorbents
2.4. Adsorption Studies
2.5. Adsorption Modeling
2.6. Characterization Methods
3. Results and Discussion
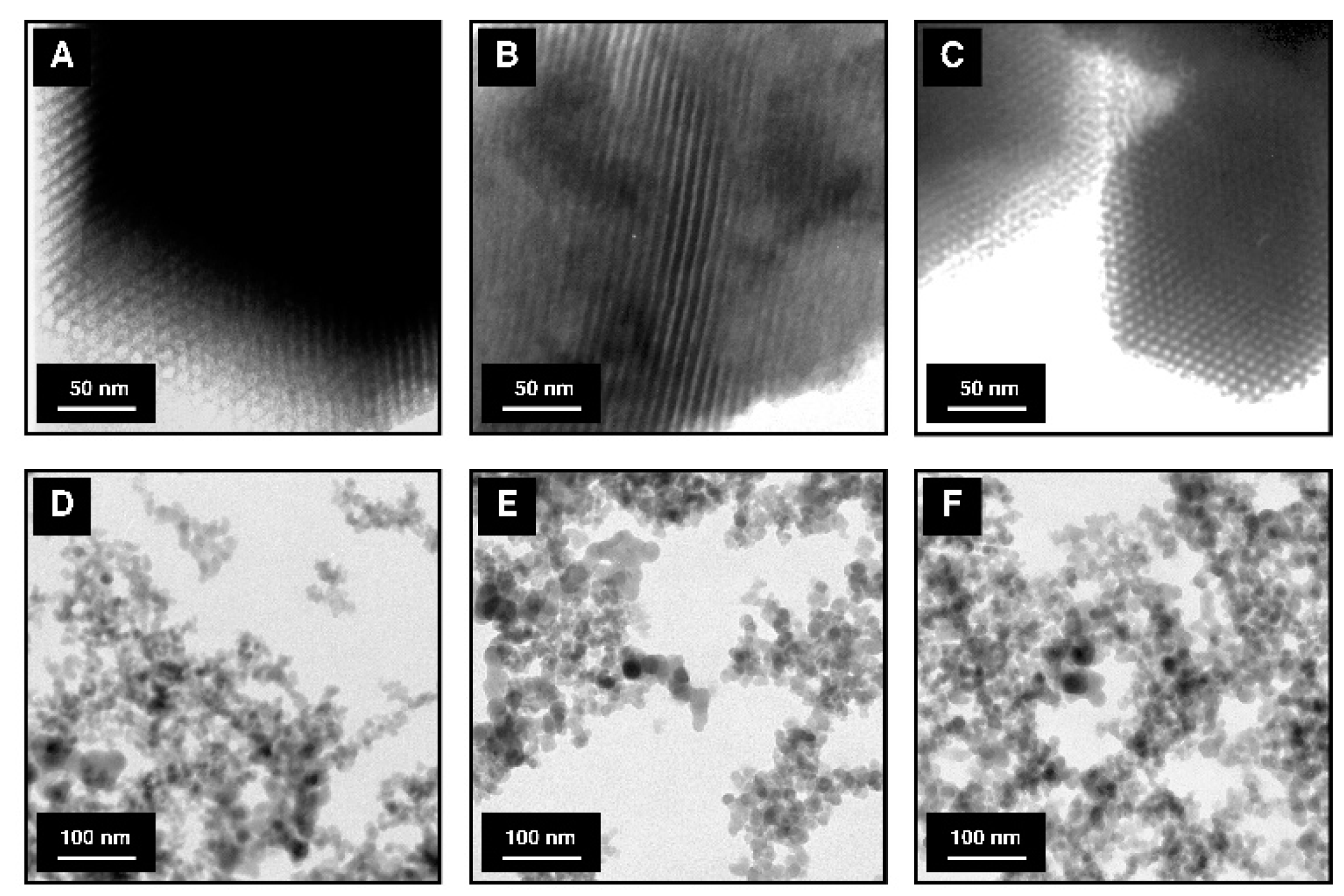
3.1. Characterization of the Adsorbents
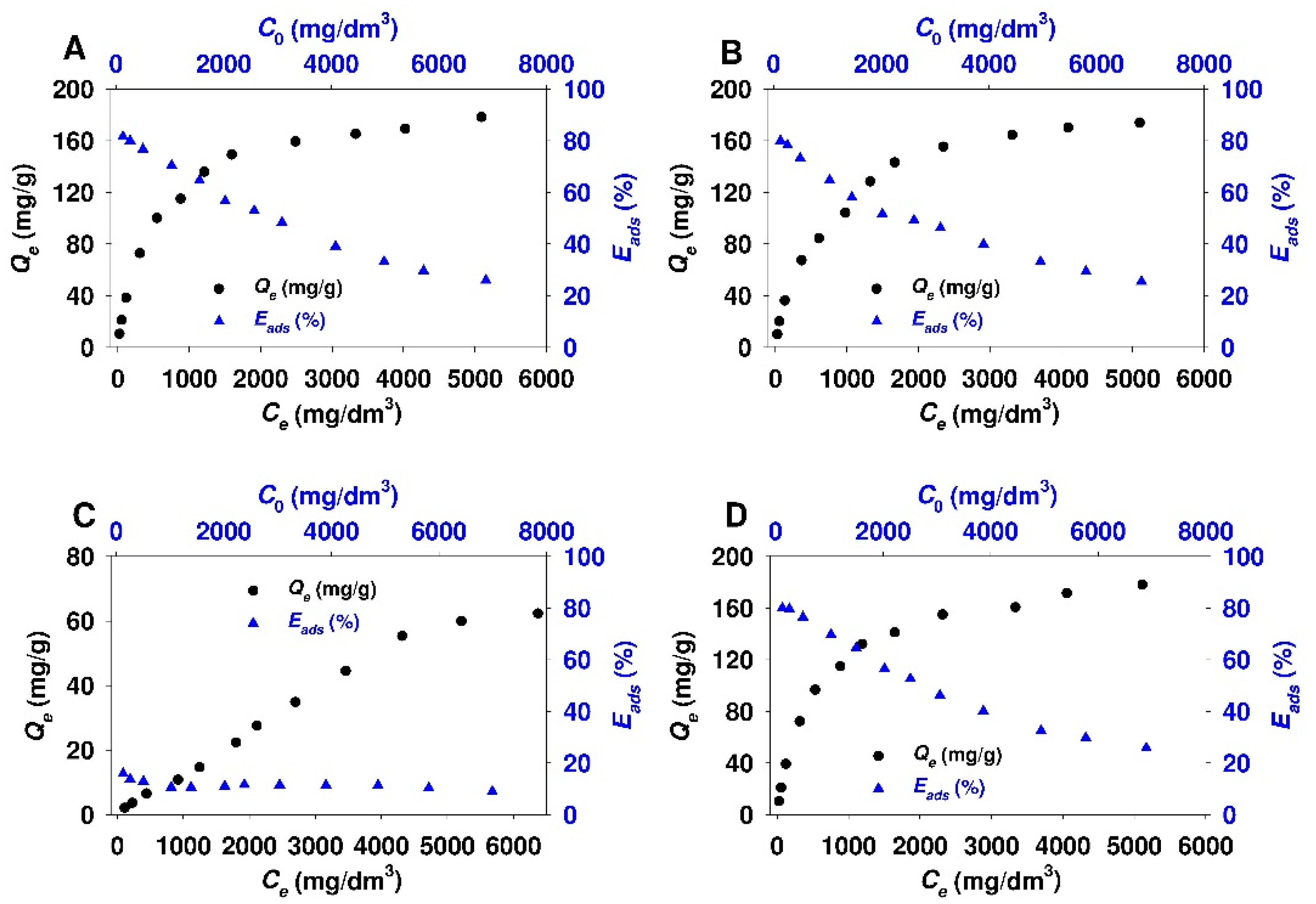
3.2. Adsorption Studies
3.3. Estimation of Isotherm Parameters Using Linear Regression
3.4. Estimation of Isotherm Parameters Using Nonlinear Fitting Analysis
4. Conclusions
- The adsorption isotherms of 18β-GA onto silicas functionalized with APTMS, MAPTMS and AEAPTMS indicate the Langmuir-type adsorption, whereas sorbents that were modified with DMAPTMS show constant distribution of the adsorbate between the adsorbent and the solution regardless of silica type.
