Effect of the Number of Anchoring and Electron-Donating Groups on the Efficiency of Free-Base- and Zn-Porphyrin-Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of New Compounds
2.1.1. Synthesis of Free-Base Porphyrins H2P-(CO2Me)n 7–9
2.1.2. Synthesis of Free-Base Porphyrins H2P-(CO2H)n 1–3:
2.1.3. Synthesis of Zinc Porphyrins ZnP-(CO2H)n 4–6
2.2. Device Preparation
2.3. Photovoltaic Characterization
3. Results and Discussion
3.1. Synthesis of New Compounds
3.2. Optical and Electrochemical Properties
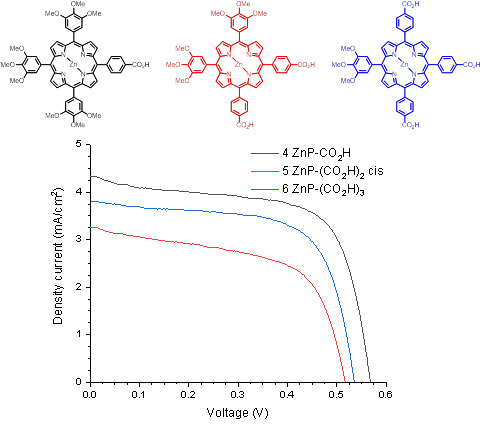
3.3. Preparation of Devices and Photovoltaic Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kundu, S.; Patra, A. Nanoscale Strategies for Light Harvesting. Chem. Rev. 2017, 117, 712–757. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Long, S.; Peh, S.B.; Dong, J.; Wang, Y.; Karmakar, A.; Yuan, Y.D.; Cheng, Y.; Zhao, D. Luminescent Metal–Organic Frameworks for the Detection and Discrimination of o-Xylene from Xylene Isomers. Inorg. Chem. 2018, 57, 13631–13639. [Google Scholar] [CrossRef]
- Acherar, S.; Colombeau, L.; Frochot, C.; Vanderesse, R. Synthesis of Porphyrin, Chlorin and Phthalocyanine Derivatives by Azide-Alkyne Click Chemistry. Curr. Med. Chem. 2015, 22, 3217–3254. [Google Scholar] [CrossRef] [PubMed]
- Stulz, E. Nanoarchitectonics with Porphyrin Functionalized DNA. Acc. Chem. Res. 2017, 50, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Hu, J.-Y.; Xiao, B.; Tang, A.; Wang, X.; Zhou, E. Recent progress in porphyrin-based materials for organic solar cells. J. Mater. Chem. A 2018, 6, 16769–16797. [Google Scholar] [CrossRef]
- Song, H.; Liu, Q.; Xie, Y. Porphyrin-sensitized solar cells: Systematic molecular optimization, coadsorption and cosensitization. Chem. Commun. 2018, 54, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Biroli, A.O.; Tessore, F.; Caramori, S.; Pizzotti, M. β-Substituted Zn II porphyrins as dyes for DSSC: A possible approach to photovoltaic windows. Coord. Chem. Rev. 2018, 358, 153–177. [Google Scholar] [CrossRef]
- Birel, Ö.; Nadeem, S.; Duman, H. Porphyrin-Based Dye-Sensitized Solar Cells (DSSCs): A Review. J. Fluoresc. 2017, 27, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Ladomenou, K.; Kitsopoulos, T.N.; Sharma, G.D.; Coutsolelos, A.G. The importance of various anchoring groups attached on porphyrins as potential dyes for DSSC applications. RSC Adv. 2014, 4, 21379–21404. [Google Scholar] [CrossRef]
- Santos, T.D.; Morandeira, A.; Koops, S.; Mozer, A.J.; Tsekouras, G.; Dong, Y.; Wagner, P.; Wallace, G.; Earles, J.C.; Gordon, K.C.; et al. Injection Limitations in a Series of Porphyrin Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 3276–3279. [Google Scholar] [CrossRef]
- Higashino, T.; Sugiura, K.; Tsuji, Y.; Nimura, S.; Ito, S.; Imahori, H. A Push–Pull Porphyrin Dimer with Multiple Electron-donating Groups for Dye-sensitized Solar Cells: Excellent Light-harvesting in Near-infrared Region. Chem. Lett. 2016, 45, 1126–1128. [Google Scholar] [CrossRef] [Green Version]
- Chaudhri, N.; Sawhney, N.; Madhusudhan, B.; Raghav, A.; Sankar, M.; Satapathi, S. Effect of functional groups on sensitization of dye-sensitized solar cells (DSSCs) using free base porphyrins. J. Porphyr. Phthalocyanines 2017, 21, 222–230. [Google Scholar] [CrossRef]
