Synthesis of Poly (Citric Acid-Co-Glycerol) and Its Application as an Inhibitor of CaCO3 Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hyperbranched CA-co-GLC
2.3. Characterizations of CA-co-GLC
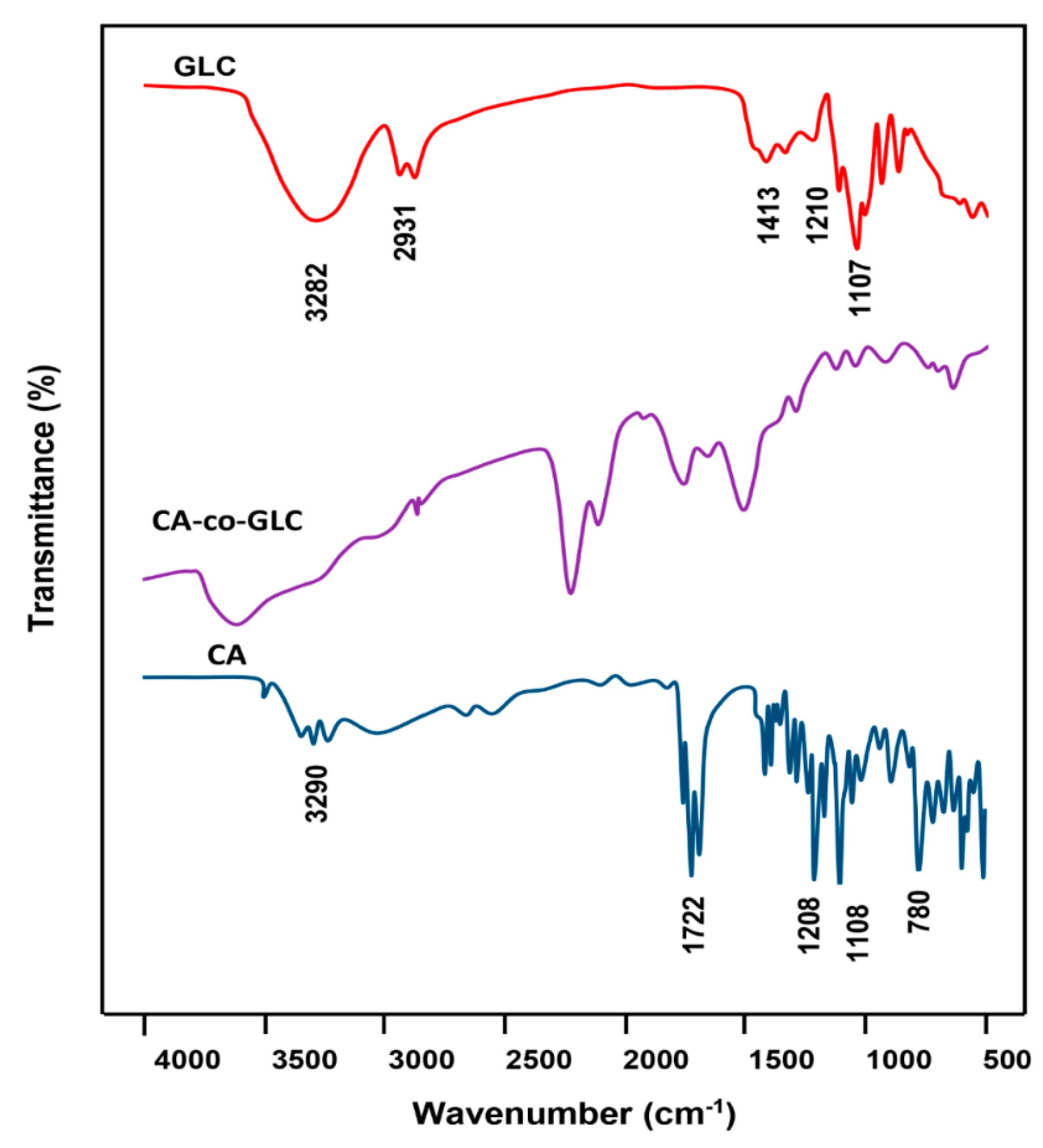
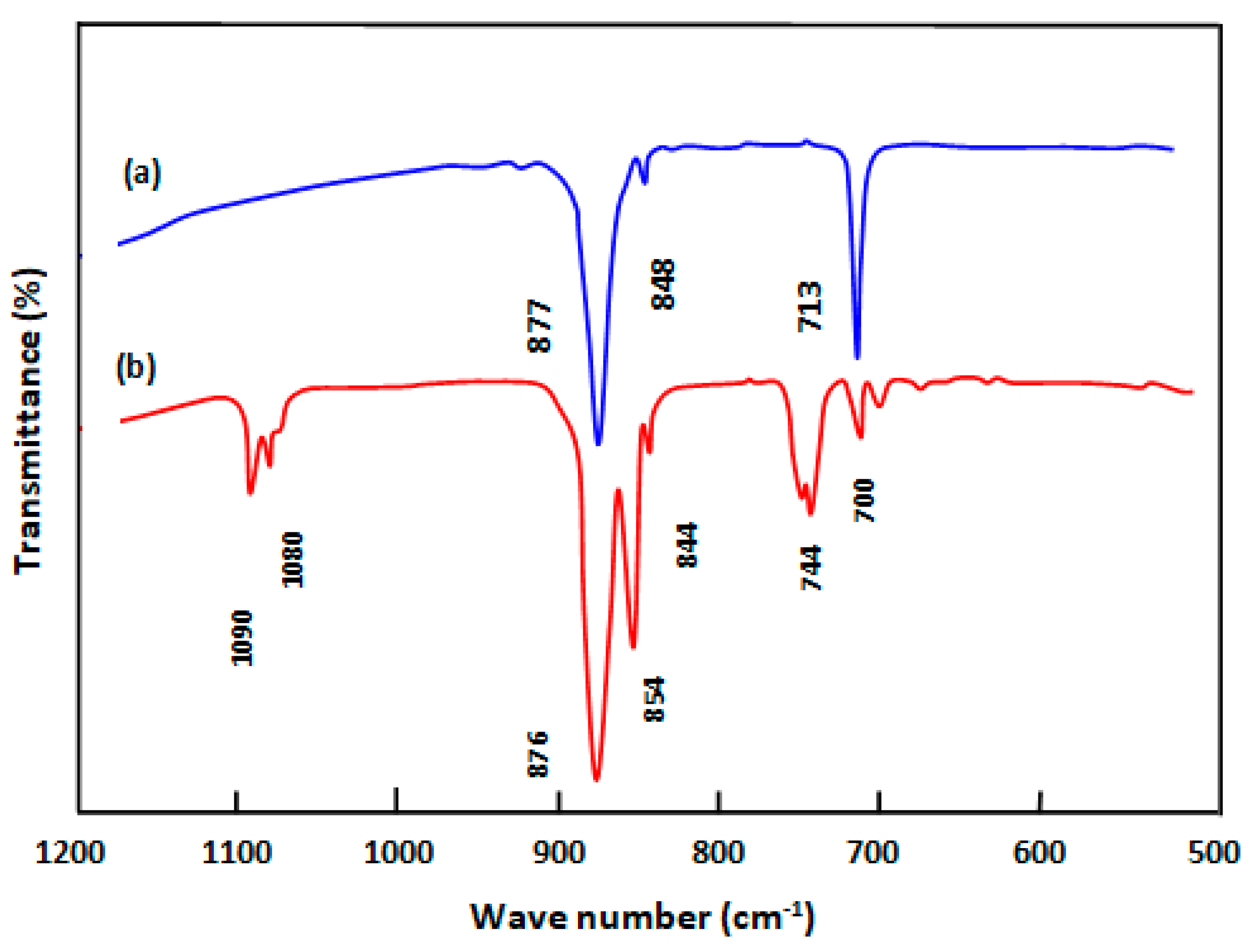
2.3.1. FT-IR Analysis
2.3.2. NMR Analysis
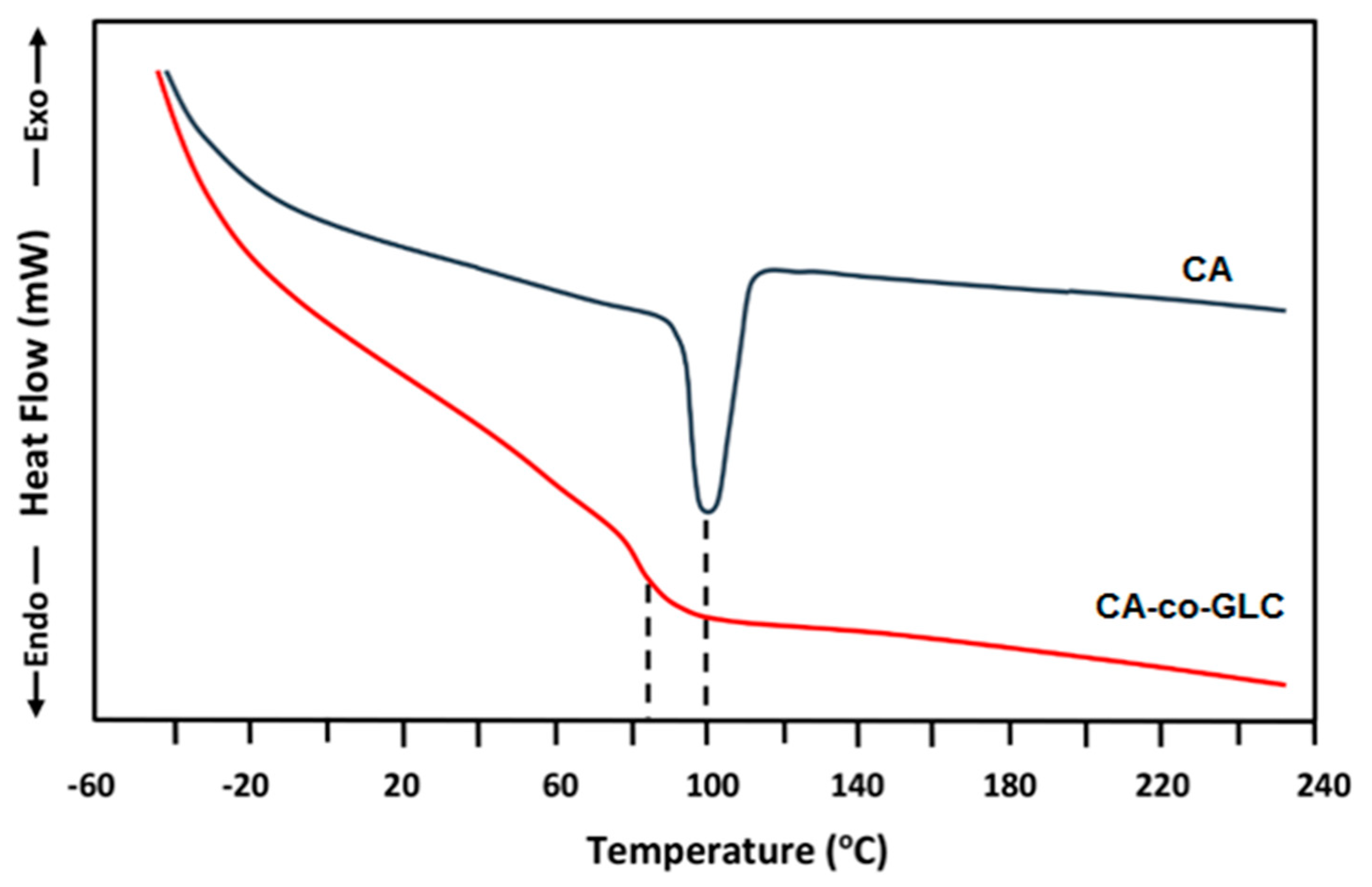
2.3.3. DSC Analysis
2.4. Scale Inhibition Evaluation
2.5. Determination of Residual Calcium Ion Concentration
2.6. Determination of Conductivity
2.7. Scale Analysis
3. Results and Discussion
3.1. Synthesis of CA-co-GLC
3.2. Characterization of CA-co-GLC
3.3. Scale Inhibition Efficiency of CA-co-GLC
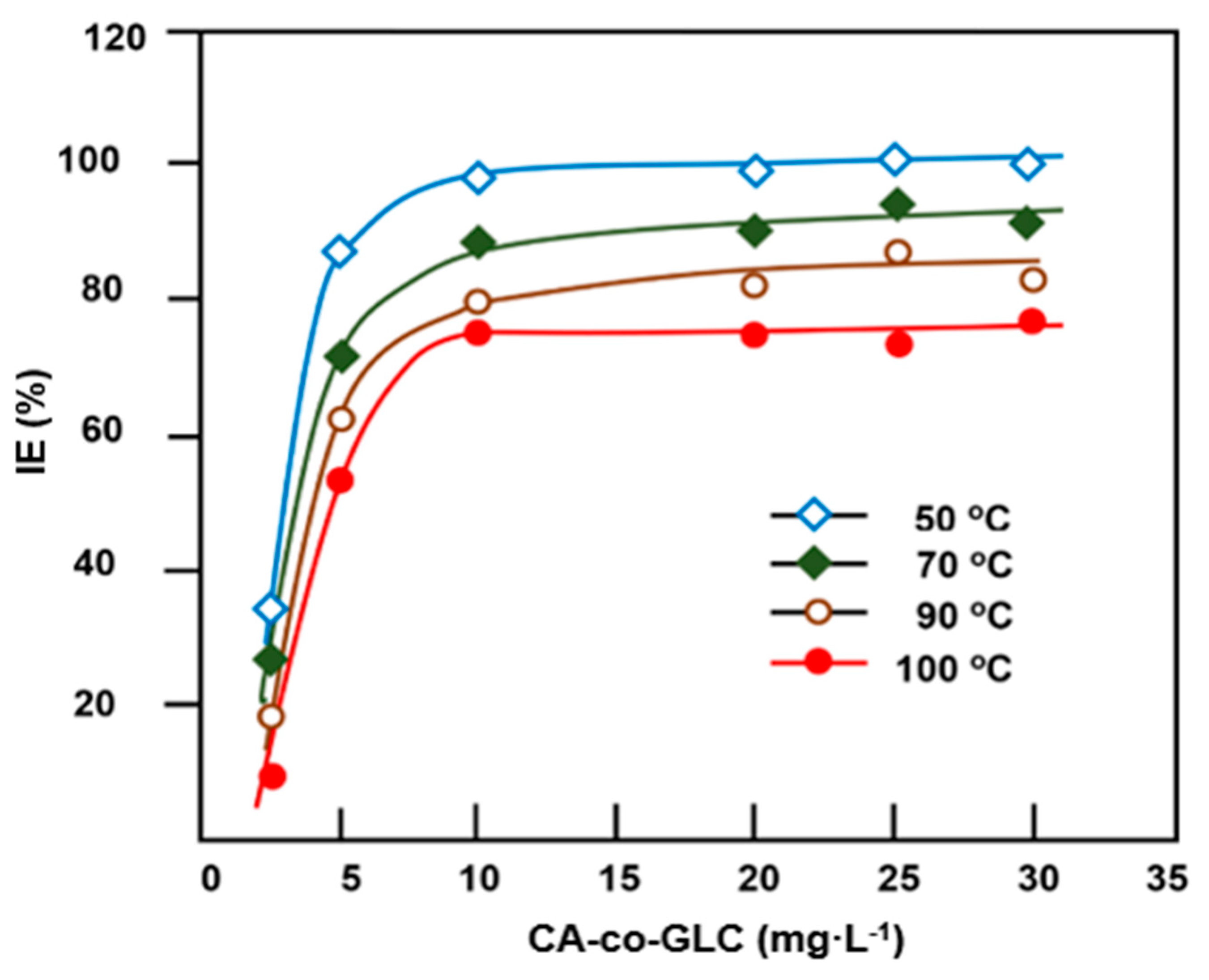
3.3.1. Effect of CA-co-GLC Concentration
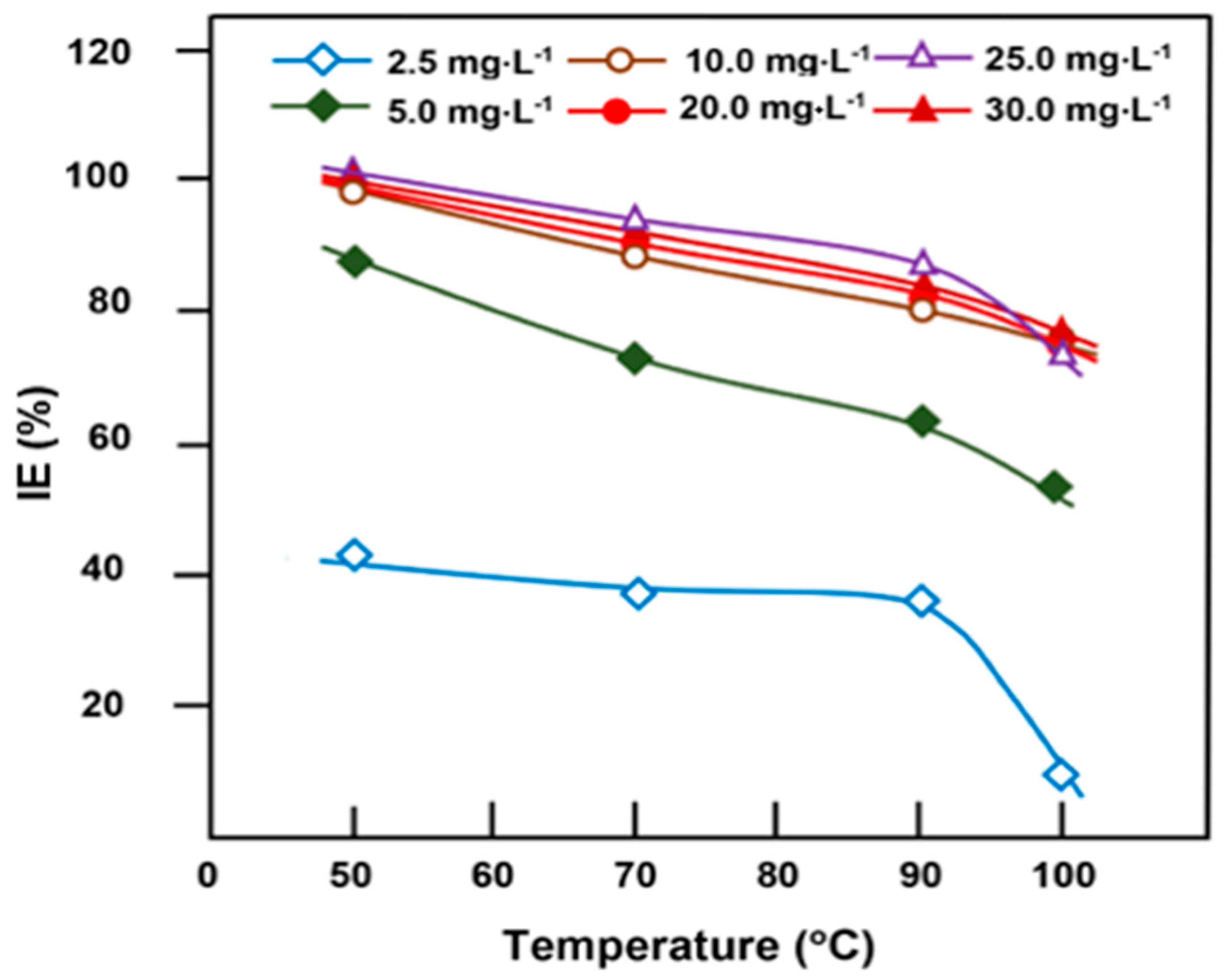
3.3.2. Effect of Temperature
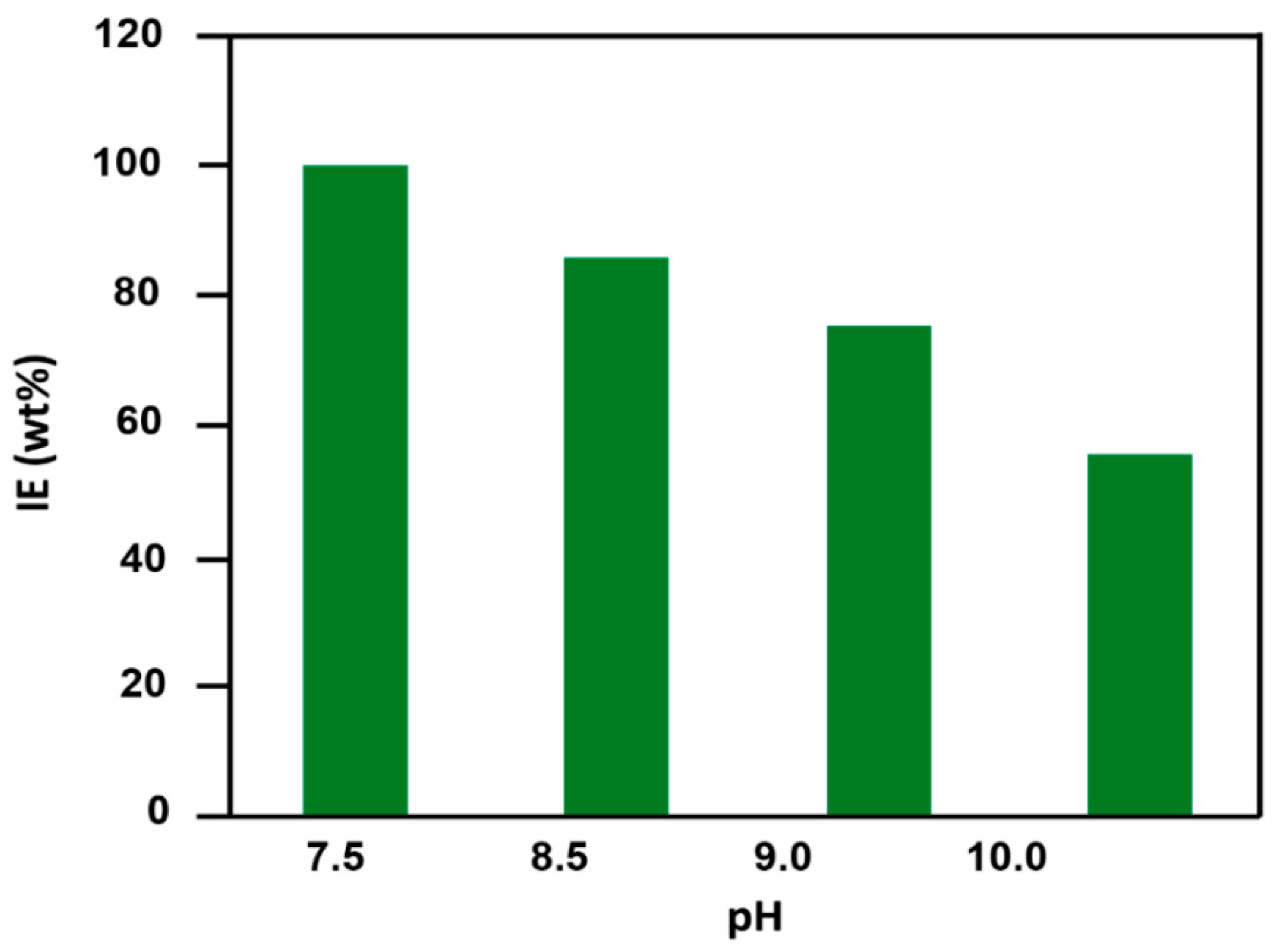
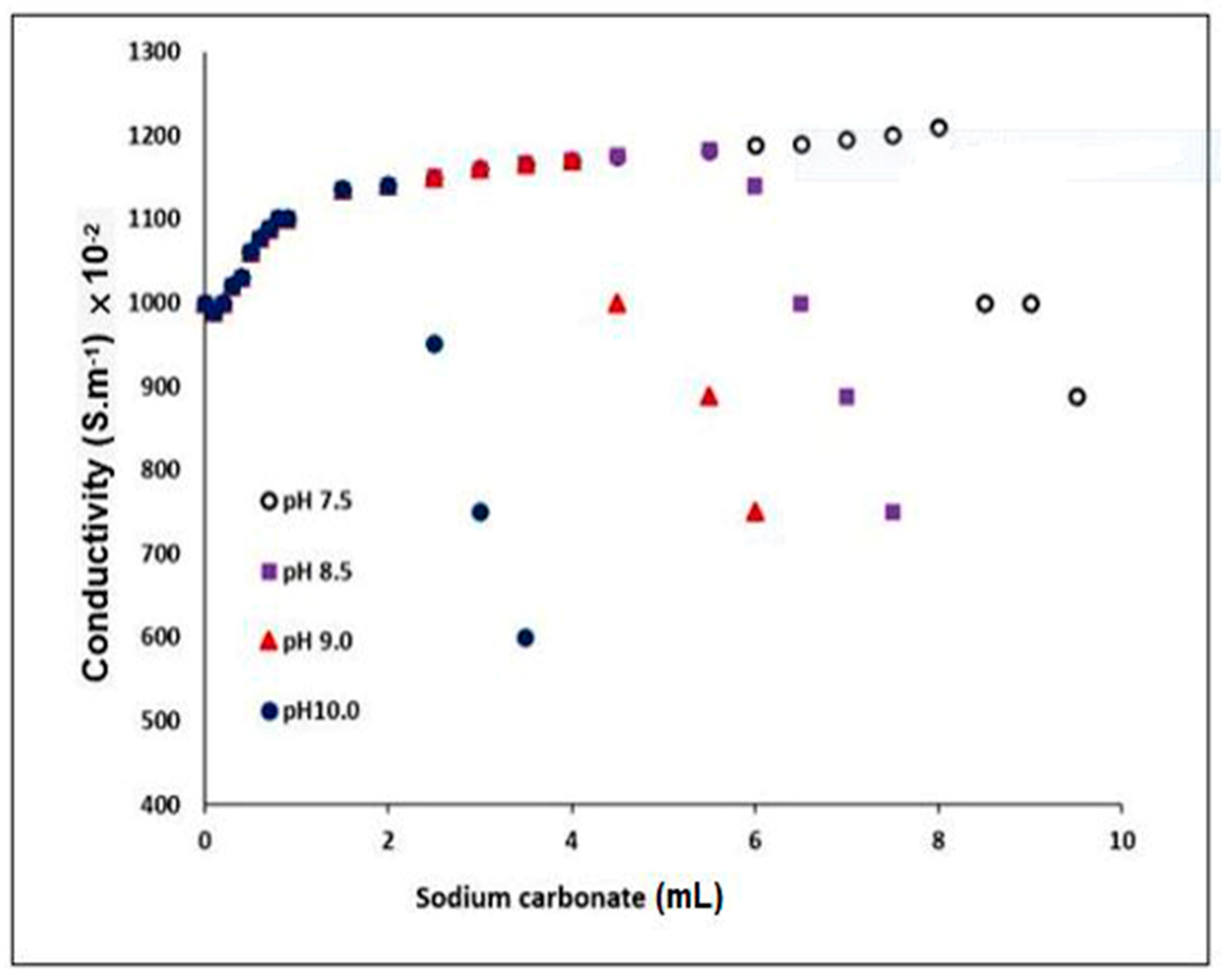
3.3.3. Effect of the pH of the Media
3.4. Solution Analysis
3.4.1. Determination of Residual Calcium Ion Concentration
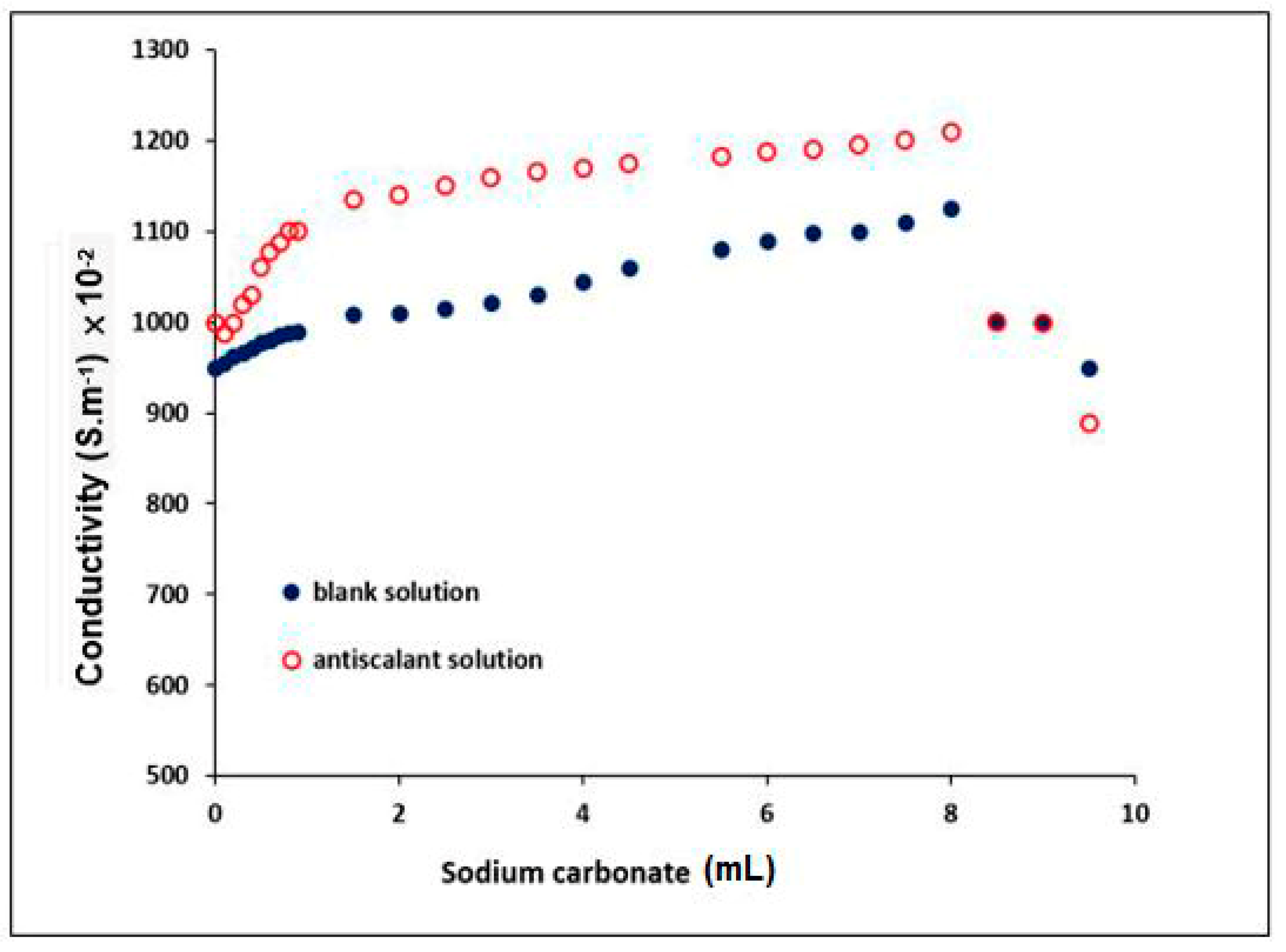
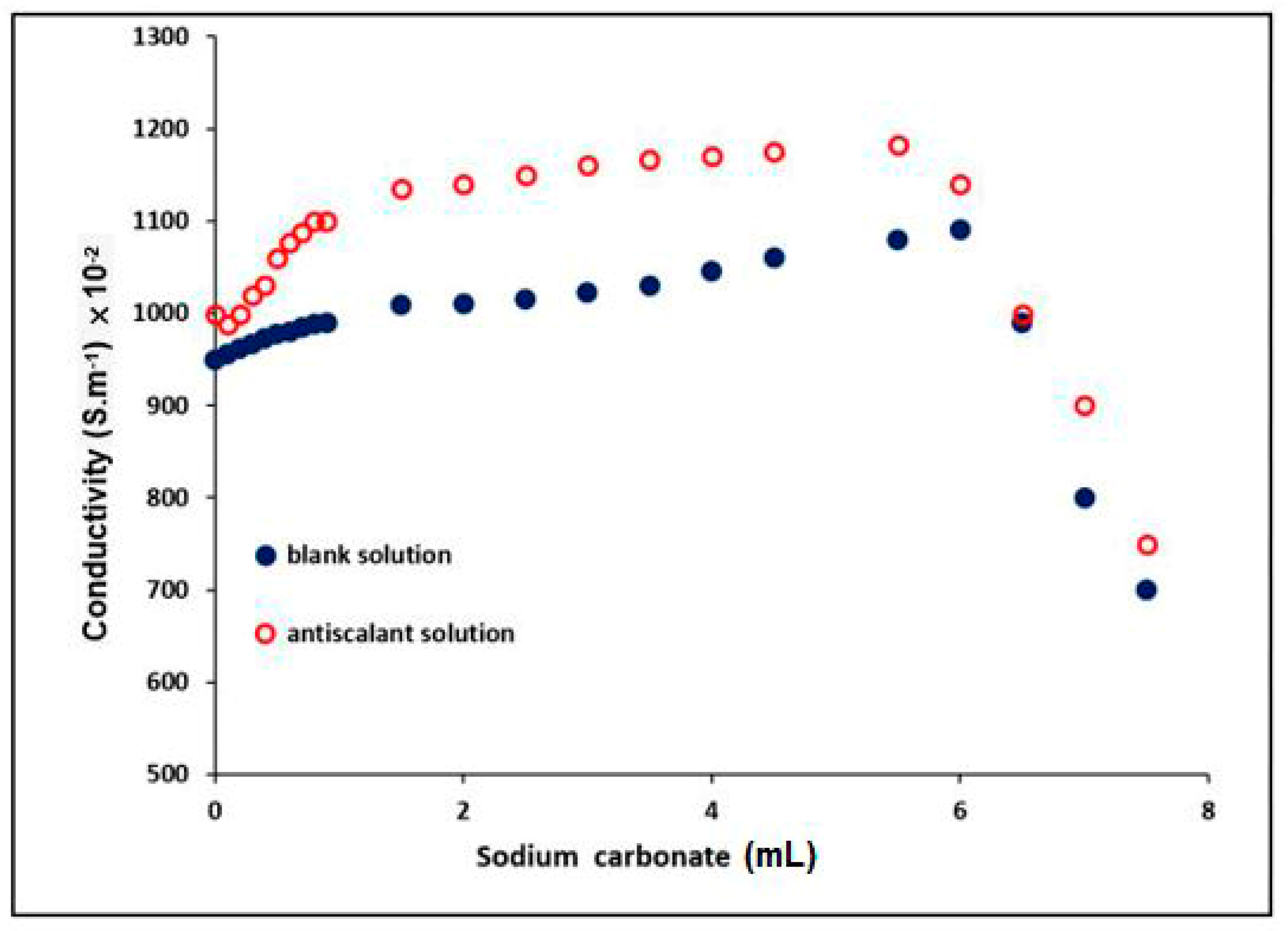
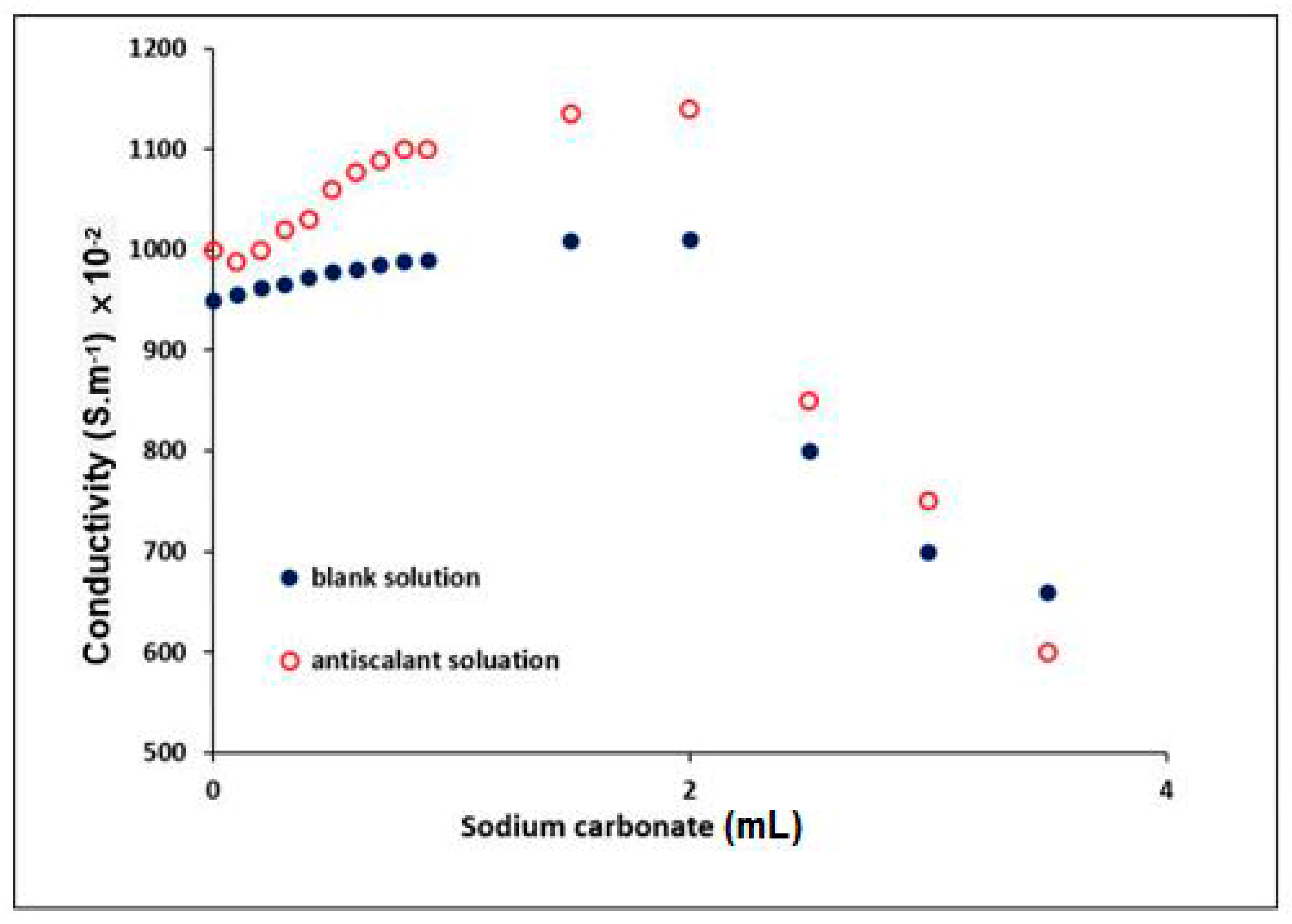
3.4.2. Determination of the Conductivity