- The Dubinin–Astakhov, Dubinin–Radushkevich, and Redlich–Peterson equations described the best the process of 18β-GA adsorption onto SBA-15 and Aerosil® silicas functionalized with APTMS, MAPTMS, and AEAPTMS regardless of the method used for estimation of isotherm parameters (linear regression or nonlinear fitting analysis).
- Based on nonlinear fitting analysis (Dubinin–Astakhov model), it can be concluded that SBA-15 sorbent modified with APTMS, MAPTMS, and AEAPTMS is characterized by twice the adsorption capacity (202.8–237.3 mg/g) as compared to functionalized Aerosil® (118.2–144.2 mg/g).
- The process of 18β-GA adsorption onto SBA-15 and Aerosil® silicas that were modified with DMAPTMS is best described by the Freundlich model.
- The Temkin isotherm is not suitable for the description of 18β-GA adsorption onto any of the used sorbents, owing to low r2 values (linear regression) or high values of MPSD error function (nonlinear fitting analysis).
- The values of mean adsorption energy (Dubinin–Astakhov model) and analysis of FT-IR spectra revealed the chemical nature of interactions between 18β-GA and siliceous surface modified with APTMS, MAPTMS, and AEAPTMS, meanwhile the adsorption of 18β-GA onto silicas that were modified with DMAPTMS has a physical nature (Dubinin-Radushkevich model).
- Higher values of molar ratio of the adsorbate to the sorbent functional groups and a higher value of surface area-normalized adsorption capacity for modified Aerosil® silica demonstrate the better exploitation of adsorption sites of non-porous sorbent when compared to the SBA-15 sample.
- The obtained adsorbents (SBA-15-AP, SBA-15-MAP, and SBA-15-AEAP) were characterized by the adsorption efficiency of 80% at the conditions of the lowest initial 18β-GA concentration (120 mg/dm3). For modified colloidal silicas, the adsorption efficiency reached 64%. The obtained results indicate that the SBA-15 material modified with trialkoxysilanes containing various amine groups (apart from the sample modified using (N,N-dimethylaminopropyl)trimethoxysilane)) is quite good adsorbent for 18β-GA. Previous studies that were also conducted in 2-propanol revealed better adsorption efficiency exceeding 90% for adsorption of carboxylic acids onto the surface of SBA-15 silica modified with 3-aminopropyl groups. It should be noted that examined adsorbates, such as diflunisal [38], caffeic acid [48], rosmarinic acid [103], and sinapic acid [104], are characterized by a slightly lower molar mass as compared to 18β-GA.
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations and Symbols
| AEAPTMS | [3-(2-aminoethylamino)propyl]trimethoxysilane |
| Aer | Aerosil® (non-porous colloidal silica) |
| APTMS | (3-aminopropyl)trimethoxysilane |
| aRP | constant of Redlich-Peterson isotherm (dm3β/mgβ) |
| bT | Temkin constant related to the adsorption heat (J g/mol mg) |
| BET | Brunauer-Emmett-Teller isotherm |
| BJH | Barrett-Joyner-Halenda isotherm |
| β | exponential constant of Redlich-Peterson isotherm |
| 18β-GA | 18β-glycyrrhetinic acid |
| C0 | initial adsorbate concentration (mg/dm3) |
| Ce | equilibrium adsorbate concentration (mg/dm3) |
| Cs | solubility (mg/dm3) |
| D-A | Dubinin-Astakhov isotherm |
| D-R | Dubinin-Radushkevich isotherm |
| DMAPTMS | (N,N-dimethylaminopropyl)trimethoxysilane |
| DTG | differential thermogravimetry |
| Eads | efficiency of adsorption (%) |
| EDA | adsorption energy calculated from Dubinin-Astakhov model (J/mol) |
| EDR | adsorption energy calculated from Dubinin-Radushkevich model (J/mol) |
| ε | Polanyi potential (J/mol) |
| F | Freundlich isotherm |
| IUPAC | International Union of Pure and Applied Chemistry |
| FT-IR | Fourier-transform infrared spectroscopy |
| KDA | constant of Dubinin-Astakhov isotherm related to the adsorption energy (molnDA/JnDA) |
| KDR | constant of Dubinin-Radushkevich isotherm related to the adsorption energy (mol2/J2) |
| KF | Freundlich constant (mg1-1/ndm 3/n/g) |
| KL | Langmuir constant (dm3/mg) |
| KRP | constant of Redlich-Peterson isotherm (dm3/g) |
| KT | Temkin binding constant (dm3/mg) |
| L | Langmuir isotherm |
| m | mass of adsorbent (g) |
| MAPTMS | [3-(methylamino)propyl]trimethoxysilane |
| M18β-GA | molar weight of 18β-glycyrrhetinic acid molecule (g/mol) |
| MPSD | Marquardt’s percent standard deviation |
| nDA | heterogeneity factor of Dubinin-Astakhov isotherm |
| nF | exponential constant of Freundlich equation |
| nFG | number of functional groups (mol) |
| nGA | number of 18β-glycyrrhetinic acid molecules (mol) |
| p/p0 | relative pressure |
| Qads(max) | maximum adsorption capacity calculated from given isotherm model (mg/g) |
| QDA(max) | maximum adsorption capacity calculated from Dubinin-Astakhov isotherm (mg/g) |
| QDR(max) | maximum adsorption capacity calculated from Dubinin-Radushkevich equation (mg/g) |
| Qe | amount of adsorbate in equilibrium solid state (mg/g) |
| QFG | content of functional groups (mol/g) |
| QL(max) | maximum adsorption capacity calculated from Langmuir equation (mg/g) |
| QS(max) | surface area-normalized maximum adsorption capacity (mg/m2) |
| R | gas constant (8.314 J/mol K) |
| R-P | Redlich-Peterson isotherm |
| r | correlation coefficient |
