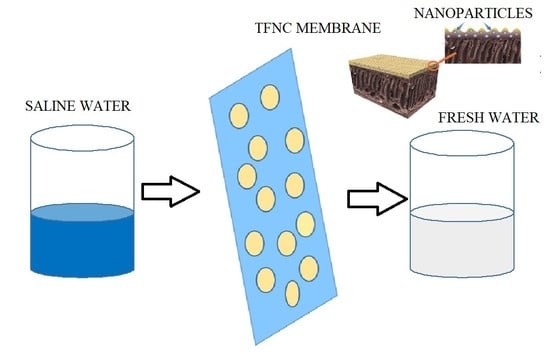
Organically Modified Nanoclay Filled Thin-Film Nanocomposite Membranes for Reverse Osmosis Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Polysulfone Support Membrane Fabrication
2.2.2. Preparation of Thin Polyamide Layer
2.3. Characterization of Membranes
2.3.1. X-Ray Diffraction (XRD)
2.3.2. Transmission Electron Microscopy (TEM)
2.3.3. Contact Angle Measurement
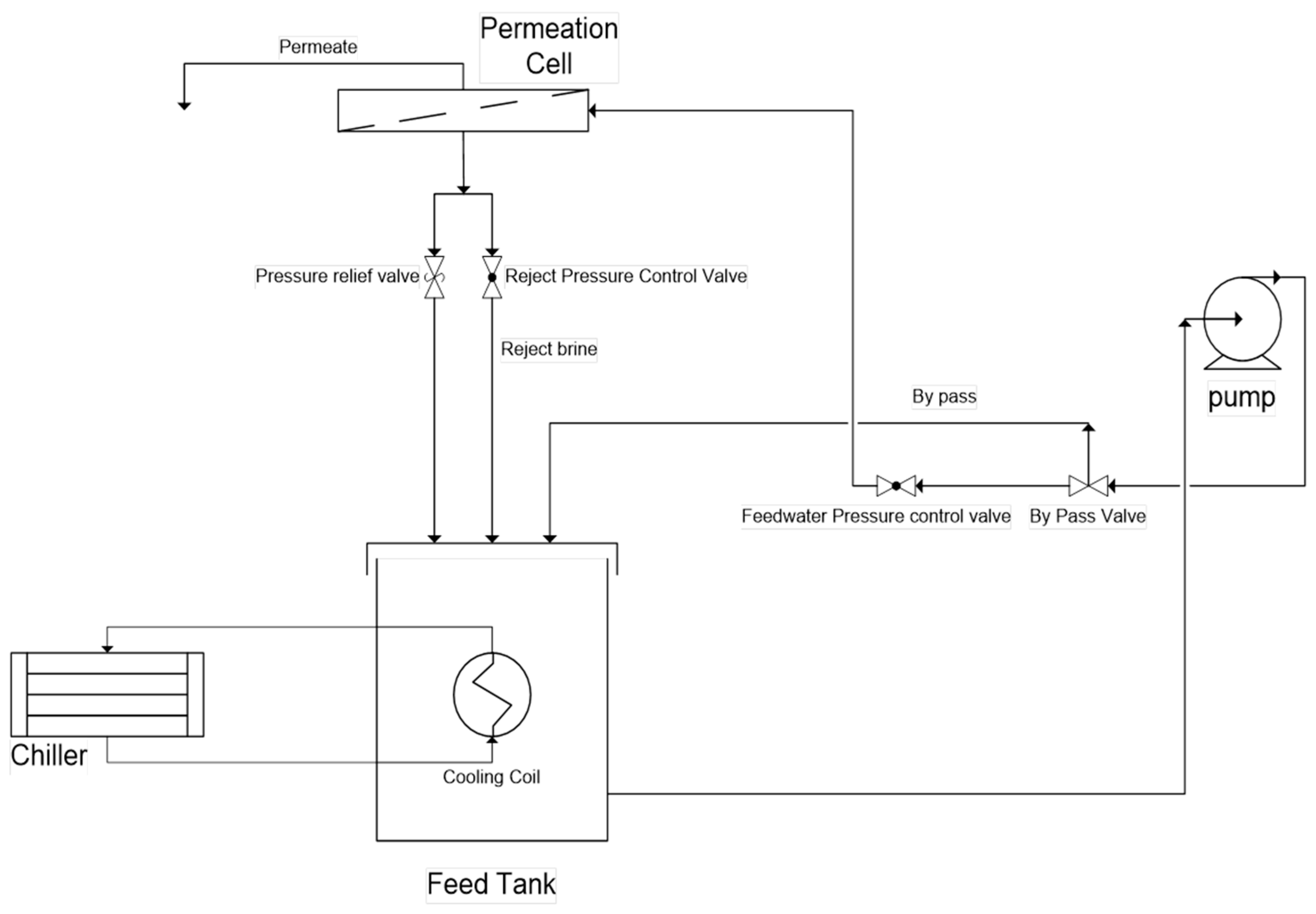
2.4. Membrane Performance Tests
3. Results and Discussion
3.1. Characterization Analyses
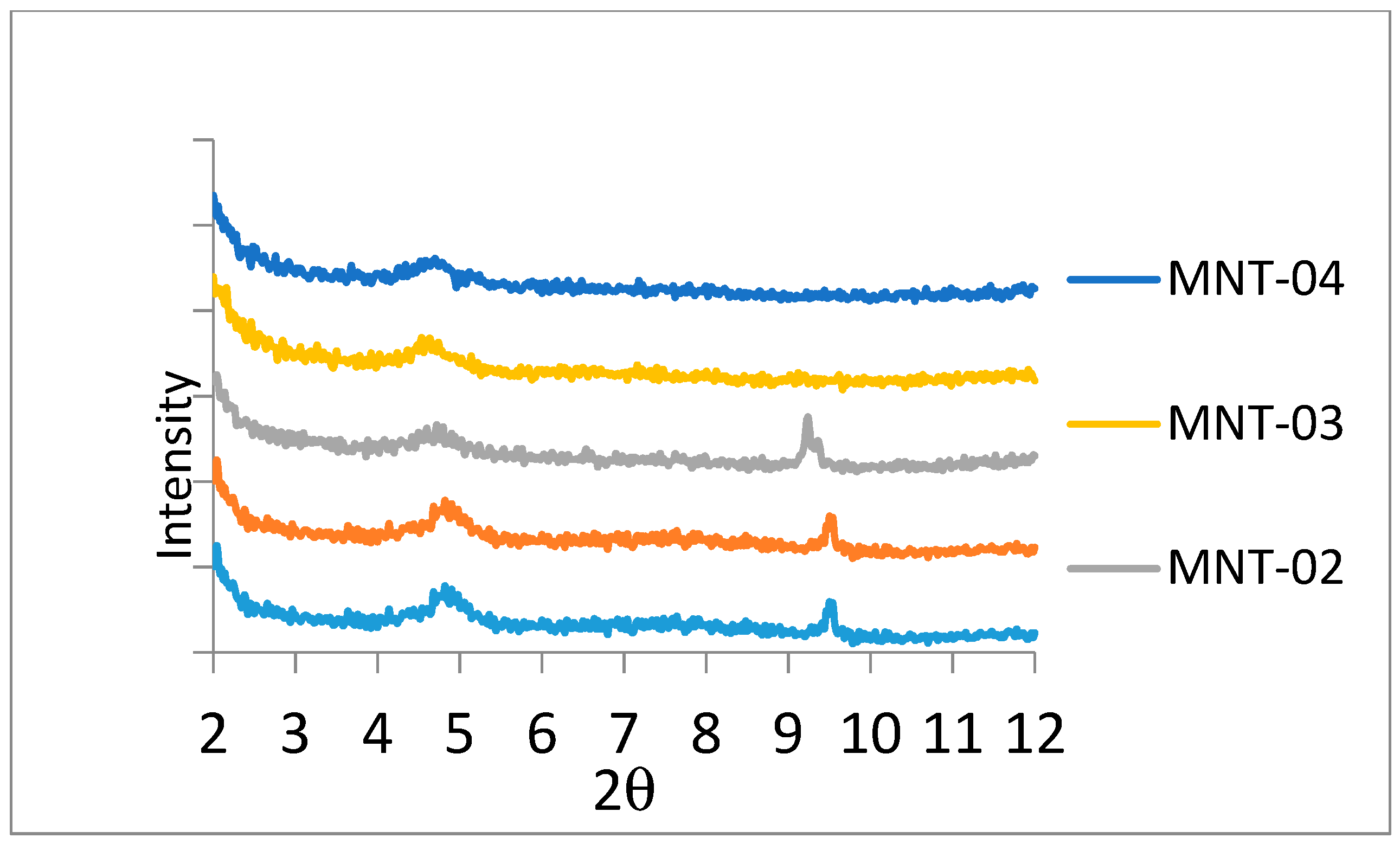
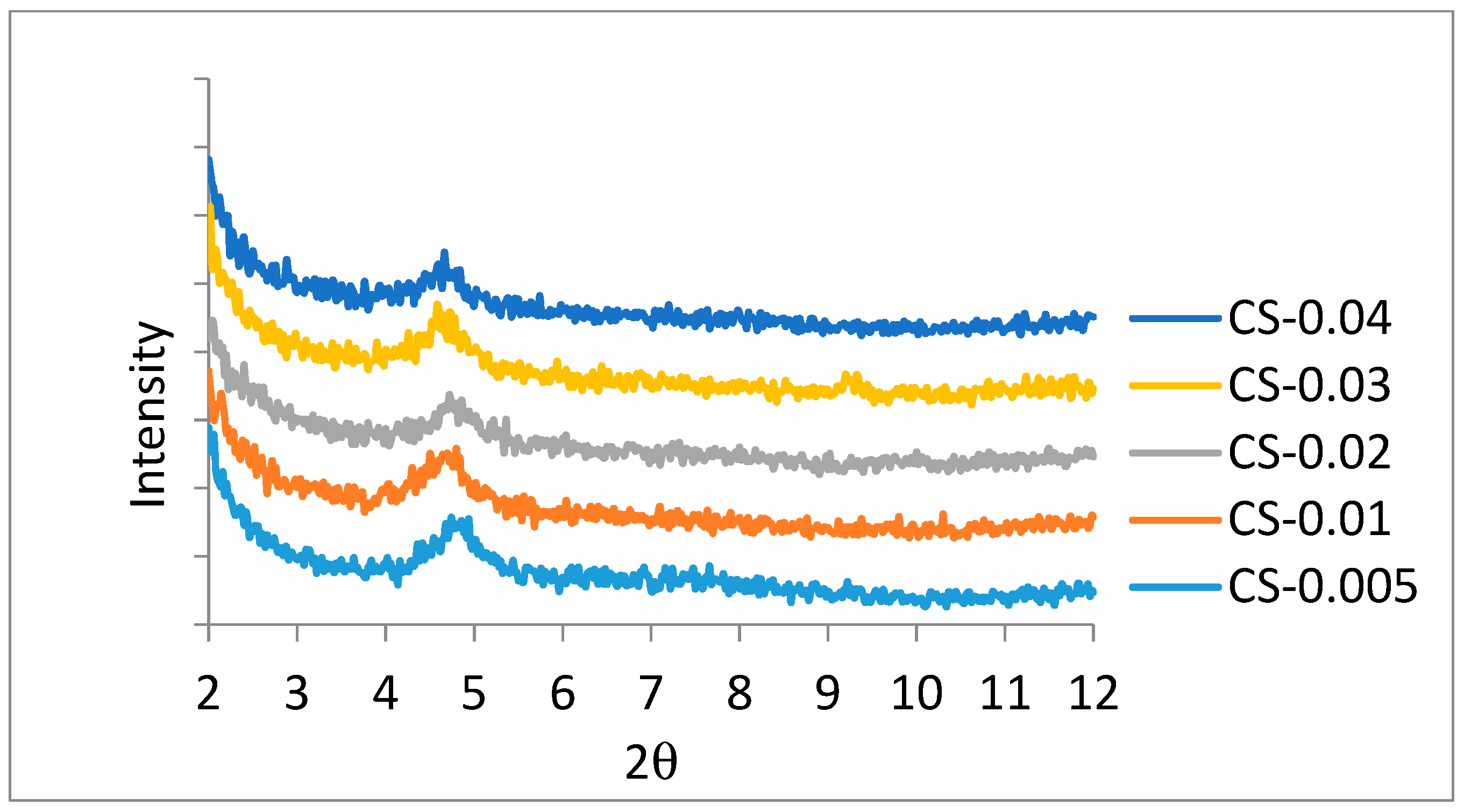
3.1.1. X-Ray Diffraction
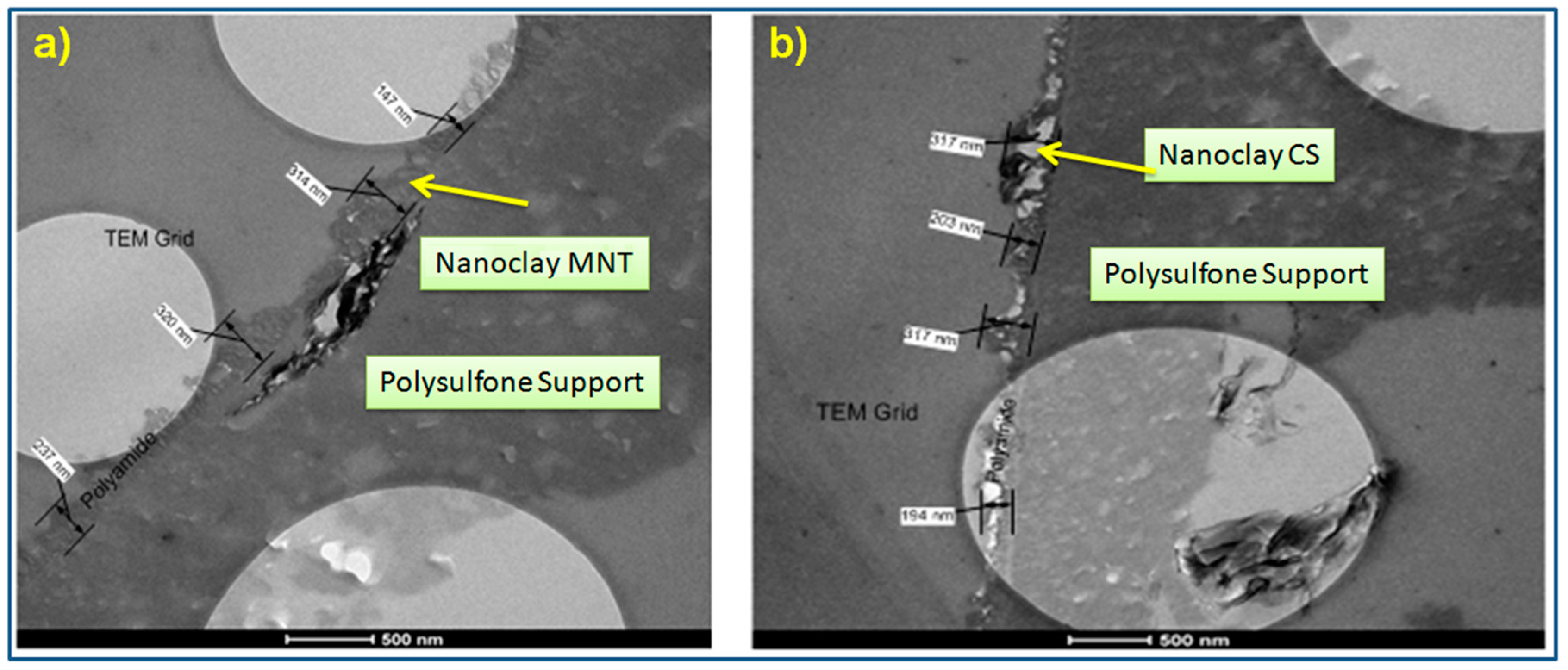
3.1.2. Transmission Electron Microscopy
3.2. Performance Analysis
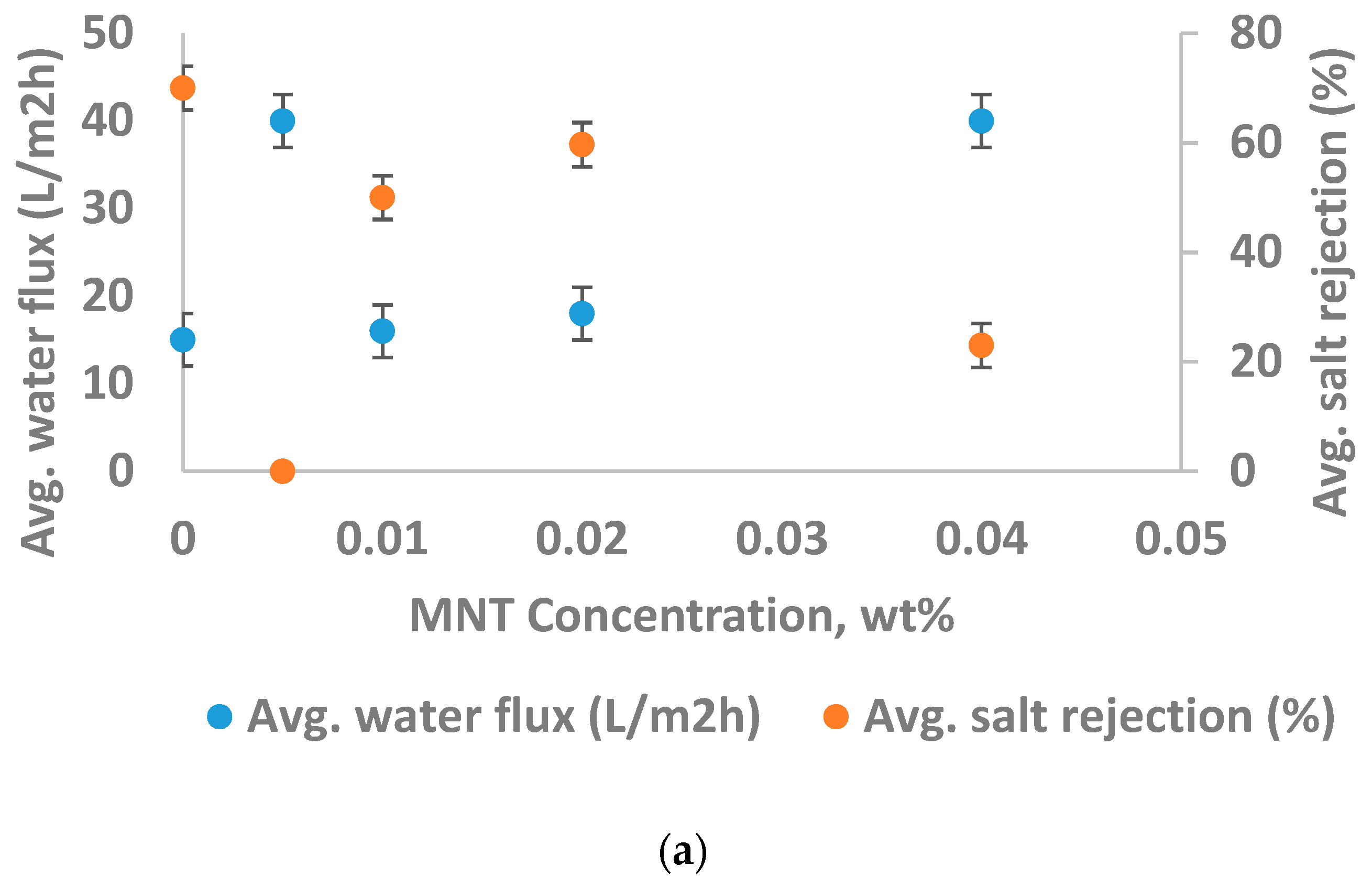
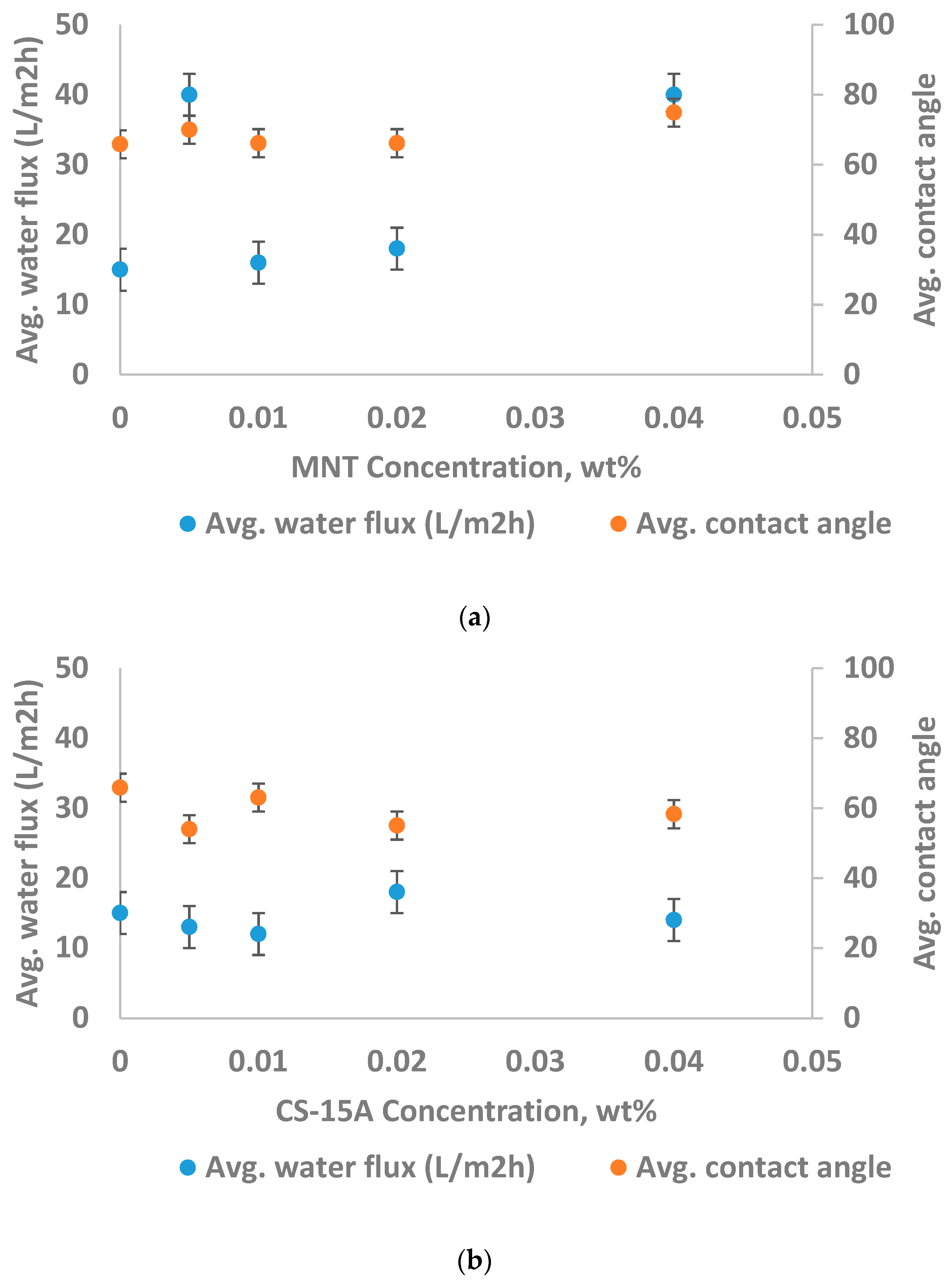
3.2.1. Nanoparticle Concentration and Performance of the Membrane
3.2.2. Membrane Hydrophilicity and Performance
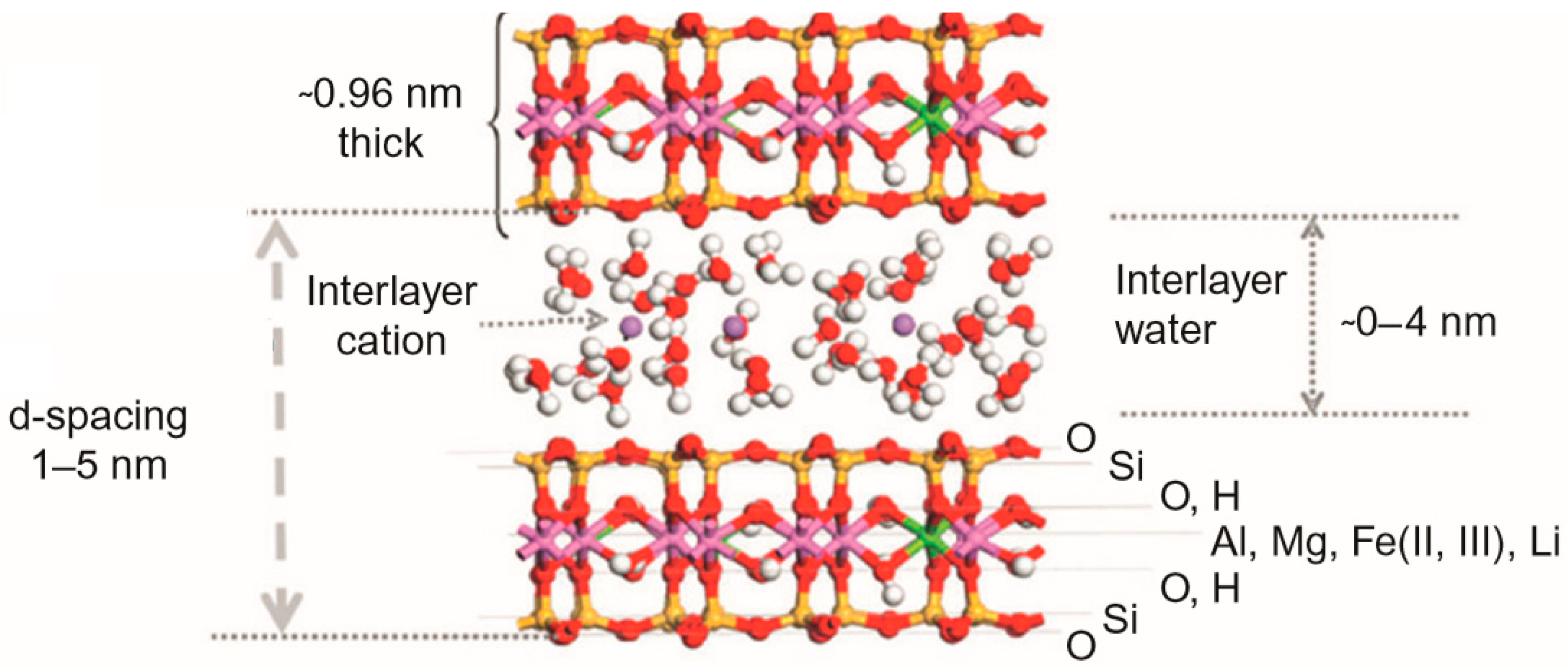
3.3. Mechanism of Water Uptake
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaffer, D.; Yip, N.; Gilron, J.; Elimelech, M. Seawater desalination for agriculture by integrated forward and reverse osmosis: Improved product water quality for potentially less energy. J. Memb. Sci. 2012, 415–416, 1–8. [Google Scholar] [CrossRef]