- Xiang, H.; Fan, W.; Li, J.H.; Li, T.; Robertson, N.; Song, X.; Wu, W.; Wang, Z.; Zhu, W.; Tian, H. High Performance Porphyrin-Based Dye-Sensitized Solar Cells with Iodine and Cobalt Redox Shuttles. ChemSusChem 2017, 10, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Parsa, Z.; Naghavi, S.S.; Safari, N. Designing Push–Pull Porphyrins for Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. A 2018, 122, 5870–5877. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Díaz, M.V.; de la Torre, G.; Torres, T. Lighting porphyrins and phthalocyanines for molecular photovoltaics. Chem. Commun. 2010, 46, 7090. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.S.K.; Liu, Y.-C.; Chou, H.-H.; Kala, K.; Wei, T.-C.; Yeh, C.-Y. Synthesis and Characterization of Novel β-Bis(N,N-diarylamino)-Substituted Porphyrin for Dye-Sensitized Solar Cells under 1 sun and Dim Light Conditions. ACS Appl. Mater. Interfaces 2018, 10, 39970–39982. [Google Scholar] [CrossRef]
- Wu, S.-L.; Lu, H.-P.; Yu, H.-T.; Chuang, S.-H.; Chiu, C.-L.; Lee, C.-W.; Diau, E.W.-G.; Yeh, C.-Y. Design and characterization of porphyrin sensitizers with a push-pull framework for highly efficient dye-sensitized solar cells. Energy Environ. Sci. 2010, 3, 949–955. [Google Scholar] [CrossRef]
- Imahori, H.; Matsubara, Y.; Iijima, H.; Umeyama, T.; Matano, Y.; Ito, S.; Niemi, M.; Tkachenko, N.V.; Lemmetyinen, H. Effects of meso-Diarylamino Group of Porphyrins as Sensitizers in Dye-Sensitized Solar Cells on Optical, Electrochemical, and Photovoltaic Properties. J. Phys. Chem. C 2010, 114, 10656–10665. [Google Scholar] [CrossRef]
- Sirithip, K.; Prachumrak, N.; Rattanawan, R.; Keawin, T.; Sudyoadsuk, T.; Namuangruk, S.; Jungsuttiwong, S.; Promarak, V. Zinc–Porphyrin Dyes with Different meso-Aryl Substituents for Dye-Sensitized Solar Cells: Experimental and Theoretical Studies. Chem. Asian J. 2015, 10, 882–893. [Google Scholar] [CrossRef]
- Keawin, T.; Tarsang, R.; Sirithip, K.; Prachumrak, N.; Sudyoadsuk, T.; Namuangruk, S.; Roncali, J.; Kungwan, N.; Promarak, V.; Jungsuttiwong, S. Anchoring number-performance relationship of zinc-porphyrin sensitizers for dye-sensitized solar cells: A combined experimental and theoretical study. Dyes Pigm. 2017, 136, 697–706. [Google Scholar] [CrossRef]
- Rangan, S.; Coh, S.; Bartynski, R.A.; Chitre, K.P.; Galoppini, E.; Jaye, C.; Fischer, D. Energy Alignment, Molecular Packing, and Electronic Pathways: Zinc(II) Tetraphenylporphyrin Derivatives Adsorbed on TiO2(110) and ZnO(11–20) Surfaces. J. Phys. Chem. C 2012, 116, 23921–23930. [Google Scholar] [CrossRef]
- Urbani, M.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-Substituted Porphyrins for Dye-Sensitized Solar Cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef]
- Ambre, R.B.; Mane, S.B.; Chang, G.-F.; Hung, C.-H. Effects of Number and Position of Meta and Para Carboxyphenyl Groups of Zinc Porphyrins in Dye-Sensitized Solar Cells: Structure–Performance Relationship. ACS Appl. Mater. Interfaces 2015, 7, 1879–1891. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Finarelli, J.D.; Goldmacher, J.; Assour, J.; Korsakoff, L. A simplified synthesis for meso-tetraphenylporphine. J. Org. Chem. 1967, 32, 476. [Google Scholar] [CrossRef]
- Gouterman, M. Spectra of Porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Gierschner, J.; Cornil, J.; Egelhaaf, H.-J. Optical Bandgaps of π-Conjugated Organic Materials at the Polymer Limit: Experiment and Theory. Adv. Mater. 2007, 19, 173–191. [Google Scholar] [CrossRef]
- Shi, W.; Peng, B.; Guo, Y.; Lin, L.; Peng, T.; Li, R. Synthesis of asymmetric zinc phthalocyanine with bulky diphenylthiophenol substituents and its photovoltaic performance for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2016, 321, 248–256. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Q.; Yang, Y.; Zhong, H.; He, C.; Sang, G.; Liu, W.; Yang, C.; Bai, F.; Li, Y. Binaphthyl-Containing Green- and Red-Emitting Molecules for Solution-Processable Organic Light-Emitting Diodes. Adv. Funct. Mater. 2008, 18, 3299–3306. [Google Scholar] [CrossRef]