| SBA-15 | Santa Barbara amorphous (silica) |
| SBET | BET surface area (specific surface) (m2/g) |
| STP | standard temperature and pressure |
| T | absolute temperature (K) |
| T | Temkin isotherm |
| TEM | transmission electron microscopy |
| TEOS | tetraethyl orthosilicate |
| TG | thermogravimetry |
| Vads N2 | volume of adsorbed nitrogen (cm3 STP/g) |
References
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special references to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Chiola, V.; Ritsko, J.E.; Vanderpool, C.D. Process for Producing Low-Bulk Density Silica. U.S. Patent 3,556,725, 19 January 1971. [Google Scholar]
- Maurin, G.; Serre, C.; Cooper, A.; Férey, G. The new age of MOFs and of their porous-related solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lofgreen, J.E.; Ozin, G.A. Why PMO? Towards functionality and utility of periodic mesoporous organosilicas. Small 2010, 6, 2634–2642. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, X.; Zhang, J. Carbazolic Porous Organic Framework as an efficient, metal-free visible-light photocatalyst for organic synthesis. ACS Catal. 2015, 5, 2250–2254. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Asl, M.S.; Mazinani, B. Modulated large-pore mesoporous silica as an efficient base catalyst for the Henry reaction. Res. Chem. Intermed. 2018, 44, 1417–1626. [Google Scholar] [CrossRef]
- Sadjadi, S.; Heravi, M.M. Current advances in the utility of functionalized SBA mesoporous silica for developing encapsulated nanocatalysts: State of the art. RSC Adv. 2017, 7, 30815–30838. [Google Scholar] [CrossRef]
- Guayaquil-Sosa, J.F.; Serrano-Rosales, B.; Valadés-Pelayo, P.J.; Lasa, H.D. Photocatalytic hydrogen production using mesoporous TiO2 doped with Pt. Appl. Catal. B 2017, 211, 337–348. [Google Scholar] [CrossRef]
- Saad, A.; Vard, C.; Abderrabba, M. Triazole/triazine-functionalized mesoporous silica as a hybrid material support for palladium nanocatalyst. Langmuir 2017, 33, 7137–7146. [Google Scholar] [CrossRef]
- Diarjani, E.S.; Rajabi, F.; Yahyazadeh, A.; Puente-Santiago, A.R.; Luque, R. Copper tridentate Schiff base complex supported on SBA-15 as efficient nanocatalyst for three-component reactions under solventless conditions. Materials 2018, 11, 2458. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Rafiaei, S.M.; Abolhosseini, S.; Shokouhimehr, M. Palladium nanocatalysts confined in mesoporous silica for heterogeneous reduction of nitroaromatics. Energy Env. Focus 2015, 4, 18–23. [Google Scholar] [CrossRef]
- Duan, L.; Fu, R.; Zhang, B.; Shi, W.; Chen, S.; Wan, Y. An efficient reusable mesoporous solid-based Pd catalyst for selective C2 arylation of indoles in water. ACS Catal. 2016, 6, 1062–1074. [Google Scholar] [CrossRef]
- Lin, T.; Chen, I.-W.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015, 350, 1508–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, F.; Abate, A.; Baena, J.P.C.; Saliba, M.; Matsui, T.; Im, S.H.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Hagfeldt, A.; Graetzel, M. Enhanced electronic properties in mesoporous TiO2 via lithium doping for high-efficiency perovskite solar cells. Nat. Commun. 2016, 7, 10379. [Google Scholar] [CrossRef]
- Libbrecht, W.; Verberckmoes, A.; Thybant, J.W.; Voort PVan Der Clercq, J. De Self templated mesoporous carbons: Tuning the porosity for the adsorption of large organic pollutants. Carbon 2017, 116, 528–546. [Google Scholar] [CrossRef]
- Jayaraman, S.; Jain, A.; Klaganathan, M.; Edison, E.; Srinivasan, M.P.; Balasubramanian, R.; Aravindan, V.; Madhavi, S. Li-ions vs. Na-ions capacitors: A performance evaluation with coconut shell derived mesoporous carbon and natural plant based hard carbon. Chem. Eng. J. 2017, 316, 506–513. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Chang, W.-C.; Saikia, D.; Wu, C.-E.; Kao, H.-M. Functionalization of cubic mesoporous silica SBA-16 with carboxylic acid via one-pot synthesis route for effective removal of cationic dyes. J. Hazard. Mater. 2016, 309, 236–248. [Google Scholar] [CrossRef]
- Popova, M.; Trendafilova, I.; Szegedi, A.; Mihály, J.; Németh, P.; Marinova, S.G.; Aleksandrov, H.A.; Vayssilov, G.N. Experimental and theoretical study of quercetin complexes formed on pure silica and Zn-modified mesoporous MCM-41 and SBA-16 materials. Microporous Mesoporous Mater. 2016, 228, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Moritz, M.; Geszke-Moritz, M. Mesoporous materials as multifunctional tools in biosciences: Principles and applications. Mater. Sci. Eng. C 2015, 49, 114–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Jiang, T. Inclusion of the poorly water-soluble drug simvastatin in mesocellular foam nanoparticles: Drug loading and release properties. Int. J. Pharm. 2011, 410, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Moritz, M.; Geszke-Moritz, M. Aminopropyl-modified mesoporous molecular sieves as efficient adsorbents for removal of auxins. Appl. Surf. Sci. 2015, 331, 415–426. [Google Scholar] [CrossRef]
- Cheng, G.; Wang, Z.-G.; Liu, Y.-L.; Zhang, J.-L.; Sun, D.-H.; Ni, J.-Z. Magnetic affinity microspheres with meso-/macroporous shells for selective enrichment and fast separation of phosphorylated biomolecules. Appl. Mater. Interfaces 2013, 5, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Rámila, A.; Pérez-Pariente, J.; Vallet-Regí, M. Influence of pore size of MCM-41 matrices on drug delivery rate. Microporous Mesoporous Mater. 2004, 68, 105–109. [Google Scholar] [CrossRef]