- Schiermeier, Q. Water: Purification with a pinch of salt. Nature 2008, 452, 260–261. [Google Scholar] [CrossRef]
- Fane, A.; Wang, R.; Hu, M. Synthetic Membranes for Water Purification: Status and Future. Angew. Chem. Int. Ed. Engl. 2015, 54, 3368–3386. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Xiong, H.; Kadhom, M.; Wang, L.; Zhu, G.; Yang, J.; Cunningham, G.; Deng, B. Physico-Chemical Processes. WER 2017, 89, 974–1028. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; Phillip, W. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Cadotte, J.E. Interfacially Synthesized Reverse Osmosis Membrane. U.S. Patent 4,277,344, 7 July 1981. [Google Scholar]
- Cadotte, J.; Petersen, R.; Larson, R.; Erickson, E. A new thin-film composite seawater reverse osmosis membrane. Desalination 1980, 32, 25–31. [Google Scholar] [CrossRef]
- Baker, R. Membrane Technology and Applications; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kadhom, M.; Deng, B. Synthesis of high-performance thin film composite (TFC) membranes by controlling the preparation conditions: Technical notes. J. Water Process Eng. 2019, 30, 100542. [Google Scholar] [CrossRef]
- Seyyed Shahabi, S.; Azizi, N.; Vatanpour, V.; Yousefimehr, N. Novel functionalized graphitic carbon nitride incorporated thin film nanocomposite membranes for high-performance reverse osmosis desalination. Sep. Purif. Technol. 2019, 235, 116134. [Google Scholar] [CrossRef]
- Duan, J.; Pan, Y.; Pacheco, F.; Litwiller, E.; Lai, Z.; Pinnau, I. High-performance polyamide thin-film-nanocomposite reverse osmosis membranes containing hydrophobic zeolitic imidazolate framework-8. J. Memb. Sci. 2015, 476, 303–310. [Google Scholar] [CrossRef]
- Seyyed Shahabi, S.; Azizi, N.; Vatanpour, V. Synthesis and characterization of novel g-C3N4 modified thin film nanocomposite reverse osmosis membranes to enhance desalination performance and fouling resistance. Sep. Purif. Technol. 2019, 215, 430–440. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.; Hamid, S.; Ramakrishna, S.; Chowdhury, Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Jeong, B.; Hoek, E.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Memb. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, H.; Kim, S.; Lee, J.; Yoon, J. Evaluation of carbon nanotube-polyamide thin-film nanocomposite reverse osmosis membrane: Surface properties, performance characteristics and fouling behavior. J. Ind. Eng. Chem. 2017, 56, 327–334. [Google Scholar] [CrossRef]