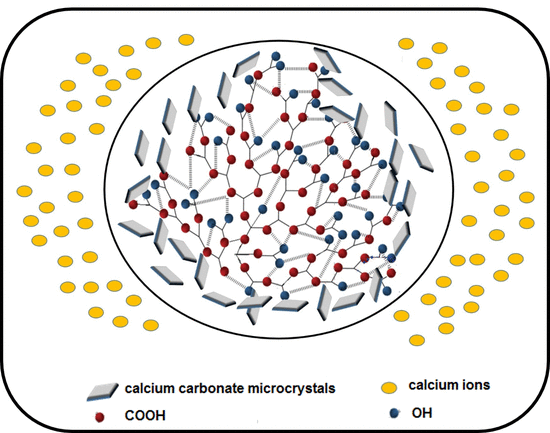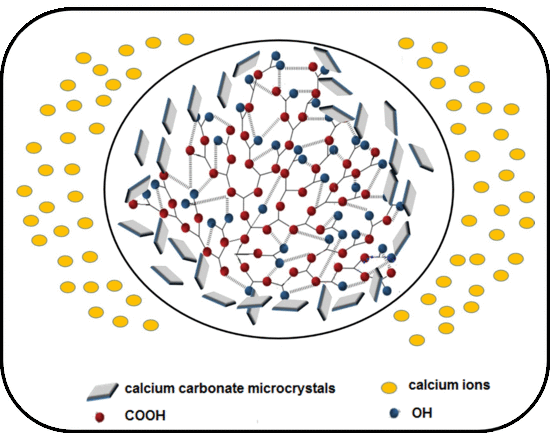
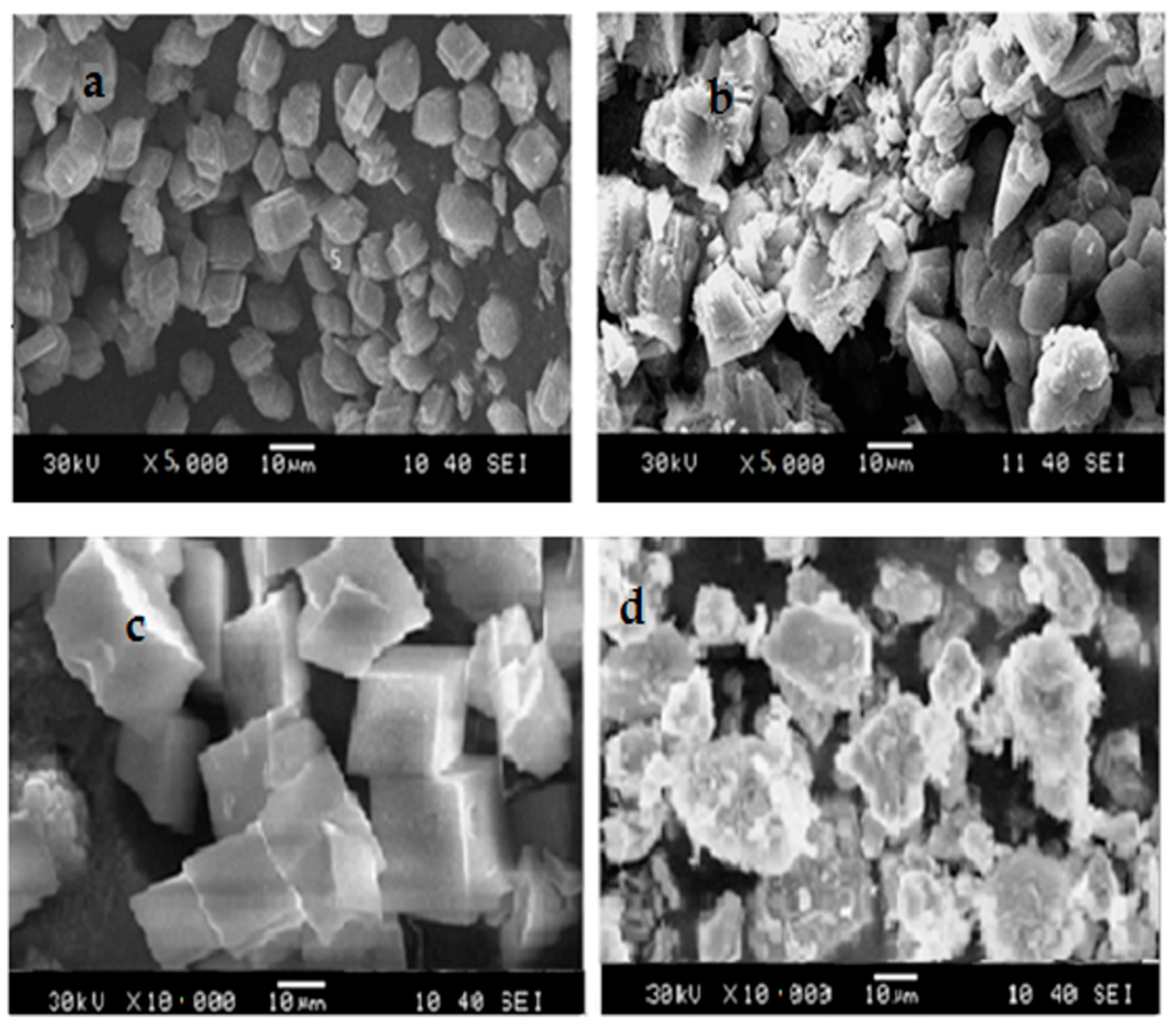
3.4.3. Calcium Carbonate Crystal Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chauhan, K.; Sharma, M.; Chauhan, G.S. Removal/Dissolution of Mineral Scale Deposits. In Mineral Scales and Deposits Scientific and Technological Approaches; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Al-Hamzah, A.A.; East, C.P.; Doherty, W.O.S.; Fellows, C.M. Inhibition of homogenous formation of calcium carbonate by poly (acrylic acid). The effect of molar mass and end-group functionality. Desalination 2014, 338, 93–105. [Google Scholar] [CrossRef]
- Al-Hamzah, A.A.; Fellows, C.M. A comparative study of novel scale inhibitors with commercial scale inhibitors used in seawater desalination. Desalination 2015, 359, 22–25. [Google Scholar] [CrossRef]
- MacAdam, J.; Parsons, S.A. Calcium carbonate scale formation and control. Rev. Environ. Sci. Biotechnol. 2004, 3, 159–169. [Google Scholar] [CrossRef]
- Bahri, S.; Endaryanto, T. Gambier extracts as an inhibitor of calcium carbonate (CaCO3) scale formation. Desalination 2011, 265, 102–106. [Google Scholar]