- Ravichandran, R.; Gandhi, S.; Sundarammurthi, D.; Sethuraman, S.; Krishnan, U.M. Hierarchical mesoporous silica nanofibers as multifunctional scaffolds for bone tissue regeneration. J. Biomater. Sci. Polym. Ed. 2013, 24, 1988–2005. [Google Scholar] [CrossRef]
- Yang, H.; Lu, N.; Qi, B.; Guo, L. Voltammetric sensor based on ordered mesoporous carbon for folic acid determination. J. Electroanal. Chem. 2011, 660, 2–7. [Google Scholar] [CrossRef]
- Chen, W.-H.; Luo, G.-F.; Lei, Q.; Cao, F.-Y.; Fan, J.-X.; Qiu, W.-X.; Jia, H.-Z.; Hong, S.; Fang, F.; Zeng, X.; et al. Rational design of multifunctional magnetic mesoporous silica nanoparticle for tumor-targeted magnetic resonance imaging and precise therapy. Biomaterials 2016, 76, 87–101. [Google Scholar] [CrossRef]
- Liu, S.; Gordiichuk, P.; Wu, Z.-S.; Liu, Z.; Wei, W.; Wagner, M.; Mohamed-Noriega, N.; Wu, D.; Mai, Y.; Herrmann, A.; et al. Patterning two-dimensional free-standing surfaces with mesoporous conducting polymers. Nat. Commun. 2015, 6, 8817. [Google Scholar] [CrossRef]
- Tsai, C.P.; Hung, Y.; Chou, Y.-H.; Huang, D.-M.; Hsiao, J.-K.; Chang, C.; Chen, Y.-C.; Mou, C.-Y. High-contrast paramegnetic fluorescent mesoporous silica nanorods as a multifunctional cell-imaging probe. Small 2008, 4, 186–191. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Chen, Z.; Tan, Y.; Xu, K.; Zhang, L.; Qin, B.; Guo, L.; Lin, Z.; Chen, G. Stimulus-response mesoporous silica nanoparticle-based chemiluminescence biosensor for cocaine determination. Biosens. Bioelectron. 2016, 75, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kempen, P.J.; Greasley, S.; Parker, K.A.; Campbell, J.L.; Cheng, H.-Y.; Jones, J.R.; Sinclair, R.; Gambhir, S.S.; Jokerst, J.V. Theranostic mesoporous silica nanoparticles biodegrade after pro-survival drug delivery and ultrasound/magnetic resonance imaging of stem cells. Theranostics 2015, 5, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, M.; Guo, Z.; Guo, P.; Chen, X.; Wang, J. Specific isolation of glycoproteins with mesoporous zirconia-polyoxometalate hybrid. Proteomics 2018, 18, 1700381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Liang, Z.; Zhaob, Z.; Sun, T.; Shi, W.; Cui, F. Adsorption of quinolone antibiotics in spherical mesoporous silica: Effects of the retained template and its alkyl chain length. J. Hazard. Mater. 2016, 305, 8–14. [Google Scholar] [CrossRef]
- Legnoverde, M.S.; Basaldella, E.I. Influence of particle size on the adsorption and release of cephalexin encapsulated in mesoporous silica SBA-15. Mater. Lett. 2016, 181, 331–334. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. APTES-modified mesoporous silicas as the carriers for poorly water-soluble drug. Modeling of diflunisal adsorption and release. Appl. Surf. Sci. 2016, 368, 348–359. [Google Scholar] [CrossRef]
- Moritz, M.; Łaniecki, M. SBA-15 mesoporous material modified with APTES as the carrier for 2-(3-benzoylphenyl)propionic acid. Appl. Surf. Sci. 2012, 258, 7523–7529. [Google Scholar] [CrossRef]
- Moritz, M. Solvent optimization for niacinamide adsorption on organo-functionalized SBA-15 mesoporous silica. Appl. Surf. Sci. 2013, 283, 537–545. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Mesoporous silica materials with different structures as the carriers for antimicrobial agent. Modeling of chlorhexidine adsorption and release. Appl. Surf. Sci. 2015, 356, 415–426. [Google Scholar] [CrossRef]
- Salis, A.; Medda, L.; Cugia, F.; Monduzzi, M. Effect of electrolytes on proteins physisorption on ordered mesoporous silica materials. Colloids Surf. B 2016, 137, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lan, J.; Li, H.; Liu, X.; Zhang, H. Fabrication of diverse pH-sensitive functional mesoporous silica for selective removal or depletion of highly abundant proteins from biological samples. Talanta 2017, 162, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.M.L.; Cecilia, J.A.; Vilarrasa-García, E.; García-Sancho, C.; Silva Junior, I.J.; Rodríguez-Castellón, E.; Azevedo, D.C.S. Adsorption of biomolecules in porous silicas modified with zirconium. Effect of the textural properties and acidity. Microporous Mesoporous Mater. 2018, 260, 146–154. [Google Scholar] [CrossRef]
- Hikosaka, R.; Nagata, F.; Tomita, M.; Kato, K. Adsorption and desorption characteristics of DNA onto the surface of amino functional mesoporous silica with various particle morphology. Colloids Surf. B 2016, 140, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Mirabi, A.; Rad, A.S.; Khanjari, Z.; Moradian, M. Preparation of SBA-15/graphene oxide nanocomposite for preconcentration and determination of trace amounts of rutoside in blood plasma and urine. Sens. Actuators B 2017, 253, 533–541. [Google Scholar] [CrossRef]