- Ghaseminezhad, S.; Barikani, M.; Salehirad, M. Development of graphene oxide-cellulose acetate nanocomposite reverse osmosis membrane for seawater desalination. Compos. Part B Eng. 2019, 161, 320–327. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Thin film nanocomposite reverse osmosis membrane modified by reduced graphene oxide/TiO2 with improved desalination performance. J. Memb. Sci. 2015, 489, 43–54. [Google Scholar] [CrossRef]
- Li, S.; Gao, B.; Wang, Y.; Jin, B.; Yue, Q.; Wang, Z. Antibacterial thin film nanocomposite reverse osmosis membrane by doping silver phosphate loaded graphene oxide quantum dots in polyamide layer. Desalination 2019, 464, 94–104. [Google Scholar] [CrossRef]
- Nidhi Maalige, R.; Aruchamy, K.; Mahto, A.; Sharma, V.; Deepika, D.; Mondal, D.; Nataraj, S.K. Low operating pressure nanofiltration membrane with functionalized natural nanoclay as antifouling and flux promoting agent. Chem. Eng. J. 2019, 358, 821–830. [Google Scholar] [CrossRef]
- Choudalakis, G.; Gotsis, A. Permeability of polymer/clay nanocomposites: A review. Eur. Polym. J. 2009, 45, 967–984. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Gupta, S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Ishiguro, M.; Matsuura, T.; Detellier, C. Reverse osmosis separation for a montmorillonite membrane. J. Memb. Sci. 1995, 107, 87–92. [Google Scholar] [CrossRef]