- Chaussemier, M.; Pourmohtasham, E.; Gelus, D.; Pécoul, N.; Perrot, H.; Lédion, J.; Cheap-Charpentier, H.; Horner, O. State of art of natural inhibitors of calcium carbonate scaling. A review article. Desalination 2015, 356, 47–55. [Google Scholar] [CrossRef]
- Belarbi, Z.; Gamby, J.; Makhloufi, L.; Sotta, B.; Tribollet, B. Inhibition of calcium carbonate precipitation by aqueous extract of Paronychia argentea. J. Cryst. Growth 2014, 386, 208–214. [Google Scholar] [CrossRef]
- Qiang, X.; Sheng, Z.; Zhang, H. Study on scale inhibition performances and interaction mechanism of modified collagen. Desalination 2013, 309, 237–242. [Google Scholar] [CrossRef]
- Wang, H.; Gao, M.; Guo, Y.; Yang, Y.; Hu, R. A natural extract of tobacco rob as scale and corrosion inhibitor in artificial seawater. Desalination 2016, 398, 198–207. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, H.; Zhang, Q.; Li, Y.; Yao, P. Synthesis and characterization of novel polyaspartic acid/urea graft copolymer with acylamino group and its scale inhibition performance. Desalination 2016, 395, 92–98. [Google Scholar] [CrossRef]
