Hollow Silica Cubes with Customizable Porosity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Pseudo-Cubic Hematite
2.3. Deposition of the Silica Shell
2.4. Extraction of the Hematite Core
2.5. Pseudomorphic Transformation
2.6. Preparation of Monolayers
2.7. Physical Measurements
3. Results and Discussion
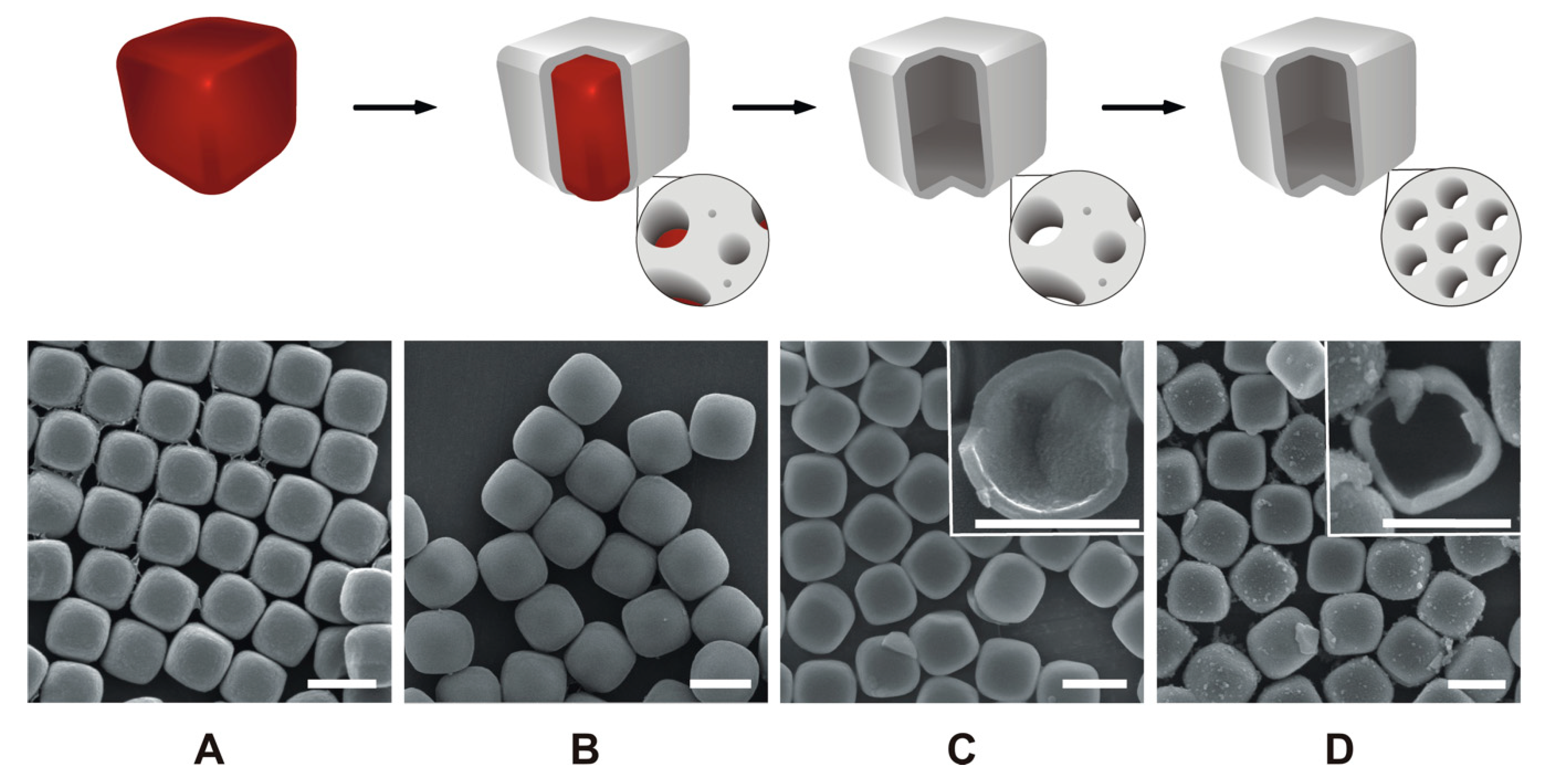
3.1. General Synthesis Pathway
3.2. Hollow Silica Cubes
3.3. Pseudomorphic Transformation
3.4. Shell Thickness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Hu, S.L.; Wu, Y.B.; Niu, Q.; Huang, Y.Y.; Wu, F.; Zhu, X.T.; Fan, J.; Yin, G.Y.; Wan, M.M.; et al. Multiple Drug Delivery from Mesoporous Coating Realizing Combination Therapy for Bare Metal Stents. Langmuir 2019, 35, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Argyo, C.; Weiss, V.; Bräuchle, C.; Bein, T. Multifunctional Mesoporous Silica Nanoparticles as a Universal Platform for Drug Delivery. Chem. Mater. 2014, 26, 435–451. [Google Scholar] [CrossRef]
- Yang, P.; Gai, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef] [PubMed]
- Barbé, C.; Bartlett, J.; Kong, L.; Finnie, K.; Lin, H.Q.; Larkin, M.; Calleja, S.; Bush, A.; Calleja, G. Silica Particles: A Novel Drug-Delivery System. Adv. Mater. 2004, 16, 1959–1966. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Silica-Bound Homogenous Catalysts as Recoverable and Reusable Catalysts in Organic Synthesis. Adv. Synth. Catal. 2006, 348, 1391–1412. [Google Scholar] [CrossRef]
- Hartmann, M. Ordered Mesoporous Materials for Bioadsorption and Biocatalysis. Chem. Mater. 2005, 17, 4577–4593. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous Catalysis in Zeolites, Mesoporous Silica, and Metal–Organic Frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, J.; Wu, D.; Li, X.; Luo, Y.; Han, C.; Ma, W.; He, S. Investigating the Heavy Metal Adsorption of Mesoporous Silica Materials Prepared by Microwave Synthesis. Nanoscale Res. Lett. 2017, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Thirumavalavan, M.; Wang, Y.-T.; Lin, L.-C.; Lee, J.-F. Monitoring of the Structure of Mesoporous Silica Materials Tailored Using Different Organic Templates and Their Effect on the Adsorption of Heavy Metal Ions. J. Phys. Chem. C 2011, 115, 8165–8174. [Google Scholar] [CrossRef]