- Dong, H.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Clay nanosheets as charged filler materials for high-performance and fouling-resistant thin film nanocomposite membranes. J. Memb. Sci. 2015, 494, 92–103. [Google Scholar] [CrossRef]
- Kadhom, M.; Deng, B. Thin film nanocomposite membranes filled with bentonite nanoparticles for brackish water desalination: A novel water uptake concept. Micropor. Mesopor. Mat. 2019, 279, 82–91. [Google Scholar] [CrossRef]
- Jaafar, J.; Ismail, A.; Matsuura, T. Preparation and barrier properties of SPEEK/Cloisite 15A®/TAP nanocomposite membrane for DMFC application. J. Memb. Sci. 2009, 345, 119–127. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.; Cauich-Rodríguez, J.; Vázquez-Torres, H.; Garfias-Mesías, L.; Paul, D. Thermal degradation of commercially available organoclays studied by TGA–FTIR. Thermochim. Acta 2007, 457, 92–102. [Google Scholar] [CrossRef]
- Singla, P.; Mehta, R.; Upadhyay, S. Microwave assisted in situ ring-opening polymerization of polylactide/clay nanocomposites: Effect of clay loading. Appl. Clay Sci. 2014, 95, 67–73. [Google Scholar] [CrossRef]
- Ghaemi, N.; Madaeni, S.; Alizadeh, A.; Rajabi, H.; Daraei, P. Preparation, characterization and performance of polyethersulfone/organically modified montmorillonite nanocomposite membranes in removal of pesticides. J. Memb. Sci. 2011, 382, 135–147. [Google Scholar] [CrossRef]
- Anadão, P.; Sato, L.; Wiebeck, H.; Valenzuela-Díaz, F. Montmorillonite as a component of polysulfone nanocomposite membranes. Appl. Clay Sci. 2010, 48, 127–132. [Google Scholar] [CrossRef]
- Villaluenga, J.; Khayet, M.; López-Manchado, M.; Valentin, J.; Seoane, B.; Mengual, J. Gas transport properties of polypropylene/clay composite membranes. Eur. Polym. J. 2007, 43, 1132–1143. [Google Scholar] [CrossRef]
- Hashemifard, S.; Ismail, A.; Matsuura, T. Effects of montmorillonite nano-clay fillers on PEI mixed matrix membrane for CO2 removal. Chem. Eng. 2011, 170, 316–325. [Google Scholar] [CrossRef]
- García, A.; Eceolaza, S.; Iriarte, M.; Uriarte, C.; Etxeberria, A. Barrier character improvement of an amorphous polyamide (Trogamid) by the addition of a nanoclay. J. Memb. Sci. 2007, 301, 190–199. [Google Scholar] [CrossRef]
- Fadhillah, F. Application of Polyelectrolyte Multilayer Reverse Osmosis Membrane in Seawater Desalination. 2012. Available online: https://search.proquest.com/openview/0857d06fdb19706a54729eeabb693f79/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 15 October 2019).