- Yangui, A.; Abderrabba, M.; Sayari, A. Amine-modified mesoporous silica for quantitative adsorption and release of hydroxytyrosol and other phenolic compounds from olive mill wastewater. J. Taiwan Inst. Chem. Eng. 2017, 70, 111–118. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Amine-modified SBA-15 and MCF mesoporous molecular sieves as promising sorbents for natural antioxidant. Modeling of caffeic acid adsorption. Mater. Sci. Eng. C 2016, 56, 411–421. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Modeling of boldine alkaloid adsorption onto pure and propyl-sulfonic acid-modified mesoporous silicas. A comparative study. Mater. Sci. Eng. C 2016, 69, 815–830. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Use of mesoporous silica modified with a sulfonic acid-derivative as adsorbent for boldine. Przem. Chem. 2017, 96, 2001–2004. [Google Scholar]
- Geszke-Moritz, M.; Moritz, M. Modeling of boldine adsorption onto PHTS mesoporous silica. Przem. Chem. 2016, 95, 1365–1368. [Google Scholar]
- Kohno, Y.; Haga, E.; Yoda, K.; Shibata, M.; Fukuhara, C.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Adsorption behavior of natural anthocyanin dye on mesoporous silica. J. Phys. Chem. Solids 2014, 75, 48–51. [Google Scholar] [CrossRef] [Green Version]
- Kohno, Y.; Kato, Y.; Shibata, M.; Fukuhara, C.; Maeda, Y.; Tomita, Y.; Kobayashi, K. Enhanced stability of natural anthocyanin incorporated in Fe-containing mesoporous silica. Microporous Mesoporous Mater. 2015, 203, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Cotea, V.V.; Luchian, C.E.; Bilba, N.; Niculaua, M. Mesoporous silica SBA-15, a new adsorbent for bioactive polyphenols from red white. Anal. Chim. Acta 2012, 732, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.K.; Siddiqi, A.; Nafees, S.; Ali, N.; Rashid, S.; Ali, R.; Shahid, A.; Sultana, S. Chemopreventive effect of 18β-glycyrrhetinic acid via modulation of inflammatory markers and induction of apoptosis in human hepatoma cell line (HepG2). Mol. Cell. Biochem. 2016, 416, 169–177. [Google Scholar] [CrossRef]
- Gupta, P.; Das, P.K.; Ukil, A. Antileishmanial effect of 18β-glycyrrhetinic acid is mediated by toll-like receptor-dependent canonical and noncanonical p38 activation. Antimicrob. Ag. Chemother. 2015, 59, 2531–2539. [Google Scholar] [CrossRef]
- Jeong, H.G.; You, H.J.; Park, S.J.; Moon, A.R.; Chung, Y.C.; Kang, S.K.; Chun, H.K. Hepatoprotective effects of 18β-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: Inhibition of cytochrome P450 2E1 expression. Pharm. Res. 2002, 46, 221–227. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Wang, C.; Jia, D.; Cai, G.; Lu, J.; Wang, D.; Zhang, Z.-J. 18β-Glycyrrhetinic acid, a novel naturally derived agent, suppresses prolactin hyperactivity and reduces antipsychotic-induced hyperprolactinemia in in vitro and in vivo models. Neurochem. Res. 2016, 41, 2233–2242. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Kao, T.-C.; Lo, W.-H.; Yen, G.-C. Glycyrrhizic acid and 18β-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-κB through PI3K p110δ and p110γ inhibitions. J. Agric. Food Chem. 2011, 59, 7726–7733. [Google Scholar] [CrossRef]
- Kao, T.-C.; Shyu, M.-H.; Yen, G.-C. Glycyrrhizic acid and 18β-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3β signaling and glucocorticoid receptor activation. J. Agric. Food Chem. 2010, 58, 8623–8629. [Google Scholar] [CrossRef]
- Kong, S.-Z.; Chen, H.-M.; Yu, X.-T.; Zhang, X.; Feng, X.-X.; Kang, X.-H.; Li, W.-J.; Huang, N.; Su, Z.-R. The protective effect of 18β-glycyrrhetinic acid against UV irradiation induced photoaging in mice. Exp. Gerontol. 2015, 61, 147–155. [Google Scholar] [CrossRef]
- Kalaiarasi, P.; Pugalendi, K.V. Antihyperglycemic effect of 18β-glycyrrhetinic acid, aglycone of glycyrrhizin, on streptozocin-diabetic rats. Eur. J. Pharm. 2009, 606, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fang, Z.-Z.; Meng, R.; Cao, Y.-F.; Tanaka, N.; Krausz, K.W.; Gonzalez, F.J. Glycyrrhizin and glycyrrhetinic acid inhibits alpha-naphthyl isothiocyanate-induced liver injury and bile acid cycle disruption. Toxicology 2017, 386, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, X.; Wang, H.; Yang, J.; Mao, S. Enhanced delivery of doxorubicin to the liver through self-assembled nanoparticles formed via conjugation of glycyrrhetinic acid to the hydroxyl group of hyaluronic acid. Carbohydr. Polym. 2018, 195, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Cheng, J.; Liu, Z.; Cheng, F.; Wei, X.; Huang, Y.; He, J. Acid-sensitive polymeric vector targeting to hepatocarcinoma cells via glycyrrhetinic acid receptor-mediated endocytosis. Mater. Sci. Eng. C 2018, 87, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, D.; Liu, Y.; Cao, Q.; Sun, Y.; Wang, Q.; Tang, H. Improving dispersive property, biocompatibility and targeting gene transfection of graphene oxide by covalent attachment of polyamidoamine dendrimer and glycyrrhetinic acid. Colloids Surf. B 2018, 171, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Abd El-Twab, S.M. Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARγ and Nrf2: Protective effect of 18β-glycyrrhetinic acid. Chem.-Biol. Interact. 2017, 270, 59–723. [Google Scholar] [CrossRef]