- Wang, C.; Shen, T.; Li, S.; Wang, X. Investigation of influence of low phosphorous co-polymer antiscalant on calcium sulfate dihydrate crystal morphologies. Desalination 2014, 348, 89–93. [Google Scholar] [CrossRef]
- Adeli, M.; Rasoulian, B.; Saadatmehr, F.; Zabihi, F. Hyperbranched poly(citric acid) and its application as anticancer drug delivery system. J. Appl. Polym. Sci. 2013, 129, 3665–3671. [Google Scholar] [CrossRef]
- Sobhani, Z.; Dinarvand, R.; Atyabi, F.; Ghahremani, M.; Adeli, M. Increased paclitaxel cytotoxicity against cancer cell lines using a novel functionalized carbon nanotube. Int. J. Nanomed. 2011, 6, 705. [Google Scholar]
- Adeli, M.; Bahari, A.; Hekmatara, H. Carbon nanotube-graft-poly (citric acid) nanocomposites. Nano 2008, 3, 37–44. [Google Scholar] [CrossRef]
- Tisserat, B.; Harry-O’kuru, R.; Hwang, H.-S.; Abdellatif, A.; Holser, R. Glycerol citrate polyesters produced through heating without catalysis. J. Appl. Polym. Sci. 2012, 125, 3429–3437. [Google Scholar] [CrossRef]
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Adeli, M. Carbon Nanotube–Graft-Poly(citric acid). Nano Brief Rep. Rev. 2008, 3, 37–44. [Google Scholar]