- Jal, P.K.; Patel, S.; Mishra, B.K. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta 2004, 62, 1005–1028. [Google Scholar] [CrossRef] [PubMed]
- Widmer, S.; Reber, M.J.; Müller, P.; Housecroft, C.E.; Constable, E.C.; Rossi, R.M.; Brühwiler, D.; Scherer, L.J.; Boesel, L.F. Incorporation of a FRET dye pair into mesoporous materials: A comparison of fluorescence spectra, FRET activity and dye accessibility. The Analyst 2015, 140, 5324–5334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Cui, G.; Li, H.; Liu, Y.; Tian, Y.; Yan, S. Multifunctional optical sensing probes based on organic–inorganic hybrid composites. J. Mater. Chem. B 2016, 4, 5194–5216. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Baeza, A.; Vallet-Regí, M. Recent applications of the combination of mesoporous silica nanoparticles with nucleic acids: Development of bioresponsive devices, carriers and sensors. Biomater. Sci. 2017, 5, 353–377. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Stöcker, M.; Hansen, E.; Akporiaye, D.; Ellestad, O.H. MCM-41: A model system for adsorption studies on mesoporous materials. Microporous Mater. 1995, 3, 443–448. [Google Scholar] [CrossRef]
- Sonwane, C.G.; Bhatia, S.K. Characterization of Pore Size Distributions of Mesoporous Materials from Adsorption Isotherms. J. Phys. Chem. B 2000, 104, 9099–9110. [Google Scholar] [CrossRef]
- Ojeda, M.L.; Esparza, J.M.; Campero, A.; Cordero, S.; Kornhauser, I.; Rojas, F. On comparing BJH and NLDFT pore-size distributions determined from N2 sorption on SBA-15 substrata. Phys. Chem. Chem. Phys. 2003, 5, 1859–1866. [Google Scholar] [CrossRef]
- Cimino, R.; Cychosz, K.A.; Thommes, M.; Neimark, A.V. Experimental and theoretical studies of scanning adsorption–desorption isotherms. Colloids Surf. Physicochem. Eng. Asp. 2013, 437, 76–89. [Google Scholar] [CrossRef]