- Yang, Z.; Guo, H.; Tang, C.Y. The upper bound of thin-film composite (TFC) polyamide membranes for desalination. J. Memb. Sci. 2019, 590, 117297. [Google Scholar] [CrossRef]
- Wang, A.; D’Souza, N.; Golden, T. Ceramic montmorillonite nanocomposites by electrochemical synthesis. Appl. Clay Sci. 2008, 42, 310–317. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef] [Green Version]
- Nikolaidis, A.; Achilias, D.; Karayannidis, G. Synthesis and Characterization of PMMA/Organomodified Montmorillonite Nanocomposites Prepared by in Situ Bulk Polymerization. Ind. Eng. Chem. Res. 2011, 50, 571–579. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface Modifications for Antifouling Membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef]
- Pielichowski, K.; Majka, T.M. Polymer Composites with Functionalized Nanoparticles: Synthesis, Properties, and Applications Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-814064-2. [Google Scholar] [CrossRef]
- Yariv, S. The effect of tetrahedral substitution of Si by Al on the surface acidity of the oxygen plane of clay minerals. Int. Rev. Phys. Chem. 1992, 11, 345–375. [Google Scholar] [CrossRef]
- Laird, D. Layer Charge Influences on the Hydration of Expandable 2:1 Phyllosilicates. Clays Clay Miner. 1999, 47, 630–636. [Google Scholar] [CrossRef]
- Hensen, E.; Smit, B. Why Clays Swell. J. Phys. Chem. B 2002, 106, 12664–12667. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.; Xiao-Feng, T.; Yu, Z.; Tao, G. Swelling of K+, Na+and Ca2+-montmorillonites and hydration of interlayer cations: A molecular dynamics simulation. Chin. Phys. B 2010, 19, 109101. [Google Scholar] [CrossRef] [Green Version]