- Popuri, S.R.; Hall, C.; Wang, C.C.; Chang, C.Y. Development of green/biodegradable polymers for water scaling applications. Int. Biodeterior. Biodegrad. 2014, 95, 225–231. [Google Scholar] [CrossRef]
- Li, X.; Gao, B.; Yue, Q.; Ma, D.; Rong, H.; Zhao, P.; Teng, P. Effect of six kinds of scale inhibitors on calcium carbonate precipitation in high salinity wastewater at high temperatures. J. Environ. Sci. (China) 2015, 29, 124–130. [Google Scholar] [CrossRef]
- Shen, Z.; Li, J.; Xu, K.; Ding, L.; Ren, H. The effect of synthesized hydrolyzed polymaleic anhydride (HPMA) on the crystal of calcium carbonate. Desalination 2012, 284, 238–244. [Google Scholar] [CrossRef]
- Shakkthivel, P.; Sathiyamoorthi, R.; Vasudevan, T. Development of acrylonitrile copolymers for scale control in cooling water systems. Desalination 2004, 164, 111–123. [Google Scholar] [CrossRef]
- Senthilmurugan, B.; Ghosh, B.; Kundu, S.S.; Haroun, M.; Kameshwari, B. Maleic acid based scale inhibitors for calcium sulfate scale inhibition in high temperature application. J. Pet. Sci. Eng. 2010, 75, 189–195. [Google Scholar] [CrossRef]