- David, R.O.; Marcolli, C.; Fahrni, J.; Qiu, Y.; Sirkin, Y.A.P.; Molinero, V.; Mahrt, F.; Brühwiler, D.; Lohmann, U.; Kanji, Z.A. Pore condensation and freezing is responsible for ice formation below water saturation for porous particles. Proc. Natl. Acad. Sci. USA 2019, 116, 8184–8189. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, F.; Gao, K.; Wu, J.; Xue, D. Recent developments in the chemical synthesis of inorganic porous capsules. J. Mater. Chem. 2009, 19, 6073–6084. [Google Scholar] [CrossRef]
- Bao, Y.; Shi, C.; Wang, T.; Li, X.; Ma, J. Recent progress in hollow silica: Template synthesis, morphologies and applications. Microporous Mesoporous Mater. 2016, 227, 121–136. [Google Scholar] [CrossRef]
- Wang, Y.; Su, X.; Ding, P.; Lu, S.; Yu, H. Shape-Controlled Synthesis of Hollow Silica Colloids. Langmuir 2013, 29, 11575–11581. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Bayindir, M. A porosity difference based selective dissolution strategy to prepare shape-tailored hollow mesoporous silica nanoparticles. J. Mater. Chem. A 2015, 3, 3839–3846. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J. Hollow-Structured Mesoporous Materials: Chemical Synthesis, Functionalization and Applications. Adv. Mater. 2014, 26, 3176–3205. [Google Scholar] [CrossRef] [PubMed]
- Castillo, S.I.R.; Ouhajji, S.; Fokker, S.; Erné, B.H.; Schneijdenberg, C.T.W.M.; Thies-Weesie, D.M.E.; Philipse, A.P. Silica cubes with tunable coating thickness and porosity: From hematite filled silica boxes to hollow silica bubbles. Microporous Mesoporous Mater. 2014, 195, 75–86. [Google Scholar] [CrossRef]
- Martin, T.; Galarneau, A.; Di Renzo, F.; Fajula, F.; Plee, D. Morphological Control of MCM-41 by Pseudomorphic Synthesis. Angew. Chem. Int. Ed. 2002, 41, 2590–2592. [Google Scholar] [CrossRef]
- Galarneau, A.; Iapichella, J.; Bonhomme, K.; Di Renzo, F.; Kooyman, P.; Terasaki, O.; Fajula, F. Controlling the Morphology of Mesostructured Silicas by Pseudomorphic Transformation: A Route Towards Applications. Adv. Funct. Mater. 2006, 16, 1657–1667. [Google Scholar] [CrossRef]