- Schoonheydt, R.A.; Johnston, C.T. Surface and Interface Chemistry of Clay Minerals; Developments in clay science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 139–172. [Google Scholar]
- Uddin, F. Montmorillonite: An Introduction to Properties and Utilization. In Current Topics in the Utilization of Clay in Industrial and Medical Applications; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
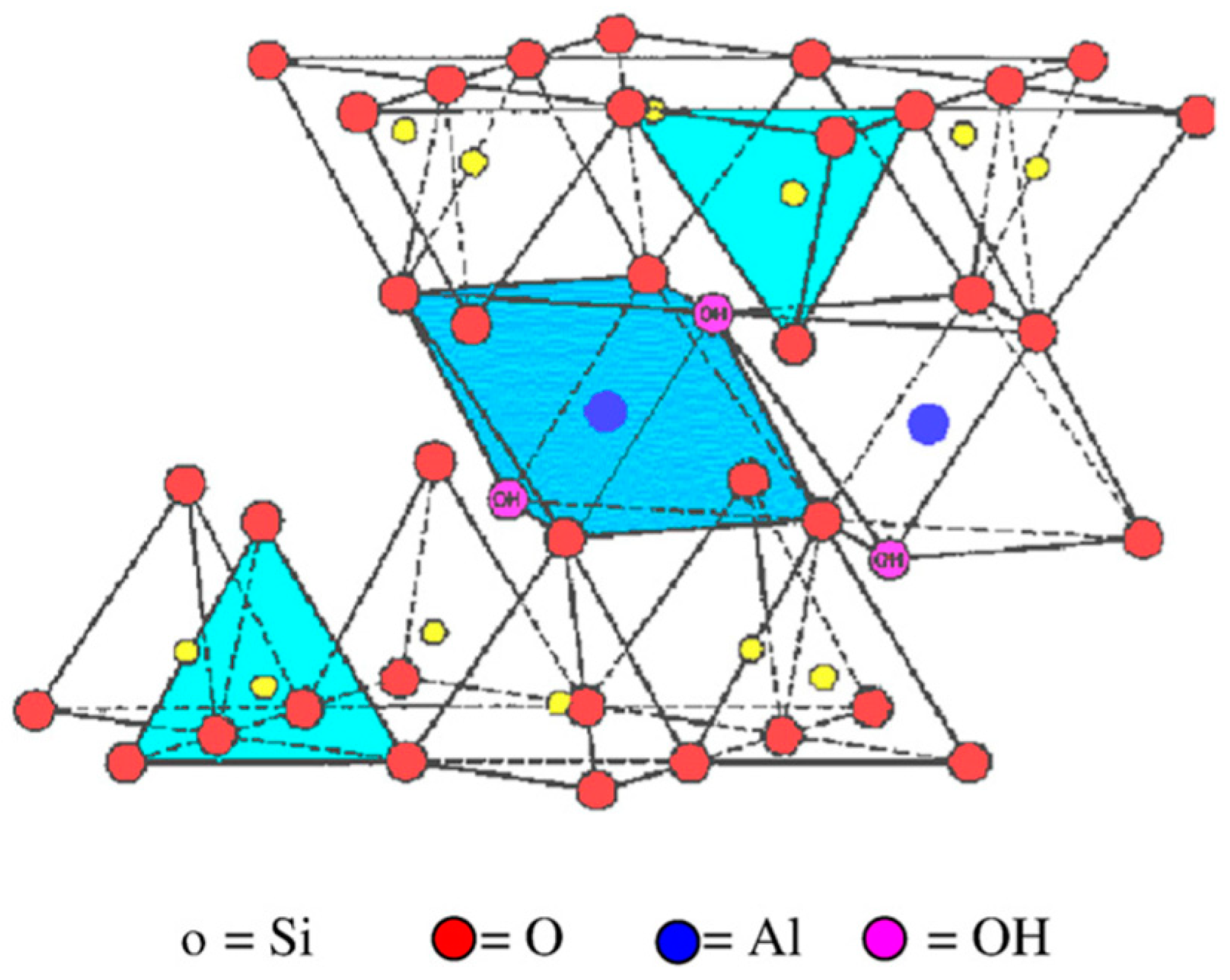
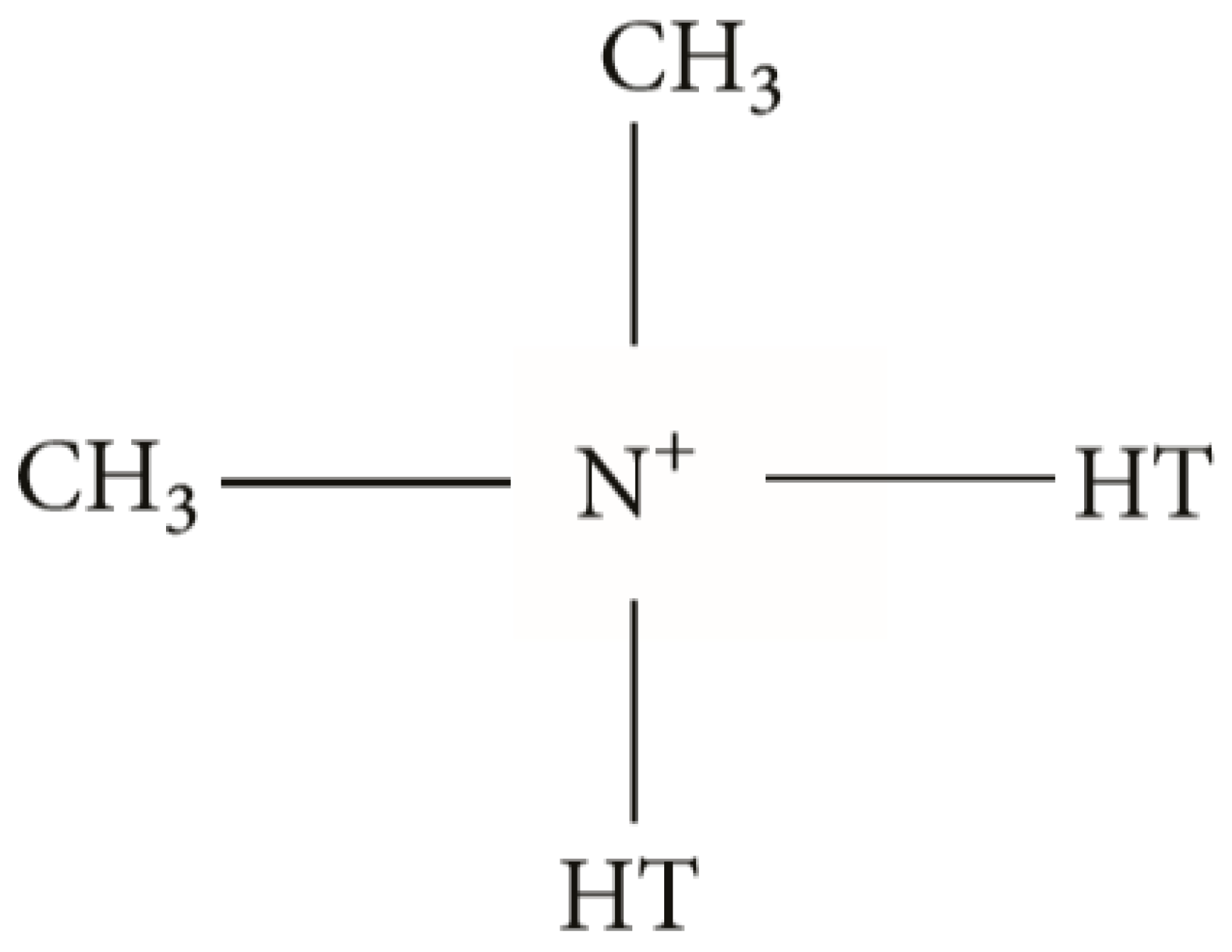


| Physical Properties | MNT |
|---|---|
| Density in g/cm3 | 2.80 |
| Particle (clay platelet) size in m | ≤2 (10%); ≤10 (50%); ≤25 (90%). |
| XRD d-Spacing in nm | 1.55 |
| Moisture content in % | ≤9.0% |
| Physical Properties | CS-15A |
|---|---|
| Density in g/cm3 | 1.66 |
| Particle (clay platelet) size in m | ≤2 (10%); ≤6 (50%); ≤13 (90%) |
| XRD d-Spacing in nm | 3.15 |
| Moisture content in % | ≤2.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaidi, S.J.; Fadhillah, F.; Saleem, H.; Hawari, A.; Benamor, A. Organically Modified Nanoclay Filled Thin-Film Nanocomposite Membranes for Reverse Osmosis Application. Materials 2019, 12, 3803. https://doi.org/10.3390/ma12223803
Zaidi SJ, Fadhillah F, Saleem H, Hawari A, Benamor A. Organically Modified Nanoclay Filled Thin-Film Nanocomposite Membranes for Reverse Osmosis Application. Materials. 2019; 12(22):3803. https://doi.org/10.3390/ma12223803
Chicago/Turabian StyleZaidi, Syed Javaid, Farid Fadhillah, Haleema Saleem, Alaa Hawari, and Abdelbaki Benamor. 2019. "Organically Modified Nanoclay Filled Thin-Film Nanocomposite Membranes for Reverse Osmosis Application" Materials 12, no. 22: 3803. https://doi.org/10.3390/ma12223803