- Shakkthivel, P.; Vasudevan, T. Acrylic acid-diphenylamine sulphonic acid copolymer threshold inhibitor for sulphate and carbonate scales in cooling water systems. Desalination 2006, 197, 179–189. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Liu, X.; Li, W.; Wang, J.; Hu, Z. Application of poly(aspartic acid-citric acid) copolymer compound inhibitor as an effective and environmental agent against calcium phosphate in cooling water systems. J. Appl. Res. Technol. 2016, 14, 425–433. [Google Scholar] [CrossRef]
- Migahed, M.A.; Rashwan, S.M.; Kamel, M.M.; Habib, R.E. Synthesis, characterization of polyaspartic acid-glycine adduct and evaluation of their performance as scale and corrosion inhibitor in desalination water plants. J. Mol. Liq. 2016, 224, 849–858. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.M.; Abd-El-Nabey, B.A.; Khamis, E.; Abd-El-Khalek, D.E. Investigation of fig leaf extract as a novel environmentally friendly antiscalent for CaCO3 calcareous deposits. Desalination 2008, 230, 314–328. [Google Scholar] [CrossRef]
- Migahed, M.A.; Rashwan, S.M.; Kamel, M.M.; Habib, R.E. Synthesized polyaspartic acid derivatives as corrosion and scale inhibitors in desalination operations. Cogent Eng. 2017, 4, 1–22. [Google Scholar] [CrossRef]