- Zhang, M.; Chang, Z.; Zhao, F.; Zhang, P.; Hao, Y.-J.; Yan, L.; Liu, N.; Wang, J.-L.; Bo, L.; Ma, P.; et al. Protective effects of 18β-glycyrrhetinic acid on monocrotaline-induced pulmonary arterial hypertension in rats. Front. Pharm. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Ma, T.; Huang, C.; Meng, X.; Li, X.; Zhang, Y.; Ji, S.; Li, J.; Ye, M.; Liang, H. A potential adjuvant chemotherapeutics, 18β-glycyrrhetinic acid, inhibits renal tubular epithelial cells apoptosis via enhancing BMP-7 epigenetically through targeting HDAC2. Sci. Rep. 2016, 6, 25396. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chen, A.-Z.; Yen, G.-C. Protective effects of glycyrrhizic acid and 18β-glycyrrhetinic acid against cisplatin-induced nephrotoxicity in BALB/c mice. J. Agric. Food Chem. 2015, 63, 1200–1209. [Google Scholar] [CrossRef]
- Ge, B.; Yang, D.; Wu, X.; Zhu, J.; Wei, W.; Yang, B. Cytoprotective effects of glycyrrhetinic acid liposomes against cyclophosphamide-induced cystitis through inhibiting inflammatory stress. Int. Immunopharmacol. 2018, 54, 139–144. [Google Scholar] [CrossRef]
- de Breij, A.; Karnaoukh, T.G.; Schrumpf, J.; Hiemstra, P.S.; Nibbering, P.H.; van Dissel, J.T.; de Visser, P.C. The licorice pentacyclic triterpenoid component 18β-glycyrrhetinic acid enhances the activity of antibiotics against strains of methicillin-resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.-Z.; Zhou, M.; Zhang, D.-D.; Li, X.-F.; Liu, W.-B. The mechanism of action of a fat regulator: Glycyrrhetinic acid (GA) stimulating fatty acid transmembrane and intracellular transport in blunt snout bream (Megalobrama amblycephala). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 226, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Hu, S.S.; Ye, L.H.; Cao, J.; Pang, X.Q.; Xu, J.J. Trace matrix solid phase dispersion using a molecular sieve as the sorbent for the determination of flavonoids in fruit peels by ultra-performance liquid chromatography. Food Chem. 2016, 190, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Yan, H.; Row, K.H. Extraction of glycyrrhizic acid and glabridin from licorice. Int. J. Mol. Sci. 2008, 9, 571–577. [Google Scholar] [CrossRef]
- Charpe, T.W.; Rathod, V.K. Extraction of glycyrrhizic acid from licorice root using ultrasound: Process intensification studies. Chem. Eng. Process. 2012, 54, 37–41. [Google Scholar] [CrossRef]
- Pan, X.; Liu, H.; Shu, Y.Y. Microwave-assisted extraction of glycyrrhizic acid from licorice root. Biochem. Eng. J. 2000, 5, 173–177. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, S.; Fu, B.; Lee, F.S.C.; Wang, X. Development of multi-stage countercurrent extraction technology for the extraction of glycyrrhizic acid (GA) from licorice (Glycyrrhiza uralensis Fisch). Biochem. Eng. J. 2004, 21, 285–292. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, C.; Sun, W.; Zhou, A.; Wang, Y.; Zhang, G.; Zhou, X.; Huo, Y.; Li, C. Boosting 11-oxo-β-amyrin and glycyrrhetinic acid synthesis in Saccharomyces cerevisiae via pairing novel oxidation and reduction system from legume plants. Metab. Eng. 2018, 45, 43–50. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, S.; Wang, J.; Li, H.; Dai, Y. Adsorption separation of 3β-D-monoglucuronyl-18β-glycyrrhetinic acid from directional biotransformation products of glycyrrhizin. Afr. J. Biotechnol. 2008, 7, 3995–4003. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Inglezakis, V.J. Solubility-normalized Dubinin-Astakhov adsorption isotherm for ion-exchange systems. Microporous Mesoporous Mater. 2007, 103, 72–81. [Google Scholar] [CrossRef]
- Hizal, J.; Demirçivi, P.; Karadirek, Ş. Investigation of individual and competitive adsorption of Cu(II), Cd(II), and Pb(II) on montmorillonite in terms of surface complexation and kinetic properties of Cu(II) adsorption. Desalin. Water Treat. 2016, 57, 22441–22453. [Google Scholar] [CrossRef]
- Ng, J.C.Y.; Cheung, W.H.; McCay, G. Equilibrium studies for the sorption of lead from effluents using chitosan. Chemosphere 2003, 52, 1021–1030. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis and equilibrium data with several isotherm models and their optimization. Chem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Yao, T.; Xiao, Y.; Wu, X.; Guo, C.; Zhao, Y.; Chen, X. Adsorption of Eu(III) on sulfonated graphene oxide: Combined macroscopic and modeling techniques. J. Mol. Liq. 2016, 215, 443–448. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Ofomaja, A.E. Biosorption thermodynamics of cadmium on coconut copra meal as biosorbent. Biochem. Eng. J. 2006, 30, 117–123. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Sanaei, D.; Ali, I.; Bhatnagar, A. Removal of chromium(VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: Kinetic modeling and isotherm studies. J. Mol. Liq. 2016, 215, 671–679. [Google Scholar] [CrossRef]
- Ayoob, A.; Gupta, A.K. Insights into isotherm making in the sorptive removal of fluoride from drinking water. J. Hazard. Mater. 2008, 152, 976–985. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the porous structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Ortiz-Bustos, J.; Martin, A.; Morales, V.; Sanz, R.; García-Muñoz, R.A. Surface-functionalization of mesoporous SBA-15 silica for controlled release of methylprednisolone sodium hemisuccinate: Influence of functionality type and strategies of incorporation. Microporous Mesoporous Mater. 2017, 240, 236–245. [Google Scholar] [CrossRef]