- Iapichella, J.; Meneses, J.-M.; Beurroies, I.; Denoyel, R.; Bayram-Hahn, Z.; Unger, K.; Galarneau, A. Characterization of mesoporous silica and its pseudomorphically transformed derivative by gas and liquid adsorption. Microporous Mesoporous Mater. 2007, 102, 111–121. [Google Scholar] [CrossRef]
- Reber, M.J.; Brühwiler, D. Mesoporous Hybrid Materials by Simultaneous Pseudomorphic Transformation and Functionalization of Silica Microspheres. Part. Part. Syst. Charact. 2015, 32, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Reber, M.J.; Brühwiler, D. Bimodal mesoporous silica with bottleneck pores. Dalton Trans. 2015, 44, 17960–17967. [Google Scholar] [CrossRef] [Green Version]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Zucchetto, N.; Reber, M.J.; Pestalozzi, L.; Schmid, R.; Neels, A.; Brühwiler, D. The structure of mesoporous silica obtained by pseudomorphic transformation of SBA-15 and SBA-16. Microporous Mesoporous Mater. 2018, 257, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L.; Sacanna, S.; Irvine, W.T.M.; Chaikin, P.M.; Pine, D.J.; Philipse, A.P. Cubic crystals from cubic colloids. Soft Matter 2011, 7, 4139–4142. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, T.; Sakata, K.; Muramatsu, A. Formation Mechanism of Monodisperse Pseudocubic α-Fe2O3 Particles from Condensed Ferric Hydroxide Gel. J. Colloid Interface Sci. 1993, 159, 372–382. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Graf, C.; Vossen, D.L.J.; Imhof, A.; van Blaaderen, A. A General Method to Coat Colloidal Particles with Silica. Langmuir 2003, 19, 6693–6700. [Google Scholar] [CrossRef]
- Liu, S.; Han, M.-Y. Silica-Coated Metal Nanoparticles. Chem.—Asian J. 2010, 5, 36–45. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Smarsly, B.; Groenewolt, M.; Ravikovitch, P.I.; Neimark, A.V. Adsorption Hysteresis of Nitrogen and Argon in Pore Networks and Characterization of Novel Micro- and Mesoporous Silicas. Langmuir 2006, 22, 756–764. [Google Scholar] [CrossRef]
- Park, S.S.; Ha, C.-S. Hollow Mesoporous Functional Hybrid Materials: Fascinating Platforms for Advanced Applications. Adv. Funct. Mater. 2018, 28, 1703814. [Google Scholar] [CrossRef]