- Roomi, Y.A.; Hussein, K.F.; Riazi, M.R. Inhibition efficiencies of synthesized anhydride based polymers as scale control additives in petroleum production. J. Pet. Sci. Eng. 2012, 81, 151–160. [Google Scholar] [CrossRef]
- Shi, W.; Xu, W.; Cang, H.; Yan, X.; Shao, R.; Zhang, Y.; Xia, M. Design and synthesis of biodegradable antiscalant based on MD simulation of antiscale mechanism: A case of itaconic acid-epoxysuccinate copolymer. Comput. Mater. Sci. 2017, 136, 118–125. [Google Scholar] [CrossRef]
- Wada, N.; Kanamura, K.; Umegaki, T. Effects of carboxylic acids on the crystallization of calcium carbonate. J. Colloid Interface Sci. 2001, 233, 65–72. [Google Scholar] [CrossRef]
- Legodi, M.A.; De Waal, D.; Potgieter, J.H.; Potgieter, S.S. Technical note rapid determination of CaCO3 in mixtures utilising FT-IR spectroscopy. Miner. Eng. 2016, 14, 1107–1111. [Google Scholar] [CrossRef]
- Shafiu Kamba, A.; Ismail, M.; Tengku Ibrahim, T.A.; Zakaria, Z.A.B. Synthesis and characterisation of calcium carbonate aragonite nanocrystals from cockle shell powder (Anadara granosa). J. Nanomater. 2013, 5. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Han, J.; Su, M.; Wu, Q. Synthesis of modified polyaspartic acid and evaluation of its scale inhibition and dispersion capacity. Desalination 2015, 358, 42–48. [Google Scholar] [CrossRef]





| Antiscalant Dose (mg·L−1) | Residual Ca2+ (mg·L−1) |
|---|---|
| 0 | 1000 |
| 5 | 990 |
| 10 | 995 |
| 15 | 994 |
| 20 | 996 |
| 30 | 994 |
| 40 | 993 |
| 50 | 998 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahlan, H.; Saeed, W.S.; Alrasheed, R.; Alandes, N.M.; Aouak, T. Synthesis of Poly (Citric Acid-Co-Glycerol) and Its Application as an Inhibitor of CaCO3 Deposition. Materials 2019, 12, 3800. https://doi.org/10.3390/ma12223800
Zahlan H, Saeed WS, Alrasheed R, Alandes NM, Aouak T. Synthesis of Poly (Citric Acid-Co-Glycerol) and Its Application as an Inhibitor of CaCO3 Deposition. Materials. 2019; 12(22):3800. https://doi.org/10.3390/ma12223800
Chicago/Turabian StyleZahlan, Hala, Waseem Sharaf Saeed, Radwan Alrasheed, Naser M. Alandes, and Taieb Aouak. 2019. "Synthesis of Poly (Citric Acid-Co-Glycerol) and Its Application as an Inhibitor of CaCO3 Deposition" Materials 12, no. 22: 3800. https://doi.org/10.3390/ma12223800