- Ek, S.; Root, A.; Peussa, M.; Niinistö, L. Determination of the hydroxyl group content in silica by thermogravimetry and a comparison with 1H NMR results. Ther. Acta 2001, 379, 201–212. [Google Scholar] [CrossRef]
- Meynen, V.; Cool, P.; Vansant, E.F. Verified syntheses of mesoporous materials. Microporous Mesoporous Mater. 2009, 125, 170–223. [Google Scholar] [CrossRef]
- Salerno, A.; Bolzinger, M.-A.; Rolland, P.; Chevalier, Y.; Josse, D.; Briançon, S. Pickering emulsions for skin decontamination. Toxicol. Vitr. 2016, 34, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, E.; Bühlmann, P.; Affolter, C. Structure Determination of Organic Compounds, Tables of Spectral Data, 3rd ed.; Springer: Berlin, Germany, 2000. [Google Scholar]
- Andrade, G.F.; Soares, D.C.F.; dos Santos, R.G.; Sousa, E.M.B. Mesoporous silica SBA-16 nanoparticles: Synthesis, physicochemical characterization, release profile, and in vitro cytocompatibility studies. Microporous Mesoporous Mater. 2013, 168, 102–110. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. 986. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 3973–3993. [Google Scholar] [CrossRef]
- Hao, J.; Sun, Y.; Wang, Q.; Tong, X.; Zhang, H.; Zhang, Q. Effect and mechanism of penetration enhancement of organic base and alcohol on Glycyrrhetinic acid in vitro. Int. J. Pharm. 2010, 399, 102–108. [Google Scholar] [CrossRef]
- Cheng, M.; Gao, X.; Wang, Y.; Chen, H.; He, B.; Xu, H.; Li, Y.; Han, J.; Zhang, Z. Synthesis of glycyrrhetinic acid-modified chitosan 5-fluorouracil nanoparticles and its inhibition of liver cancer characteristics in vitro and in vivo. Mar. Drugs 2013, 11, 3517–3536. [Google Scholar] [CrossRef]
- Cestari, A.R.; Vieira, E.F.S.; Vieira, G.S.; Almeida, L.E. Aggregation and adsorption of reactive dyes in the presence of an anionic surfactant on mesoporous aminopropyl silica. J. Colloid Interface Sci. 2007, 309, 402–411. [Google Scholar] [CrossRef]
- Kaur, S.; Rani, S.; MAhajan, R.K.; Asif, M.; Gupta, V.K. Synthesis and adsorption properties of mesoporous material for the removal of dye safranin: Kinetics, equilibrium, and thermodynamics. J. Ind. Eng. Chem. 2015, 22, 19–27. [Google Scholar] [CrossRef]
- Iriel, A.; Bruneel, S.P.; Schenone, N.; Cirelli, A.F. The removal of fluoride from aqueous solution by a lateritic soil adsorption: Kinetic and equilibrium studies. Ecotoxicol. Env. Saf. 2018, 149, 166–172. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Modeling of rosmarinic acid adsorption onto (3-aminopropyl)triethoxysilane-modified SBA-15 silica. Przem. Chem. 2017, 96, 1775–1779. [Google Scholar]
- Geszke-Moritz, M.; Moritz, M. Use of trialkoxysilane-modified mesoporous silicas for adsorption of sinapic acid. Przem. Chem. 2018, 97, 1941–1944. [Google Scholar]













| Isotherm Model | Non-Linear Expression | Linear Transform | ||
|---|---|---|---|---|
| Equation | ||||
| Langmuir | (3) | (9) | ||
| Freundlich | (4) | (10) | ||
| Redlich-Peterson | (5) | (11) | ||
| Temkin | (6) | (12) | ||
| Dubinin-Radushkevich | (7) | (13) | ||
| Dubinin-Astakhov | (8) | (14) | ||
| Adsorbent | Modifying Agent | Amount of Functional Groups, QFG (mol/g) a | BET Surface Area (m2/g) | BJH Pore Volume (cm3/g) b | Pore Diameter (nm) b |
|---|---|---|---|---|---|
| SBA-15 | − | − | 770 | 0.96 | 5.8 |
| SBA-15-AP | APTMS | 1.55 × 10−3 | 438 | 0.67 | 5.4 |
| SBA-15-MAP | MAPTMS | 1.42 × 10−3 | 430 | 0.67 | 5.4 |
| SBA-15-DMAP | DMAPTMS | 1.30 × 10−3 | 425 | 0.68 | 5.4 |
| SBA-15-AEAP | AEAPTMS | 1.47 × 10−3 | 382 | 0.66 | 5.3 |
| Aer | − | − | 181 | − | − |
| Aer-AP | APTMS | 5.63 × 10−4 | 168 | − | − |
| Aer-MAP | MAPTMS | 4.99 × 10−4 | 163 | − | − |
| Aer-DMAP | DMAPTMS | 4.32 × 10−4 | 158 | − | − |
| Aer-AEAP | AEAPTMS | 6.10 × 10−4 | 149 | − | − |
| Adsorption model | Parameter | Adsorbent | |||
|---|---|---|---|---|---|
| SBA-15-AP | SBA-15-MAP | SBA-15-DMAP | SBA-15-AEAP | ||
| Langmuir | QL(max) (mg/g) | 169.5 | 151.5 | 61.0 | 178.6 |
| KL (dm3/mg) | 2.745 × 10−3 | 2.794 × 10−3 | 3.072 × 10−4 | 2.384 × 10−3 | |
| r2 | 0.9992 | 0.9970 | 0.9903 | 0.9993 | |
| Freundlich | KF (mg1−1/ndm3/n/g) | 2.973 | 2.413 | 2.923 × 10−2 | 2.868 |
| nF | 1.948 | 1.879 | 1.126 | 1.938 | |
| r2 | 0.9440 | 0.9680 | 0.9941 | 0.9422 | |
| Redlich−Peterson | KRP (dm3/g) | 0.488 | 0.479 | − | 0.441 |
| aRP (dm3β/mgβ) | 6.698 × 10−3 | 1.581 × 10−2 | − | 4.144 × 10−3 | |
| β | 0.883 | 0.778 | − | 0.931 | |
| r2 | 0.9993 | 0.9978 | − | 0.9949 | |
| Temkin | KT (dm3/mg) | 3.765 × 10−2 | 3.032 × 10−2 | 4.372 × 10−3 | 3.571 × 10−2 |
| bT (J g/mol mg) | 72.74 | 72.42 | 157.1 | 72.93 | |
| r2 | 0.9839 | 0.9659 | 0.8247 | 0.9883 | |
| Dubinin–Radushkevich | QDR(max) (mg/g) | 286.3 | 272.9 | 98.4 | 284.6 |
| KDR (mol2/J2) | 8.110 × 10−9 | 8.369 × 10−9 | 1.616 × 10−8 | 8.209 × 10−9 | |
| EDR (kJ/mol) | 7.85 | 7.73 | 5.56 | 7.80 | |
| r2 | 0.9901 | 0.9971 | 0.9703 | 0.9891 | |
| Dubinin–Astakhov | QDA(max) (mg/g) | 210.4 | 237.9 | − | 203.0 |
| KDA (molnDA/JnDA) | 5.462 × 10−12 | 6.442 × 10−10 | − | 2.030 × 10−12 | |
| nDA | 2.733 | 2.257 | − | 2.834 | |
| EDA (kJ/mol) | 9.34 | 8.35 | − | 9.45 | |
| r2 | 0.9988 | 0.9982 | − | 0.9996 | |
| Adsorption model | Parameter | Adsorbent | |||
|---|---|---|---|---|---|
| Aer-AP | Aer-MAP | Aer-DMAP | Aer-AEAP | ||
| Langmuir | QL(max) (mg/g) | 89.3 | 84.0 | − | 89.3 |