- Meot-Ner, M.; Adler, A.D. Substituent effects in noncoplanar pi systems: ms-Porphyrins. J. Am. Chem. Soc. 1975, 97, 5107–5111. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Eu, S.; Katoh, T.; Umeyama, T.; Matano, Y.; Imahori, H. Synthesis of sterically hindered phthalocyanines and their applications to dye-sensitized solar cells. Dalton Trans. 2008, 5476–5483. [Google Scholar] [CrossRef] [PubMed]
- Pavlishchuk, V.V.; Addison, A.W. Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25 °C. Inorg. Chim. Acta 2000, 298, 97–102. [Google Scholar] [CrossRef]
- Tan, Q.; Zhang, X.; Mao, L.; Xin, G.; Zhang, S. Novel zinc porphyrin sensitizers for dye-sensitized solar cells: Synthesis and spectral, electrochemical, and photovoltaic properties. J. Mol. Struct. 2013, 1035, 400–406. [Google Scholar] [CrossRef]
- Martin-Gomis, L.; Fernández-Lázaro, F.; Sastre-Santos, Á. Advances in phthalocyanine-sensitized solar cells (PcSSCs). J. Mater. Chem. A 2014, 2, 15672–15682. [Google Scholar] [CrossRef]
- Cherian, S.; Wamser, C.C. Adsorption and Photoactivity of Tetra(4-carboxyphenyl)porphyrin (TCPP) on Nanoparticulate TiO2. J. Phys. Chem. B 2000, 104, 3624–3629. [Google Scholar] [CrossRef]
- Rochford, J.; Chu, D.; Hagfeldt, A.; Galoppini, E. Tetrachelate Porphyrin Chromophores for Metal Oxide Semiconductor Sensitization: Effect of the Spacer Length and Anchoring Group Position. J. Am. Chem. Soc. 2007, 129, 4655–4665. [Google Scholar] [CrossRef]









| Dye | λabs/nm (log ε) | λedge (nm) | Egopt (eV) |
|---|---|---|---|
| H2P-CO2H 1 | 420 (5.38), 515 (4.13), 550 (3.70), 592 (3.70), 650 (3.30) | 665.2 | 1.86 |
| cis-H2P-(CO2H)2 2-c | 420 (5.59), 515 (4.27), 550 (3.93), 591 (3.78), 649 (3.65) | 665.2 | 1.86 |
| H2P-(CO2H)3 3 | 419 (5.52), 515 (4.18), 549 (3.78), 590 (3.54), 649 (3.40) | 665.2 | 1.86 |
| ZnP-CO2H 4 | 426 (5.63), 557 (4.20), 597 (3.65) | 615.0 | 2.02 |
| cis-ZnP-(CO2H)2 5-c | 426 (5.56), 557 (4.29), 597 (3.80) | 615.0 | 2.02 |
| ZnP-(CO2H)3 6 | 426 (5.70),557 (4.33), 598 (3.89) | 615.0 | 2.02 |
| Dye | H2P-CO2H 1 | cis-H2P-(CO2H)2 2-c | H2P-(CO2H)3 3 | ZnP-CO2H 4 | cis-ZnP-(CO2H)2 5-c | ZnP-(CO2H)3 6 |
|---|---|---|---|---|---|---|
| Eox (V) | 0.60 | 0.60 | 0.84 | 0.29 | 0.39 | 0.42 |
| Dye | Jsc (mA/cm2) | Voc (V) | FF | Efficiency (%) |
|---|---|---|---|---|
| H2P-CO2H 1 | 0.36 | 0.32 | 0.32 | 0.04 |
| cis-H2P-(CO2H)2 2-c | 0.82 | 0.46 | 0.52 | 0.20 |
| H2P-(CO2H)3 3 | 0.87 | 0.44 | 0.38 | 0.15 |
| ZnP-CO2H 4 | 4.34 | 0.57 | 0.65 | 1.62 |
| cis-ZnP-(CO2H)2 5-c | 3.27 | 0.52 | 0.59 | 0.99 |
| ZnP-(CO2H)3 6 | 3.79 | 0.54 | 0.67 | 1.36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasrollahi, R.; Martín-Gomis, L.; Fernández-Lázaro, F.; Zakavi, S.; Sastre-Santos, Á. Effect of the Number of Anchoring and Electron-Donating Groups on the Efficiency of Free-Base- and Zn-Porphyrin-Sensitized Solar Cells. Materials 2019, 12, 650. https://doi.org/10.3390/ma12040650
Nasrollahi R, Martín-Gomis L, Fernández-Lázaro F, Zakavi S, Sastre-Santos Á. Effect of the Number of Anchoring and Electron-Donating Groups on the Efficiency of Free-Base- and Zn-Porphyrin-Sensitized Solar Cells. Materials. 2019; 12(4):650. https://doi.org/10.3390/ma12040650
Chicago/Turabian StyleNasrollahi, Raheleh, Luis Martín-Gomis, Fernando Fernández-Lázaro, Saeed Zakavi, and Ángela Sastre-Santos. 2019. "Effect of the Number of Anchoring and Electron-Donating Groups on the Efficiency of Free-Base- and Zn-Porphyrin-Sensitized Solar Cells" Materials 12, no. 4: 650. https://doi.org/10.3390/ma12040650