- Tanamura, Y.; Uchida, T.; Teramae, N.; Kikuchi, M.; Kusaba, K.; Onodera, Y. Ship-in-a-Bottle Synthesis of Copper Phthalocyanine Molecules within Mesoporous Channels of MCM-41 by a Chemical Vapor Deposition Method. Nano Lett. 2001, 1, 387–390. [Google Scholar] [CrossRef]
- Guardado-Alvarez, T.M.; Chen, W.; Norton, A.E.; Russell, M.M.; Connick, W.B.; Zink, J.I. Analyte-responsive gated hollow mesoporous silica nanoparticles exhibiting inverse functionality and an AND logic response. Nanoscale 2016, 8, 18296–18300. [Google Scholar] [CrossRef]
- Shu, X.; Jin, R.; Zhao, Z.; Cheng, T.; Liu, G. An integrated immobilization strategy manipulates dual active centers to boost enantioselective tandem reactions. Chem. Commun. 2018, 54, 13244–13247. [Google Scholar] [CrossRef]
- Shakeri, M.; Klein Gebbink, R.J.M.; de Jongh, P.E.; de Jong, K.P. Tailoring the Window Sizes to Control the Local Concentration and Activity of (salen)Co Catalysts in Plugged Nanochannels of SBA-15 Materials. Angew. Chem. Int. Ed. 2013, 52, 10854–10857. [Google Scholar] [CrossRef]

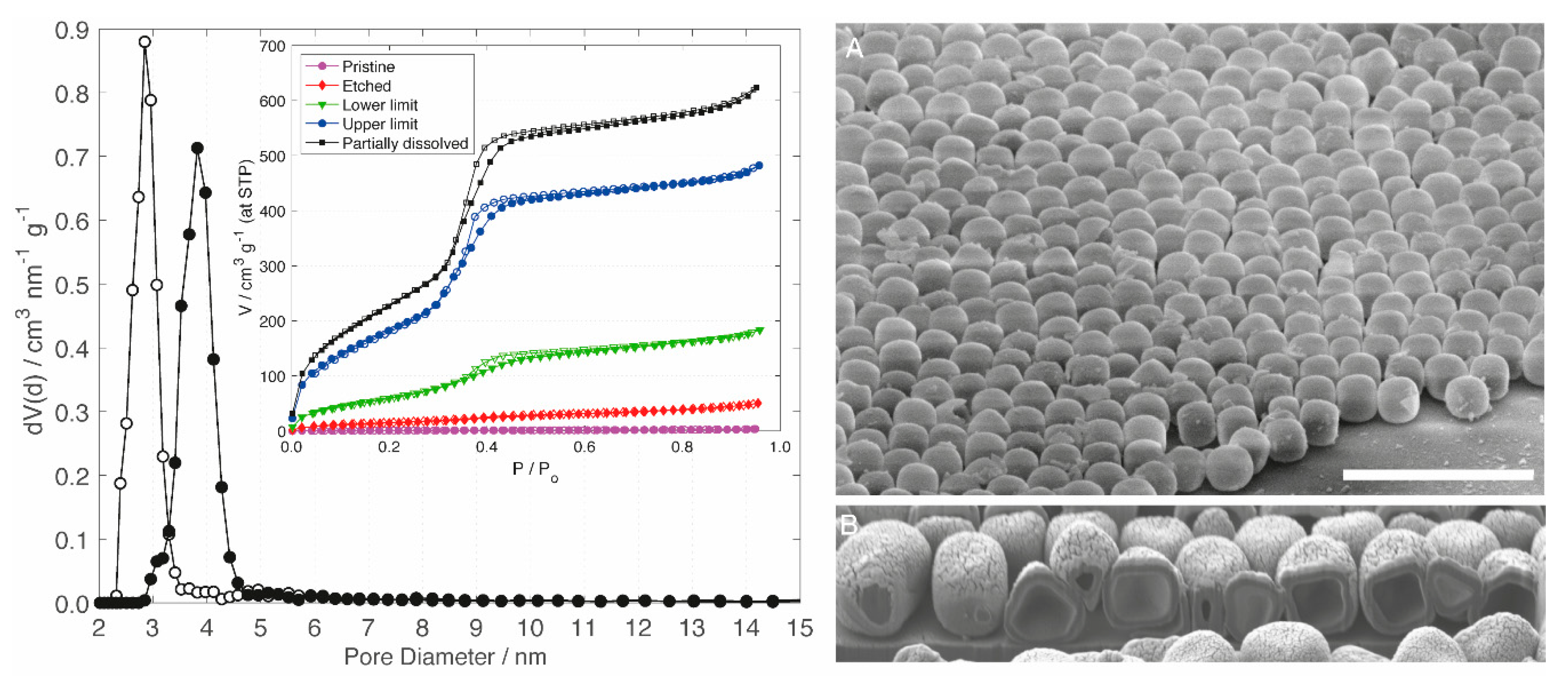
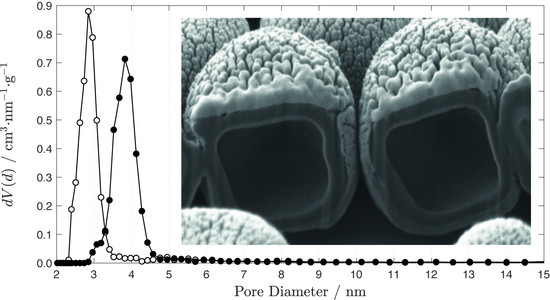
| m (NaOH)/mg | SBET/m2 g−1 | Vtot/cm3 g−1 | Vp/cm3 g−1 | |
|---|---|---|---|---|
| Macroporous shell (pristine) | — | 7 | 0.01 | 0.00 |
| Etched shell | 1.2 | 52 | 0.06 | 0.04 |
| Lower limit of PT | 6.4 | 221 | 0.23 | 0.19 |
| Upper limit of PT | 9.0 | 651 | 0.61 | 0.53 |
| Partially dissolved shell | 12.0 | 801 | 0.79 | 0.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallagher, S.H.; Trussardi, O.; Lipp, O.; Brühwiler, D. Hollow Silica Cubes with Customizable Porosity. Materials 2020, 13, 2474. https://doi.org/10.3390/ma13112474
Gallagher SH, Trussardi O, Lipp O, Brühwiler D. Hollow Silica Cubes with Customizable Porosity. Materials. 2020; 13(11):2474. https://doi.org/10.3390/ma13112474
Chicago/Turabian StyleGallagher, Samuel Hugh, Olivier Trussardi, Oliver Lipp, and Dominik Brühwiler. 2020. "Hollow Silica Cubes with Customizable Porosity" Materials 13, no. 11: 2474. https://doi.org/10.3390/ma13112474