| KL (dm3/mg) | 2.822 × 10−3 | 2.663 × 10−3 | − | 2.046 × 10−3 | |
| r2 | 0.9952 | 0.9910 | − | 0.9949 | |
| Freundlich | KF (mg1−1/ndm3/n/g) | 2.179 | 1.767 | 1.606 x 10−2 | 1.362 |
| nF | 2.152 | 2.058 | 1.101 | 1.924 | |
| r2 | 0.9533 | 0.9730 | 0.9840 | 0.9772 | |
| Redlich−Peterson | KRP (dm3/g) | 0.288 | 0.284 | − | 0.221 |
| aRP (dm3β/mgβ) | 1.250 × 10−2 | 2.345 × 10−2 | − | 1.691 × 10−2 | |
| β | 0.820 | 0.744 | − | 0.748 | |
| r2 | 0.9987 | 0.9982 | − | 0.9992 | |
| Temkin | KT (dm3/mg) | 2.942 × 10−2 | 2.294 × 10−2 | − | 1.851 × 10−2 |
| bT (J g/mol mg) | 128.3 | 124.9 | − | 116.6 | |
| r2 | 0.9870 | 0.9731 | − | 0.9703 | |
| Dubinin−Radushkevich | QDR(max) (mg/g) | 144.9 | 141.7 | 65.0 | 149.8 |
| KDR (mol2/J2) | 7.809 × 10−9 | 8.169 × 10−9 | 1.648 × 10−8 | 8.828 × 10−9 | |
| EDR (kJ/mol) | 8.00 | 7.82 | 5.51 | 7.53 | |
| r2 | 0.9940 | 0.9980 | 0.9376 | 0.9993 | |
| Dubinin−Astakhov | QDA(max) (mg/g) | 118.2 | 134.0 | − | 144.3 |
| KDA (molnDA/JnDA) | 4.273 × 10−11 | 2.622 × 10−9 | − | 4.375 × 10−9 | |
| nDA | 2.525 | 2.114 | − | 2.071 | |
| EDA (kJ/mol) | 9.04 | 8.11 | − | 7.69 | |
| r2 | 0.9987 | 0.9982 | − | 0.9994 | |
| Adsorption model | Parameter | Adsorbent | |||
|---|---|---|---|---|---|
| SBA-15-AP | SBA-15-MAP | SBA-15-DMAP | SBA-15-AEAP | ||
| Langmuir | QL(max) (mg/g) | 183.7 | 176.7 | 227.3 | 182.0 |
| KL (dm3/mg) | 2.357 × 10−3 | 2.033 × 10−3 | 6.453 × 10−5 | 2.278 × 10−3 | |
| MPSD | 4.66 | 10.53 | 11.98 | 4.03 | |
| Freundlich | KF (mg1−1/ndm3/n/g) | 2.634 | 2.268 | 2.921 x 10−2 | 2.479 |
| nF | 1.918 | 1.867 | 1.128 | 1.895 | |
| MPSD | 23.15 | 18.15 | 9.36 | 23.45 | |
| Redlich−Peterson | KRP (dm3/g) | 0.471 | 0.468 | 3.971 | 0.446 |
| aRP (dm3β/mgβ) | 5.068 ×10−3 | 1.412 × 10−2 | 135.1 | 4.516 × 10−3 | |
| β | 0.914 | 0.790 | 0.113 | 0.922 | |
| MPSD | 2.72 | 5.01 | 9.87 | 2.11 | |
| Temkin | KT (dm3/mg) | 5.728 × 10−2 | 5.412 × 10−2 | − | 5.193 × 10−2 |
| bT (J g/mol mg) | 91.96 | 99.00 | − | 89.23 | |
| MPSD | 22.22 | 24.26 | − | 19.16 | |
| Dubinin-Radushkevich | QDR(max) (mg/g) | 282.9 | 271.5 | 97.0 | 282.0 |
| KDR (mol2/J2) | 8.107 × 10−9 | 8.358 × 10−9 | 1.654 × 10−8 | 8.234 × 10−9 | |
| EDR (kJ/mol) | 7.85 | 7.73 | 5.50 | 7.79 | |
| MPSD | 9.74 | 5.30 | 20.62 | 10.16 | |
| Dubinin−Astakhov | QDA(max) (mg/g) | 210.3 | 237.3 | − | 202.8 |
| KDA (molnDA/JnDA) | 5.448 × 10−12 | 6.414 × 10−10 | − | 2.035 × 10−12 | |
| nDA | 2.733 | 2.257 | − | 2.834 | |
| EDA (kJ/mol) | 9.35 | 8.37 | − | 9.44 | |
| MPSD | 3.58 | 4.33 | − | 1.99 | |
| Adsorption model | Parameter | Adsorbent | |||
|---|---|---|---|---|---|
| Aer-AP | Aer-MAP | Aer-DMAP | Aer-AEAP | ||
| Langmuir | QL(max) (mg/g) | 99.6 | 98.1 | − | 104.1 |
| KL (dm3/mg) | 2.191 × 10−3 | 1.835 × 10−3 | − | 1.468 × 10−3 | |
| MPSD | 7.47 | 11.28 | − | 10.26 | |
| Freundlich | KF (mg1−1/ndm3/n/g) | 2.011 | 1.705 | 1.380 × 10−2 | 1.295 |
| nF | 2.125 | 2.052 | 1.081 | 1.908 | |
| MPSD | 17.73 | 13.93 | 15.21 | 13.33 | |
| Redlich−Peterson | KRP (dm3/g) | 0.269 | 0.269 | 3.910 | 0.219 |
| aRP (dm3β/mgβ) | 8.902 × 10−3 | 1.916 × 10−2 | 282.5 | 1.629 × 10−2 | |
| β | 0.853 | 0.762 | 0.075 | 0.751 | |
| MPSD | 3.59 | 4.65 | 16.04 | 2.82 | |
| Temkin | KT (dm3/mg) | 4.093 × 10−2 | 3.689 × 10−2 | − | 3.117 × 10−2 |
| bT (J g/mol mg) | 148.7 | 154.9 | − | 150.1 | |
| MPSD | 14.43 | 17.03 | − | 18.60 | |
| Dubinin–Radushkevich | QDR(max) (mg/g) | 144.2 | 141.4 | 64.0 | 149.6 |
| KDR (mol2/J2) | 7.806 × 10−9 | 8.159 × 10−9 | 1.740 × 10−8 | 8.825 × 10−9 | |
| EDR (kJ/mol) | 8.00 | 7.83 | 5.36 | 7.53 | |
| MPSD | 6.31 | 3.70 | 28.55 | 2.29 | |
| Dubinin–Astakhov | QDA(max) (mg/g) | 118.2 | 133.7 | - | 144.2 |
| KDA (molnDA/JnDA) | 4.282 × 10−11 | 2.628 × 10−9 | - | 4.363 × 10−9 | |
| nDA | 2.525 | 2.114 | - | 2.071 | |
| EDA (kJ/mol) | 9.03 | 8.10 | - | 7.70 | |
| MPSD | 3.03 | 3.65 | - | 2.27 | |
| Adsorbent | Fitting | |
|---|---|---|
| Linear Regression | Nonlinear Analysis | |
| SBA-15-AP | R-P ≈ L ≈ D-A ≈ D-R > T > F | R-P ≈ D-A ≈ L > D-R > T ≈ F |
| SBA-15-MAP | D-A ≈ R-P ≈ D-R ≈ L > F ≈ T | D-A ≈ R-P ≈ D-R > L > F > T |
| SBA-15-DMAP | F > L > D-R > T | F ≈ R-P > L > D-R |
| SBA-15-AEAP | D-A ≈ L ≈ R-P > D-R ≈ T > F | D-A ≈ R-P ≈ L > D-R > T > F |
| Aer-AP | D-A > R-P ≈ L ≈ D-R > T > F | D-A ≈ R-P > D-R ≈ L > T > F |
| Aer-MAP | D-A = R-P ≈ D-R ≈ L > T ≈ F | D-A ≈ D-R ≈ R-P > L > F > T |
| Aer-DMAP | F > D-R | F ≈ R-P > D-R |
| Aer-AEAP | D-A ≈ D-R ≈ R-P ≈ L > F ≈ T | D-A ≈ D-R ≈ R-P > L > F > T |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moritz, M.; Geszke-Moritz, M. The Effect of SBA-15 Surface Modification on the Process of 18β-Glycyrrhetinic Acid Adsorption: Modeling of Experimental Adsorption Isotherm Data. Materials 2019, 12, 3671. https://doi.org/10.3390/ma12223671
Moritz M, Geszke-Moritz M. The Effect of SBA-15 Surface Modification on the Process of 18β-Glycyrrhetinic Acid Adsorption: Modeling of Experimental Adsorption Isotherm Data. Materials. 2019; 12(22):3671. https://doi.org/10.3390/ma12223671
Chicago/Turabian StyleMoritz, Michał, and Małgorzata Geszke-Moritz. 2019. "The Effect of SBA-15 Surface Modification on the Process of 18β-Glycyrrhetinic Acid Adsorption: Modeling of Experimental Adsorption Isotherm Data" Materials 12, no. 22: 3671. https://doi.org/10.3390/ma12223671